

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294714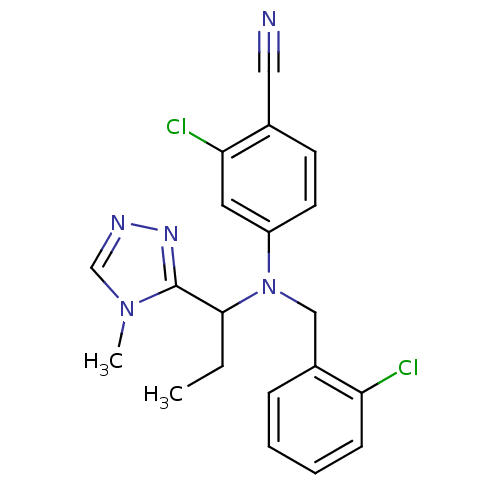 (4-((2-chlorobenzyl)(1-(4-methyl-4H-1,2,4-triazol-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells by alkaline phosphatase release based reporter gene assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294702 (4-((2-chlorobenzyl)(1-(1-methyl-1H-tetrazol-5-yl)e...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells by alkaline phosphatase release based reporter gene assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294705 (4-((2-chlorobenzyl)(1-(1-methyl-1H-tetrazol-5-yl)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells by alkaline phosphatase release based reporter gene assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294715 (4-((2-chlorobenzyl)(1-(4-methyl-4H-1,2,4-triazol-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells by alkaline phosphatase release based reporter gene assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration on alkaline phosphatase activity in human T47D breast carcinoma cell line. | Bioorg Med Chem Lett 13: 1313-6 (2003) BindingDB Entry DOI: 10.7270/Q2SN09HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM8903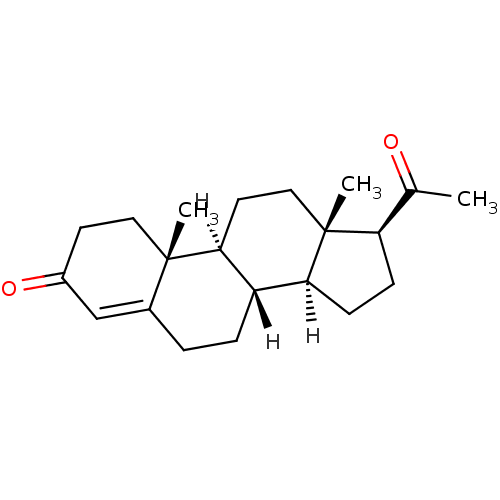 ((1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activation of progesterone receptor in human T47D cells after 20 hrs by PRE-tagged luciferase reporter gene assay | J Nat Prod 72: 1944-8 (2009) Article DOI: 10.1021/np9004882 BindingDB Entry DOI: 10.7270/Q2R49RQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50304978 ((S)-methyl 3-((3-chloro-4-cyanophenyl)(2-chloroben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at PR in human T47D cells | Bioorg Med Chem Lett 20: 371-4 (2010) Article DOI: 10.1016/j.bmcl.2009.10.092 BindingDB Entry DOI: 10.7270/Q2057G17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50219260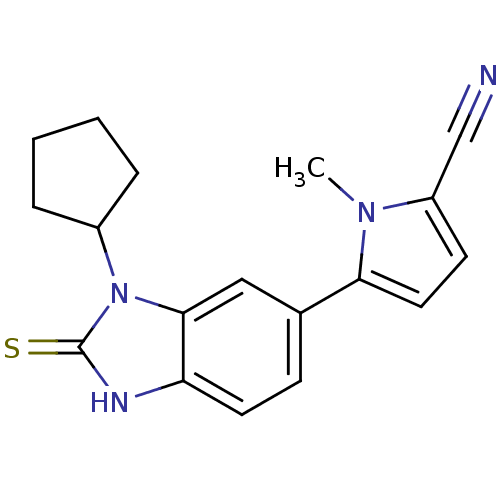 (5-(3-cyclopentyl-2-thioxo-2,3-dihydro-1H-benzimida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor expressed in T47D cells by alkaline phosphatase assay | Bioorg Med Chem 15: 6556-64 (2007) Article DOI: 10.1016/j.bmc.2007.07.011 BindingDB Entry DOI: 10.7270/Q2CV4HGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375436 (CHEMBL260869) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
NV Organon Curated by ChEMBL | Assay Description Agonist activity at human PRB expressed in CHO cells | Bioorg Med Chem 16: 2753-63 (2008) Article DOI: 10.1016/j.bmc.2008.01.010 BindingDB Entry DOI: 10.7270/Q2Z89D9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonistic activity against progesterone receptor in alkaline phosphatase assay using human T47D breast carcinoma cell line | Bioorg Med Chem Lett 12: 787-90 (2002) BindingDB Entry DOI: 10.7270/Q2WS8TSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration against PR (progesterone receptor) | Bioorg Med Chem Lett 13: 1313-6 (2003) BindingDB Entry DOI: 10.7270/Q2SN09HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50129152 ((6S,8S,10R,14S,15S)-17-Acetyl-6,10-dimethyl-13-(R)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory concentration for antagonistic activity towards human progesterone receptor (hPR) using the cotransfection assay in CV-1 cells | Bioorg Med Chem Lett 13: 2071-4 (2003) BindingDB Entry DOI: 10.7270/Q2SX6DSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375441 (CHEMBL260330) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
NV Organon Curated by ChEMBL | Assay Description Agonist activity at human PRB expressed in CHO cells | Bioorg Med Chem 16: 2753-63 (2008) Article DOI: 10.1016/j.bmc.2008.01.010 BindingDB Entry DOI: 10.7270/Q2Z89D9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Agonistic activity against human progesterone receptor expressed in CV-1 cells | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375821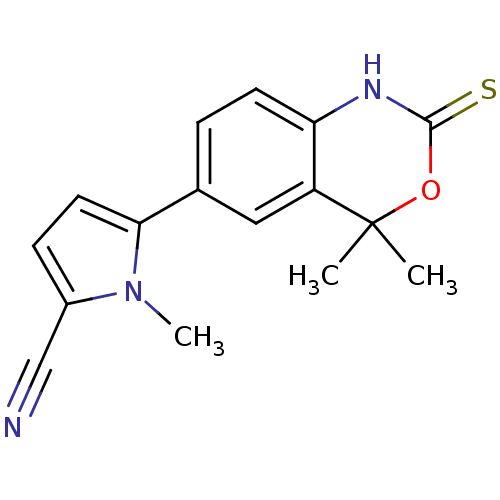 (TANAPROGET) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonist activity at human PR expressed in human T47D cells assessed as stimulation of alkaline phosphatase | J Med Chem 51: 1861-73 (2008) Article DOI: 10.1021/jm701080t BindingDB Entry DOI: 10.7270/Q2G44R56 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Effective concentration (EC50) against human progesterone receptor expressed in CV-1 cell | J Med Chem 41: 4354-9 (1998) Article DOI: 10.1021/jm980366a BindingDB Entry DOI: 10.7270/Q2V40TB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonistic activity to the human progesterone receptor(hPR)assayed in CV-1 cells in cotransfection assay. | J Med Chem 41: 291-302 (1998) Article DOI: 10.1021/jm9705768 BindingDB Entry DOI: 10.7270/Q29W0DMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonistic activity was measured for modulation of hPR-B (human progesterone receptor) in co-transfected CV-1 cells. | J Med Chem 41: 303-10 (1998) Article DOI: 10.1021/jm9705770 BindingDB Entry DOI: 10.7270/Q2RV0PC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Compound was tested for agonistic activity by cotransfection assay against human Progesterone receptor in CV-1 cells | J Med Chem 42: 1466-72 (1999) Article DOI: 10.1021/jm980723h BindingDB Entry DOI: 10.7270/Q2NK3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonist activity was determined against hPR (human progesterone receptor) compared to that of progesterone (100%) | Bioorg Med Chem Lett 8: 3365-70 (1999) BindingDB Entry DOI: 10.7270/Q2BC402X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM8903 ((1S,2R,10S,11S,14S,15S)-14-acetyl-2,15-dimethyltet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at PR in human T47D cells using p-nitrophenyl phosphate as substrate assessed as induction of alkaline phosphatase activity incubate... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116425 BindingDB Entry DOI: 10.7270/Q22F7SBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375438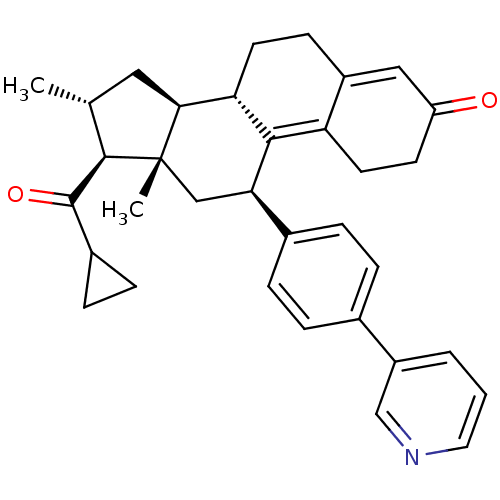 (CHEMBL410923) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
NV Organon Curated by ChEMBL | Assay Description Agonist activity at human PRB expressed in CHO cells | Bioorg Med Chem 16: 2753-63 (2008) Article DOI: 10.1016/j.bmc.2008.01.010 BindingDB Entry DOI: 10.7270/Q2Z89D9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110516 (4-Methyl-5-(2,4,4-trimethyl-1,4-dihydro-2H-benzo[d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonistic activity against progesterone receptor in alkaline phosphatase assay using human T47D breast carcinoma cell line | Bioorg Med Chem Lett 12: 787-90 (2002) BindingDB Entry DOI: 10.7270/Q2WS8TSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375442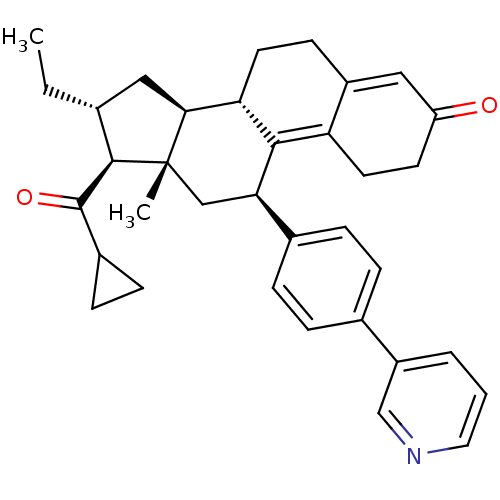 (CHEMBL260764) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a |
NV Organon Curated by ChEMBL | Assay Description Agonist activity at human PRB expressed in CHO cells | Bioorg Med Chem 16: 2753-63 (2008) Article DOI: 10.1016/j.bmc.2008.01.010 BindingDB Entry DOI: 10.7270/Q2Z89D9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375425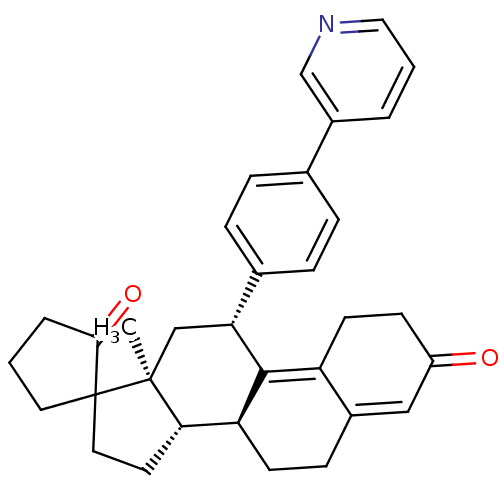 (CHEMBL259879) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a |
NV Organon Curated by ChEMBL | Assay Description Agonist activity at human PRB expressed in CHO cells | Bioorg Med Chem 16: 2753-63 (2008) Article DOI: 10.1016/j.bmc.2008.01.010 BindingDB Entry DOI: 10.7270/Q2Z89D9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298208 ((S)-2-chloro-4-((2-chloro-5-fluorobenzyl)(1-ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298206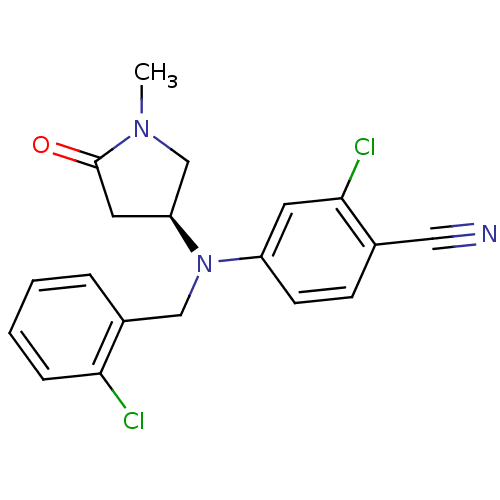 ((S)-2-chloro-4-((2-chlorobenzyl)(1-methyl-5-oxopyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50298207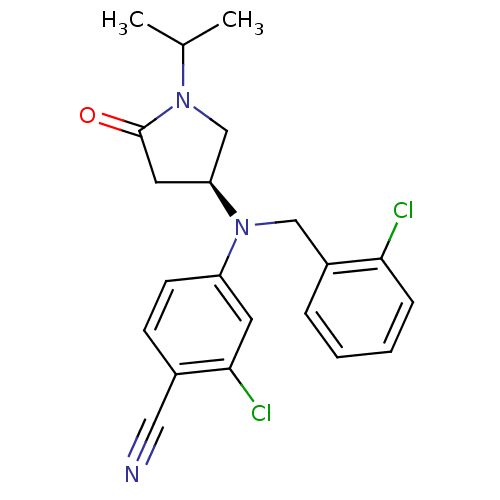 ((S)-2-chloro-4-((2-chlorobenzyl)(1-isopropyl-5-oxo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity | Bioorg Med Chem Lett 19: 4664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.06.081 BindingDB Entry DOI: 10.7270/Q25H7G9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Antagonistic activity at human progesterone receptor in CV-1 cells. | Bioorg Med Chem Lett 13: 2075-8 (2003) BindingDB Entry DOI: 10.7270/Q2P55P2M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404221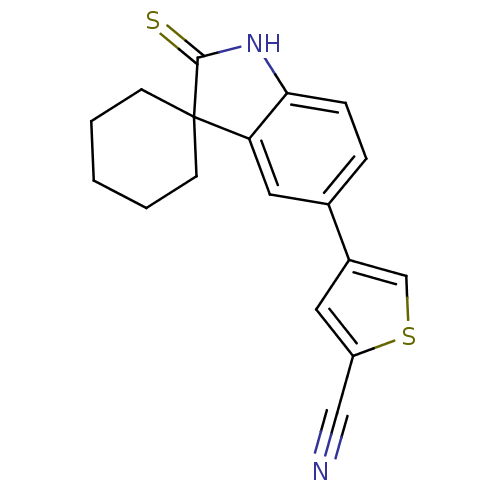 (CHEMBL285139) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50312005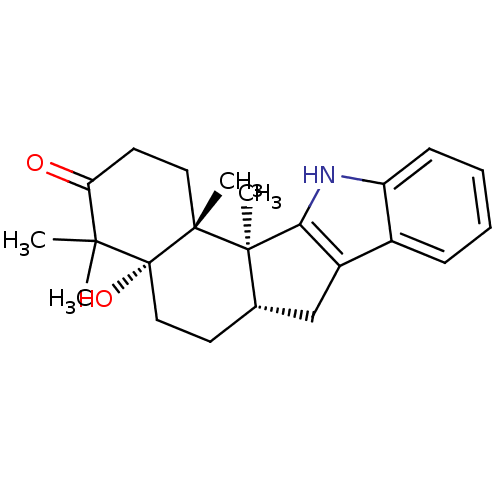 (CHEMBL1087512 | Lecanindole B) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activation of progesterone receptor in human T47D cells after 20 hrs by PRE-tagged luciferase reporter gene assay | J Nat Prod 72: 1944-8 (2009) Article DOI: 10.1021/np9004882 BindingDB Entry DOI: 10.7270/Q2R49RQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404182 (CHEMBL25463) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration on alkaline phosphatase activity in human T47D breast carcinoma cell line. | Bioorg Med Chem Lett 13: 1313-6 (2003) BindingDB Entry DOI: 10.7270/Q2SN09HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Agonistic activity against human progesterone receptor in T47D breast cancer cells | J Med Chem 46: 4104-12 (2003) Article DOI: 10.1021/jm020477g BindingDB Entry DOI: 10.7270/Q2QZ29C6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Agonistic activity by cotransfection assay against human Progesterone receptor in T47D cells | J Med Chem 42: 1466-72 (1999) Article DOI: 10.1021/jm980723h BindingDB Entry DOI: 10.7270/Q2NK3FQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110501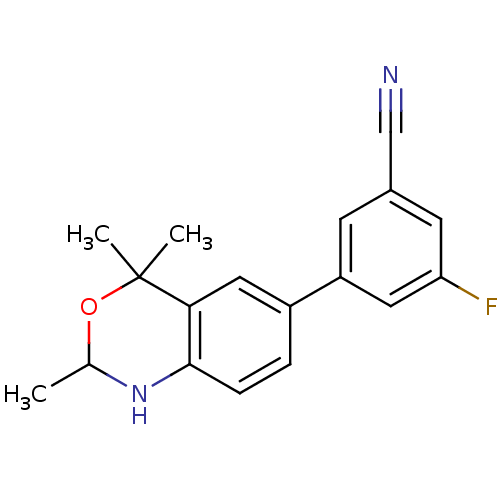 (3-Fluoro-5-(2,4,4-trimethyl-1,4-dihydro-2H-benzo[d...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonist activity against Progesterone receptor (PR) in transcriptional activation assay in human T47D breast carcinoma cell line | Bioorg Med Chem Lett 12: 787-90 (2002) BindingDB Entry DOI: 10.7270/Q2WS8TSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50110520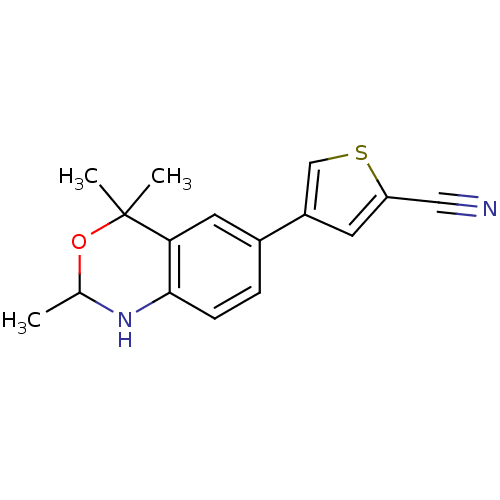 (4-(2,4,4-Trimethyl-1,4-dihydro-2H-benzo[d][1,3]oxa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonistic activity against progesterone receptor in alkaline phosphatase assay using human T47D breast carcinoma cell line | Bioorg Med Chem Lett 12: 787-90 (2002) BindingDB Entry DOI: 10.7270/Q2WS8TSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50126158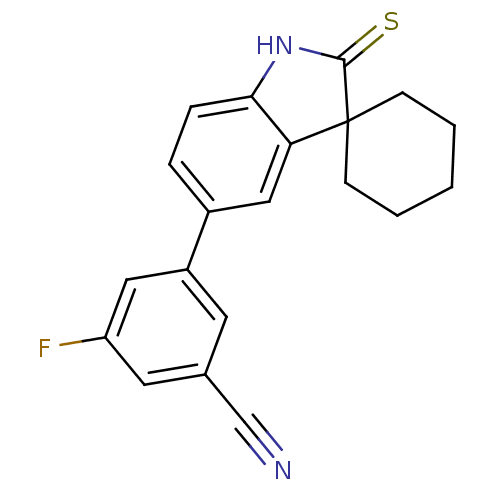 (3-fluoro-5-(2'-thioxo-1',2'-dihydrospiro[cyclohexa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375429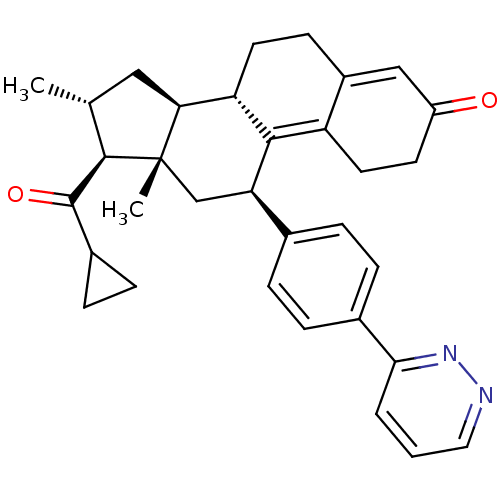 (CHEMBL438593) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
NV Organon Curated by ChEMBL | Assay Description Agonist activity at human PRB expressed in CHO cells | Bioorg Med Chem 16: 2753-63 (2008) Article DOI: 10.1016/j.bmc.2008.01.010 BindingDB Entry DOI: 10.7270/Q2Z89D9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50126111 (4-(4,4-Dimethyl-2-thioxo-1,4-dihydro-2H-benzo[d][1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration against PR (progesterone receptor) | Bioorg Med Chem Lett 13: 1313-6 (2003) BindingDB Entry DOI: 10.7270/Q2SN09HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50264311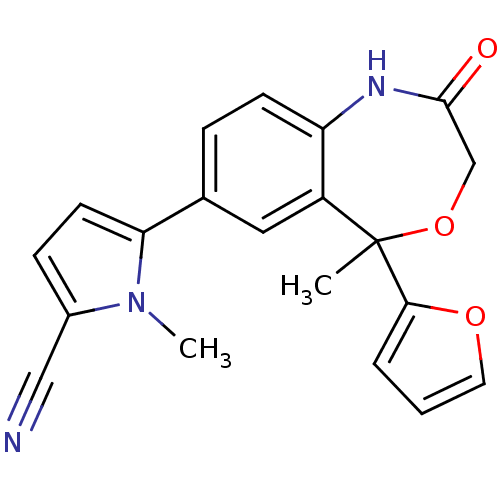 (5-(5-(furan-2-yl)-5-methyl-2-oxo-1,2,3,5-tetrahydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Activity at progesterone receptor assessed as alkaline phosphatase activity in human T47D cells | Bioorg Med Chem Lett 18: 5015-7 (2008) Article DOI: 10.1016/j.bmcl.2008.08.015 BindingDB Entry DOI: 10.7270/Q25B03D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50404206 (CHEMBL280613) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration on alkaline phosphatase activity in human T47D breast carcinoma cell line. | Bioorg Med Chem Lett 13: 1313-6 (2003) BindingDB Entry DOI: 10.7270/Q2SN09HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50126111 (4-(4,4-Dimethyl-2-thioxo-1,4-dihydro-2H-benzo[d][1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory activity against GR (glucocorticoid receptor) | Bioorg Med Chem Lett 13: 1313-6 (2003) BindingDB Entry DOI: 10.7270/Q2SN09HR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50243440 ((-)-7,9-difluoro-5-(6-methyl-7-oxa-bicyclo[4.1.0]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Agonist activity at human progesterone receptor | J Med Chem 51: 3696-9 (2008) Article DOI: 10.1021/jm8004256 BindingDB Entry DOI: 10.7270/Q2H131VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50304987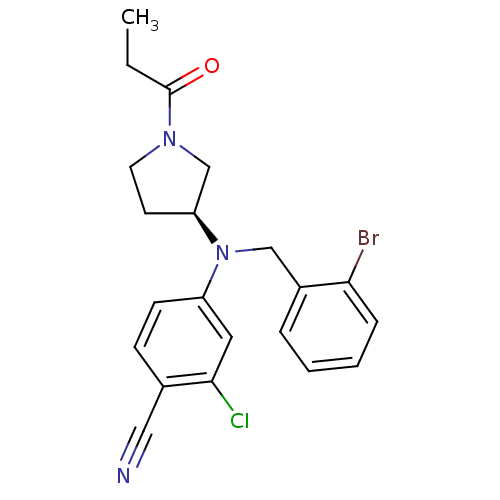 ((S)-4-((2-bromobenzyl)(1-propionylpyrrolidin-3-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at PR in human T47D cells | Bioorg Med Chem Lett 20: 371-4 (2010) Article DOI: 10.1016/j.bmcl.2009.10.092 BindingDB Entry DOI: 10.7270/Q2057G17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Agonist activity at human progesterone receptor in human T47D cells assessed as alkaline phosphatase activity | J Med Chem 51: 3696-9 (2008) Article DOI: 10.1021/jm8004256 BindingDB Entry DOI: 10.7270/Q2H131VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375439 (CHEMBL410304) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
NV Organon Curated by ChEMBL | Assay Description Agonist activity at human PRB expressed in CHO cells | Bioorg Med Chem 16: 2753-63 (2008) Article DOI: 10.1016/j.bmc.2008.01.010 BindingDB Entry DOI: 10.7270/Q2Z89D9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50375432 (CHEMBL258521) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
NV Organon Curated by ChEMBL | Assay Description Agonist activity at human PRB expressed in CHO cells | Bioorg Med Chem 16: 2753-63 (2008) Article DOI: 10.1016/j.bmc.2008.01.010 BindingDB Entry DOI: 10.7270/Q2Z89D9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Effective concentration of progesterone receptor agonist induction of alkaline phosphatase activity in human T47D breast carcinoma cells | Bioorg Med Chem Lett 13: 1317-20 (2003) BindingDB Entry DOI: 10.7270/Q2WS8SNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50219261 (5-(3-cyclobutyl-2-thioxo-2,3-dihydro-1H-benzimidaz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor expressed in T47D cells by alkaline phosphatase assay | Bioorg Med Chem 15: 6556-64 (2007) Article DOI: 10.1016/j.bmc.2007.07.011 BindingDB Entry DOI: 10.7270/Q2CV4HGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50294703 (4-((2-chlorobenzyl)(1-(4-methyl-4H-1,2,4-triazol-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at progesterone receptor in human T47D cells by alkaline phosphatase release based reporter gene assay | Bioorg Med Chem Lett 19: 2637-41 (2009) Article DOI: 10.1016/j.bmcl.2009.03.146 BindingDB Entry DOI: 10.7270/Q2FX79GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 692 total ) | Next | Last >> |