 Found 1040 hits of ec50 data for polymerid = 50001135,9850,9854
Found 1040 hits of ec50 data for polymerid = 50001135,9850,9854 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Agonist activity at mouse melanocortin 5 receptor expressed in HEK293 cells after 48 hrs by cAMP based beta-galactosidase reporter gene assay |
J Med Chem 54: 1379-90 (2011)
Article DOI: 10.1021/jm101425m
BindingDB Entry DOI: 10.7270/Q2FB537C |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50121261
(5-(2-Acetylamino-hexanoylamino)-19-[3-(diaminometh...)Show SMILES CCCC[C@@H](NC(C)=O)C(=O)N[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=[NH2+])NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(O)[C@H]2CCCN2C1=O)C(N)=O Show InChI InChI=1S/C53H73N13O9/c1-3-4-16-39(60-31(2)67)47(70)65-43-29-45(68)57-23-10-9-18-38(46(54)69)61-50(73)42(28-35-30-59-37-17-8-7-15-36(35)37)63-48(71)40(19-11-24-58-53(55)56)62-49(72)41(64-51(74)44-20-12-25-66(44)52(43)75)27-32-21-22-33-13-5-6-14-34(33)26-32/h5-8,13-15,17,21-22,26,30,38-44,51,59,64,74H,3-4,9-12,16,18-20,23-25,27-29H2,1-2H3,(H2,54,69)(H,57,68)(H,60,67)(H,61,73)(H,62,72)(H,63,71)(H,65,70)(H4,55,56,58)/p+1/t38-,39+,40+,41-,42-,43+,44+,51?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against human Melanocortin 5 receptor |
J Med Chem 45: 5287-94 (2002)
BindingDB Entry DOI: 10.7270/Q20G3KW8 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50211392
(Ac-Nle4-cyclo(Asp5-Pro6-D-4,4'-Bip7-Pro8-Trp9-Lys1...)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@]1([H])CCCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC2=O)C(N)=O)NC(=O)[C@H](CCCC)NC(C)=O Show InChI InChI=1S/C54H68N10O9/c1-3-4-17-41(58-33(2)65)49(68)61-44-31-47(66)56-26-11-10-19-40(48(55)67)59-50(69)42(30-37-32-57-39-18-9-8-16-38(37)39)60-51(70)45-20-12-27-63(45)53(72)43(62-52(71)46-21-13-28-64(46)54(44)73)29-34-22-24-36(25-23-34)35-14-6-5-7-15-35/h5-9,14-16,18,22-25,32,40-46,57H,3-4,10-13,17,19-21,26-31H2,1-2H3,(H2,55,67)(H,56,66)(H,58,65)(H,59,69)(H,60,70)(H,61,68)(H,62,71)/t40?,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human MC5R expressed in CHO cells assessed as cAMP accumulation |
J Med Chem 50: 2520-6 (2007)
Article DOI: 10.1021/jm0614275
BindingDB Entry DOI: 10.7270/Q2JQ10QM |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031479
(CHEMBL3358545)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CCCc2cn(CC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC1=O)C(N)=O)nn2 |r| Show InChI InChI=1S/C51H69N17O8/c1-3-4-16-38(59-30(2)69)45(71)61-39-18-10-14-33-28-68(67-66-33)22-20-37(44(52)70)60-49(75)42(24-32-26-57-36-17-9-8-15-35(32)36)64-47(73)40(19-11-21-56-51(53)54)62-48(74)41(23-31-12-6-5-7-13-31)63-50(76)43(65-46(39)72)25-34-27-55-29-58-34/h5-9,12-13,15,17,26-29,37-43,57H,3-4,10-11,14,16,18-25H2,1-2H3,(H2,52,70)(H,55,58)(H,59,69)(H,60,75)(H,61,71)(H,62,74)(H,63,76)(H,64,73)(H,65,72)(H4,53,54,56)/t37-,38-,39-,40-,41+,42-,43-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0420 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031473
(CHEMBL3358549)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CCn2cc(CCC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC1=O)C(N)=O)nn2 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0450 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031488
(CHEMBL3358541)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCN=[N+]=[N-])NC(=O)[C@H](CCCC)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C50H71N17O8/c1-4-6-17-36(43(51)69)61-48(74)41(25-32-27-57-35-19-12-11-16-34(32)35)65-45(71)38(20-13-22-56-50(52)53)62-47(73)40(24-31-14-9-8-10-15-31)64-49(75)42(26-33-28-55-29-58-33)66-46(72)39(21-23-59-67-54)63-44(70)37(18-7-5-2)60-30(3)68/h8-12,14-16,19,27-29,36-42,57H,4-7,13,17-18,20-26H2,1-3H3,(H2,51,69)(H,55,58)(H,60,68)(H,61,74)(H,62,73)(H,63,70)(H,64,75)(H,65,71)(H,66,72)(H4,52,53,56)/t36-,37-,38-,39-,40+,41-,42-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells by beta-galactosidase assay |
J Med Chem 47: 6702-10 (2004)
Article DOI: 10.1021/jm0492756
BindingDB Entry DOI: 10.7270/Q27D2VZK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Maximum agonist response for mouse melanocortin 5 receptor (MC5R) |
J Med Chem 47: 1514-26 (2004)
Article DOI: 10.1021/jm030452x
BindingDB Entry DOI: 10.7270/Q2XP75PK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50410399

(CHEMBL2096712)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-35(58-29(2)66)43(68)64-41-25-42(67)65-45(70)36(18-10-11-20-51)59-47(72)39(23-31-26-56-34-17-9-8-15-33(31)34)62-44(69)37(19-12-21-55-50(52)53)60-46(71)38(22-30-13-6-5-7-14-30)61-48(73)40(63-49(41)74)24-32-27-54-28-57-32/h5-9,13-15,17,26-28,35-41,56H,3-4,10-12,16,18-25,51H2,1-2H3,(H,54,57)(H,58,66)(H,59,72)(H,60,71)(H,61,73)(H,62,69)(H,63,74)(H,64,68)(H4,52,53,55)(H,65,67,70)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Effective concentration for mouse Melanocortin-5 receptor |
J Med Chem 48: 3060-75 (2005)
Article DOI: 10.1021/jm049010r
BindingDB Entry DOI: 10.7270/Q2PR7WS4 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50121267
(5-(2-Acetylamino-hexanoylamino)-19-[3-(diaminometh...)Show SMILES CCCC[C@@H](NC(C)=O)C(=O)N[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=[NH2+])NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H]2CCCN2C1=O)C(N)=O Show InChI InChI=1S/C53H71N13O9/c1-3-4-16-39(60-31(2)67)47(70)65-43-29-45(68)57-23-10-9-18-38(46(54)69)61-50(73)42(28-35-30-59-37-17-8-7-15-36(35)37)63-48(71)40(19-11-24-58-53(55)56)62-49(72)41(64-51(74)44-20-12-25-66(44)52(43)75)27-32-21-22-33-13-5-6-14-34(33)26-32/h5-8,13-15,17,21-22,26,30,38-44,59H,3-4,9-12,16,18-20,23-25,27-29H2,1-2H3,(H2,54,69)(H,57,68)(H,60,67)(H,61,73)(H,62,72)(H,63,71)(H,64,74)(H,65,70)(H4,55,56,58)/p+1/t38-,39+,40+,41-,42-,43+,44+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against human Melanocortin 5 receptor |
J Med Chem 45: 5287-94 (2002)
BindingDB Entry DOI: 10.7270/Q20G3KW8 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031469
(CHEMBL3358552)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CCn2cc(CC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC1=O)C(N)=O)nn2 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Activity in mouse melanocortin-5 receptor stably expressed in HEK293 cells |
J Med Chem 45: 5736-44 (2002)
BindingDB Entry DOI: 10.7270/Q2Q2410R |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Agonist activity towards mouse Melanocortin-5 receptor (mMC5R) |
J Med Chem 45: 3073-81 (2002)
BindingDB Entry DOI: 10.7270/Q2KK9CHF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Effective concentration against mouse Melanocortin 5 receptor |
J Med Chem 47: 2194-207 (2004)
Article DOI: 10.1021/jm0303608
BindingDB Entry DOI: 10.7270/Q2474BM0 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0710 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
In vitro activaction of mouse recombinant Melanocortin-5 receptor. |
J Med Chem 45: 2801-10 (2002)
BindingDB Entry DOI: 10.7270/Q27945DV |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50565811
(CHEMBL4792986)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H]2CCCN2C1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50141018
(1N-(1-carbamoyl-2-hydroxypropyl)-7-[3-amino(imino)...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H]1CCCCNC(=O)C[C@H](NC(=O)[C@@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N1)C(N)=O Show InChI InChI=1S/C55H72N16O10/c1-31(72)46(47(57)74)71-50(77)39-19-10-11-21-61-45(73)27-44(67-48(75)37(56)23-32-13-4-2-5-14-32)54(81)70-43(26-35-29-60-30-64-35)53(80)68-41(24-33-15-6-3-7-16-33)51(78)66-40(20-12-22-62-55(58)59)49(76)69-42(52(79)65-39)25-34-28-63-38-18-9-8-17-36(34)38/h2-9,13-18,28-31,37,39-44,46,63,72H,10-12,19-27,56H2,1H3,(H2,57,74)(H,60,64)(H,61,73)(H,65,79)(H,66,78)(H,67,75)(H,68,80)(H,69,76)(H,70,81)(H,71,77)(H4,58,59,62)/t31-,37+,39-,40+,41-,42-,43+,44+,46+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Maximum agonist response for mouse melanocortin 5 receptor (MC5R) |
J Med Chem 47: 1514-26 (2004)
Article DOI: 10.1021/jm030452x
BindingDB Entry DOI: 10.7270/Q2XP75PK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells |
J Med Chem 51: 1026-34 (2008)
Article DOI: 10.1021/jm701093y
BindingDB Entry DOI: 10.7270/Q29W0GC3 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
In vitro agonist potency for Mouse Melanocortin 5 receptor |
J Med Chem 48: 3328-36 (2005)
Article DOI: 10.1021/jm0490843
BindingDB Entry DOI: 10.7270/Q29K4C0B |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at mouse melanocortin receptor 5 expressed in HEK293 cells assessed as stimulation of cAMP accumulation incubated for 2 hrs by cAMP ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00561
BindingDB Entry DOI: 10.7270/Q2N301KK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Functional activity at the mouse melanocortin 5 receptor |
J Med Chem 44: 2247-52 (2001)
BindingDB Entry DOI: 10.7270/Q2GB24S4 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031492
(CHEMBL3358539)Show SMILES CCCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCN=[N+]=[N-])NC(=O)[C@H](CCCC)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C51H73N17O8/c1-4-6-9-20-37(44(52)70)62-49(75)42(26-33-28-58-36-19-13-12-17-35(33)36)66-46(72)39(21-14-23-57-51(53)54)63-48(74)41(25-32-15-10-8-11-16-32)65-50(76)43(27-34-29-56-30-59-34)67-47(73)40(22-24-60-68-55)64-45(71)38(18-7-5-2)61-31(3)69/h8,10-13,15-17,19,28-30,37-43,58H,4-7,9,14,18,20-27H2,1-3H3,(H2,52,70)(H,56,59)(H,61,69)(H,62,75)(H,63,74)(H,64,71)(H,65,76)(H,66,72)(H,67,73)(H4,53,54,57)/t37-,38-,39-,40-,41+,42-,43-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50121900

(Ac-Ser-Try-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pr...)Show SMILES CCCC[C@H](NC(=O)C(CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@@H]1C(=O)N[C@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61?,62+,65+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Effective concentration for mouse Melanocortin-5 receptor |
J Med Chem 48: 3060-75 (2005)
Article DOI: 10.1021/jm049010r
BindingDB Entry DOI: 10.7270/Q2PR7WS4 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50141023

(1N-(1-carbamoyl-2-hydroxypropyl)-15-[3-amino(imino...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@H](Cc2cnc[nH]2)C(=O)N[C@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N1)C(N)=O Show InChI InChI=1S/C51H65N15O9S2/c1-28(67)42(43(53)68)66-50(75)41-26-77-76-25-40(64-44(69)34(52)19-29-11-4-2-5-12-29)49(74)63-39(22-32-24-56-27-59-32)48(73)61-37(20-30-13-6-3-7-14-30)46(71)60-36(17-10-18-57-51(54)55)45(70)62-38(47(72)65-41)21-31-23-58-35-16-9-8-15-33(31)35/h2-9,11-16,23-24,27-28,34,36-42,58,67H,10,17-22,25-26,52H2,1H3,(H2,53,68)(H,56,59)(H,60,71)(H,61,73)(H,62,70)(H,63,74)(H,64,69)(H,65,72)(H,66,75)(H4,54,55,57)/t28-,34-,36+,37-,38-,39-,40+,41-,42+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Maximum agonist response for mouse melanocortin 5 receptor (MC5R) |
J Med Chem 47: 1514-26 (2004)
Article DOI: 10.1021/jm030452x
BindingDB Entry DOI: 10.7270/Q2XP75PK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50268792
(AC-Nle-c[Asp-His-DPhe-Pro-Trp-Lys]-NH2 | CHEMBL501...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C49H64N12O9/c1-3-4-16-36(55-29(2)62)44(65)58-39-25-42(63)52-20-11-10-18-35(43(50)64)56-45(66)37(23-31-26-53-34-17-9-8-15-33(31)34)59-48(69)41-19-12-21-61(41)49(70)40(22-30-13-6-5-7-14-30)60-46(67)38(57-47(39)68)24-32-27-51-28-54-32/h5-9,13-15,17,26-28,35-41,53H,3-4,10-12,16,18-25H2,1-2H3,(H2,50,64)(H,51,54)(H,52,63)(H,55,62)(H,56,66)(H,57,68)(H,58,65)(H,59,69)(H,60,67)/t35-,36-,37-,38-,39-,40+,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC5R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50268794
(Ac-Nle-c[Asp-His-DPhe-cis-4-guanidinyl-Pro-Trp-Lys...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H]2C[C@@H](CN2C(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)N=C(N)N)C(N)=O |r,wU:46.49,21.76,12.11,41.72,4.4,wD:57.61,25.25,39.45,(20.33,-40.8,;18.97,-40.08,;18.92,-38.54,;17.56,-37.81,;17.51,-36.27,;18.82,-35.46,;18.77,-33.92,;20.08,-33.11,;17.41,-33.19,;16.15,-35.55,;16.1,-34.01,;14.85,-36.36,;13.49,-35.64,;12.18,-36.45,;12.23,-37.99,;13.59,-38.71,;10.92,-38.8,;9.56,-38.08,;8.26,-38.89,;6.9,-38.17,;5.59,-38.98,;4.23,-38.25,;4.18,-36.71,;2.82,-35.99,;1.52,-36.8,;2.77,-34.45,;1.41,-33.72,;1.36,-32.19,;2.59,-31.24,;2.06,-29.79,;.52,-29.85,;-.55,-28.75,;-2.04,-29.13,;-2.45,-30.61,;-1.37,-31.7,;.1,-31.32,;4.08,-33.64,;4.74,-34.72,;5.06,-36.27,;5.44,-34.36,;6.06,-35.94,;7.6,-35.89,;8.02,-34.41,;6.75,-33.55,;6.7,-32.01,;5.34,-31.28,;8,-31.2,;7.95,-29.66,;6.59,-28.93,;6.55,-27.39,;5.19,-26.67,;3.88,-27.48,;3.94,-29.03,;5.29,-29.75,;9.36,-31.92,;10.67,-31.11,;10.62,-29.57,;12.03,-31.83,;13.33,-31.02,;13.29,-29.48,;14.51,-28.54,;14,-27.09,;12.46,-27.13,;12.02,-28.61,;12.08,-33.37,;13.44,-34.1,;14.74,-33.28,;8.54,-37.11,;10.04,-36.73,;10.8,-37.54,;10.65,-35.47,;2.93,-39.07,;1.57,-38.34,;2.98,-40.61,)| Show InChI InChI=1S/C50H67N15O9/c1-3-4-15-36(58-28(2)66)44(69)62-39-23-42(67)55-18-11-10-17-35(43(51)68)60-45(70)37(20-30-24-56-34-16-9-8-14-33(30)34)63-48(73)41-22-32(59-50(52)53)26-65(41)49(74)40(19-29-12-6-5-7-13-29)64-46(71)38(61-47(39)72)21-31-25-54-27-57-31/h5-9,12-14,16,24-25,27,32,35-41,56H,3-4,10-11,15,17-23,26H2,1-2H3,(H2,51,68)(H,54,57)(H,55,67)(H,58,66)(H,60,70)(H,61,72)(H,62,69)(H,63,73)(H,64,71)(H4,52,53,59)/t32-,35-,36-,37-,38-,39-,40+,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC5R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50268805
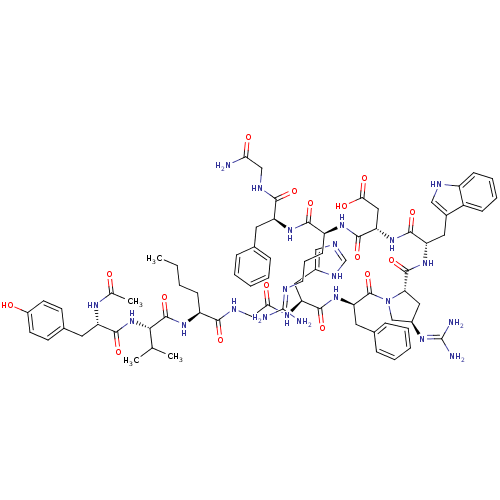
(Ac-Tyr-Val-Nle-Gly-His-DPhe-trans-Xaa-Trp-Asp-Arg-...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O)N=C(N)N |r,wU:8.8,45.46,59.63,96.101,35.35,77.82,wD:4.4,57.117,12.21,63.66,85.90,(3.89,-26.77,;2.57,-27.55,;1.22,-26.79,;-.1,-27.57,;-1.45,-26.81,;-2.78,-27.6,;-2.76,-29.14,;-1.41,-29.89,;-4.09,-29.92,;-4.07,-31.46,;-5.4,-32.25,;-6.73,-31.49,;-5.38,-33.78,;-4.04,-34.54,;-2.72,-33.75,;-2.73,-32.22,;-1.4,-31.43,;-.06,-32.19,;1.27,-31.4,;-.04,-33.74,;-1.37,-34.52,;-6.71,-34.57,;-6.71,-36.11,;-8.02,-36.89,;-5.35,-36.86,;-5.43,-29.17,;-5.44,-27.63,;-6.74,-29.95,;-1.46,-25.28,;-2.8,-24.52,;-.14,-24.49,;-.16,-22.95,;1.17,-22.16,;2.52,-22.92,;1.16,-20.62,;2.48,-19.84,;2.46,-18.3,;1.13,-17.55,;.96,-16.02,;-.56,-15.71,;-1.31,-17.05,;-.27,-18.18,;3.82,-20.6,;3.85,-22.14,;5.15,-19.82,;6.5,-20.57,;6.51,-22.12,;7.85,-22.87,;7.87,-24.42,;9.21,-25.18,;10.53,-24.39,;10.51,-22.85,;9.17,-22.09,;7.86,-19.86,;9.11,-20.76,;7.93,-18.32,;6.9,-17.17,;7.69,-15.84,;9.19,-16.18,;9.34,-17.71,;10.66,-18.5,;10.65,-20.04,;12,-17.74,;13.32,-18.54,;13.31,-20.08,;14.59,-20.94,;16.03,-20.4,;16.98,-21.61,;16.14,-22.89,;16.51,-24.38,;15.41,-25.47,;13.93,-25.05,;13.55,-23.56,;14.65,-22.48,;14.67,-17.78,;14.68,-16.24,;15.99,-18.57,;17.33,-17.82,;17.34,-16.27,;18.68,-15.52,;20.02,-16.29,;18.7,-13.97,;18.66,-18.59,;18.64,-20.14,;20,-17.84,;21.32,-18.63,;21.3,-20.18,;22.62,-20.95,;22.61,-22.5,;23.94,-23.27,;23.91,-24.81,;22.59,-25.56,;25.26,-25.58,;22.66,-17.88,;22.67,-16.34,;23.99,-18.65,;25.33,-17.89,;25.35,-16.35,;26.69,-15.6,;28.02,-16.38,;29.36,-15.63,;29.38,-14.09,;28.05,-13.3,;26.71,-14.06,;26.66,-18.67,;26.64,-20.22,;28,-17.92,;29.32,-18.7,;30.66,-17.95,;31.99,-18.73,;30.68,-16.41,;7.08,-14.43,;7.99,-13.19,;7.38,-11.78,;9.52,-13.37,)| Show InChI InChI=1S/C77H102N22O16/c1-5-6-21-53(93-74(114)65(42(2)3)98-72(112)56(89-43(4)100)30-46-24-26-50(101)27-25-46)66(106)87-39-63(103)91-58(33-48-37-83-41-88-48)70(110)97-60(31-45-18-11-8-12-19-45)75(115)99-40-49(90-77(81)82)34-61(99)73(113)96-57(32-47-36-85-52-22-14-13-20-51(47)52)69(109)95-59(35-64(104)105)71(111)92-54(23-15-28-84-76(79)80)68(108)94-55(67(107)86-38-62(78)102)29-44-16-9-7-10-17-44/h7-14,16-20,22,24-27,36-37,41-42,49,53-61,65,85,101H,5-6,15,21,23,28-35,38-40H2,1-4H3,(H2,78,102)(H,83,88)(H,86,107)(H,87,106)(H,89,100)(H,91,103)(H,92,111)(H,93,114)(H,94,108)(H,95,109)(H,96,113)(H,97,110)(H,98,112)(H,104,105)(H4,79,80,84)(H4,81,82,90)/t49-,53+,54+,55+,56+,57+,58+,59+,60-,61+,65+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC5R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50268803
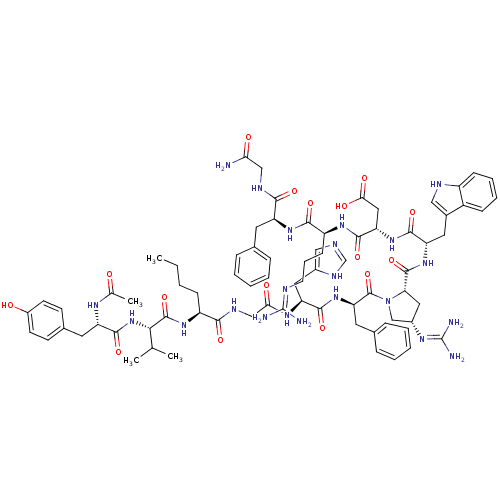
(Ac-Tyr-Val-Nle-Gly-His-DPhe-cis-Xaa-Trp-Asp-Arg-Ph...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O)N=C(N)N |r,wU:8.8,45.46,59.63,57.117,96.101,35.35,77.82,wD:4.4,12.21,63.66,85.90,(2.53,-50.05,;1.21,-50.83,;-.13,-50.07,;-1.45,-50.85,;-2.8,-50.09,;-4.13,-50.88,;-4.11,-52.42,;-2.77,-53.17,;-5.44,-53.2,;-5.43,-54.75,;-6.75,-55.53,;-8.09,-54.77,;-6.74,-57.06,;-5.4,-57.82,;-4.07,-57.04,;-4.09,-55.5,;-2.76,-54.71,;-1.41,-55.47,;-.08,-54.68,;-1.39,-57.02,;-2.73,-57.8,;-8.07,-57.85,;-8.06,-59.39,;-9.38,-60.18,;-6.71,-60.14,;-6.78,-52.45,;-6.8,-50.91,;-8.1,-53.23,;-2.82,-48.56,;-4.16,-47.8,;-1.49,-47.77,;-1.51,-46.23,;-.18,-45.44,;1.17,-46.2,;-.2,-43.9,;1.13,-43.12,;1.11,-41.58,;-.23,-40.83,;-.4,-39.3,;-1.91,-38.99,;-2.66,-40.33,;-1.63,-41.47,;2.47,-43.88,;2.49,-45.42,;3.8,-43.1,;5.14,-43.85,;5.16,-45.4,;6.5,-46.15,;6.51,-47.7,;7.85,-48.46,;9.17,-47.68,;9.16,-46.14,;7.81,-45.37,;6.5,-43.14,;7.76,-44.04,;6.57,-41.61,;5.55,-40.45,;6.33,-39.12,;7.84,-39.46,;7.99,-40.99,;9.31,-41.78,;9.3,-43.32,;10.65,-41.02,;11.97,-41.82,;11.95,-43.36,;13.23,-44.22,;14.68,-43.68,;15.63,-44.89,;14.78,-46.18,;15.16,-47.67,;14.06,-48.75,;12.58,-48.33,;12.19,-46.84,;13.3,-45.76,;13.31,-41.06,;13.32,-39.52,;14.64,-41.85,;15.98,-41.1,;15.99,-39.56,;17.33,-38.8,;18.67,-39.57,;17.35,-37.26,;17.3,-41.87,;17.29,-43.42,;18.65,-41.12,;19.96,-41.91,;19.94,-43.46,;21.27,-44.24,;21.26,-45.78,;22.59,-46.55,;22.56,-48.09,;21.24,-48.84,;23.9,-48.86,;21.3,-41.16,;21.31,-39.62,;22.64,-41.93,;23.98,-41.17,;23.99,-39.64,;25.33,-38.88,;26.66,-39.66,;28.01,-38.91,;28.02,-37.37,;26.7,-36.58,;25.36,-37.34,;25.31,-41.95,;25.29,-43.5,;26.65,-41.2,;27.97,-41.99,;29.31,-41.23,;30.64,-42.01,;29.32,-39.69,;5.72,-37.71,;6.64,-36.47,;6.03,-35.06,;8.17,-36.65,)| Show InChI InChI=1S/C77H102N22O16/c1-5-6-21-53(93-74(114)65(42(2)3)98-72(112)56(89-43(4)100)30-46-24-26-50(101)27-25-46)66(106)87-39-63(103)91-58(33-48-37-83-41-88-48)70(110)97-60(31-45-18-11-8-12-19-45)75(115)99-40-49(90-77(81)82)34-61(99)73(113)96-57(32-47-36-85-52-22-14-13-20-51(47)52)69(109)95-59(35-64(104)105)71(111)92-54(23-15-28-84-76(79)80)68(108)94-55(67(107)86-38-62(78)102)29-44-16-9-7-10-17-44/h7-14,16-20,22,24-27,36-37,41-42,49,53-61,65,85,101H,5-6,15,21,23,28-35,38-40H2,1-4H3,(H2,78,102)(H,83,88)(H,86,107)(H,87,106)(H,89,100)(H,91,103)(H,92,111)(H,93,114)(H,94,108)(H,95,109)(H,96,113)(H,97,110)(H,98,112)(H,104,105)(H4,79,80,84)(H4,81,82,90)/t49-,53-,54-,55-,56-,57-,58-,59-,60+,61-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC5R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50268800
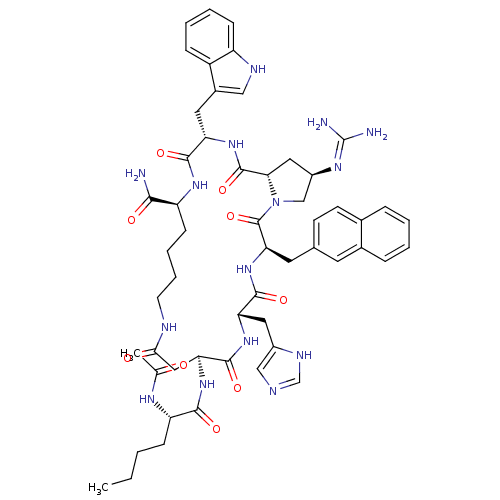
(Ac-Nle-c[Asp-His-DNal(2')-cis-4-guanidinyl-Pro-Trp...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2C[C@H](CN2C(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)N=C(N)N)C(N)=O |r,wU:46.49,21.81,12.11,4.4,39.45,wD:61.66,25.25,41.77,(17.08,-28.2,;15.72,-27.49,;15.65,-25.95,;14.29,-25.23,;14.22,-23.7,;15.52,-22.87,;15.46,-21.33,;16.76,-20.51,;14.09,-20.62,;12.85,-22.98,;12.79,-21.44,;11.56,-23.81,;10.19,-23.09,;8.89,-23.93,;8.95,-25.47,;10.32,-26.17,;7.66,-26.29,;6.29,-25.58,;4.99,-26.4,;3.62,-25.69,;2.33,-26.52,;.96,-25.8,;.9,-24.26,;-.47,-23.55,;-1.77,-24.38,;-.53,-22.01,;-1.9,-21.3,;-1.97,-19.76,;-.75,-18.81,;-1.29,-17.36,;-2.83,-17.43,;-3.91,-16.34,;-5.39,-16.73,;-5.79,-18.22,;-4.71,-19.3,;-3.24,-18.91,;.77,-21.19,;1.43,-22.26,;1.77,-23.81,;2.13,-21.9,;2.76,-23.47,;4.3,-23.41,;4.72,-21.93,;3.43,-21.07,;3.37,-19.54,;2,-18.82,;4.67,-18.71,;4.6,-17.17,;3.24,-16.46,;1.94,-17.29,;.57,-16.58,;.5,-15.04,;-.85,-14.32,;-.92,-12.79,;.39,-11.96,;1.75,-12.68,;1.81,-14.22,;3.17,-14.92,;6.03,-19.42,;7.33,-18.6,;7.26,-17.05,;8.7,-19.3,;10,-18.48,;9.94,-16.94,;11.15,-15.99,;10.63,-14.55,;9.09,-14.6,;8.66,-16.08,;8.76,-20.84,;10.13,-21.56,;11.43,-20.73,;5.25,-24.61,;6.76,-24.38,;7.52,-24.99,;7.32,-22.97,;-.34,-26.63,;-1.71,-25.91,;-.27,-28.17,)| Show InChI InChI=1S/C54H69N15O9/c1-3-4-14-40(62-30(2)70)48(73)66-43-25-46(71)59-19-10-9-16-39(47(55)72)64-49(74)41(22-34-26-60-38-15-8-7-13-37(34)38)67-52(77)45-24-36(63-54(56)57)28-69(45)53(78)44(21-31-17-18-32-11-5-6-12-33(32)20-31)68-50(75)42(65-51(43)76)23-35-27-58-29-61-35/h5-8,11-13,15,17-18,20,26-27,29,36,39-45,60H,3-4,9-10,14,16,19,21-25,28H2,1-2H3,(H2,55,72)(H,58,61)(H,59,71)(H,62,70)(H,64,74)(H,65,76)(H,66,73)(H,67,77)(H,68,75)(H4,56,57,63)/t36-,39+,40+,41+,42+,43+,44-,45+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC5R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50268804
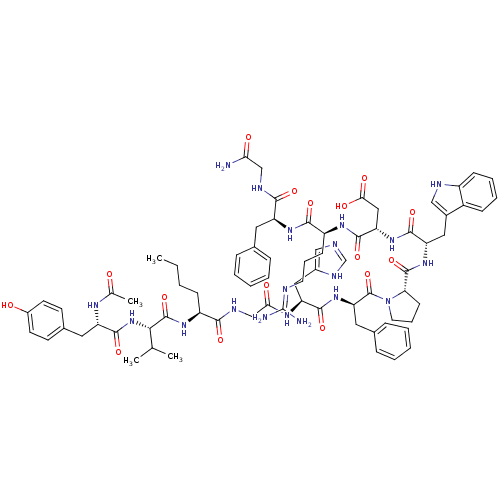
(Ac-Tyr-Val-Nle-Gly-His-DPhe-Pro-Trp-Asp-Arg-Phe-Gl...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |r,wU:8.8,45.46,59.63,96.101,35.35,77.82,wD:4.4,12.21,63.66,85.90,(2.68,-6.73,;1.36,-7.52,;.01,-6.75,;-1.31,-7.53,;-2.65,-6.78,;-3.99,-7.57,;-3.97,-9.11,;-2.62,-9.86,;-5.3,-9.89,;-5.28,-11.43,;-6.61,-12.22,;-7.94,-11.46,;-6.59,-13.75,;-5.25,-14.51,;-3.92,-13.72,;-3.94,-12.19,;-2.61,-11.4,;-1.26,-12.16,;.06,-11.37,;-1.25,-13.71,;-2.58,-14.49,;-7.92,-14.54,;-7.92,-16.08,;-9.23,-16.86,;-6.56,-16.83,;-6.64,-9.14,;-6.65,-7.59,;-7.95,-9.92,;-2.67,-5.24,;-4.01,-4.49,;-1.35,-4.46,;-1.37,-2.92,;-.03,-2.13,;1.32,-2.89,;-.05,-.59,;1.27,.2,;1.26,1.73,;-.08,2.49,;-.25,4.02,;-1.77,4.33,;-2.52,2.99,;-1.48,1.85,;2.62,-.57,;2.64,-2.11,;3.94,.22,;5.29,-.54,;5.31,-2.09,;6.64,-2.84,;6.66,-4.39,;8,-5.15,;9.32,-4.36,;9.3,-2.82,;7.96,-2.06,;6.65,.17,;7.9,-.73,;6.72,1.71,;5.7,2.87,;6.48,4.19,;7.98,3.86,;8.13,2.32,;9.45,1.54,;9.44,-0,;10.8,2.29,;12.11,1.49,;12.1,-.05,;13.38,-.9,;14.82,-.37,;15.78,-1.58,;14.93,-2.86,;15.31,-4.35,;14.21,-5.43,;12.73,-5.02,;12.34,-3.52,;13.44,-2.45,;13.46,2.25,;13.47,3.79,;14.78,1.46,;16.13,2.22,;16.14,3.76,;17.48,4.52,;18.82,3.75,;17.5,6.06,;17.45,1.44,;17.43,-.1,;18.79,2.2,;20.11,1.4,;20.09,-.14,;21.42,-.92,;21.41,-2.46,;22.74,-3.24,;22.71,-4.78,;21.38,-5.53,;24.05,-5.55,;21.45,2.16,;21.46,3.7,;22.79,1.39,;24.13,2.15,;24.14,3.68,;25.48,4.44,;26.81,3.66,;28.16,4.41,;28.17,5.95,;26.84,6.74,;25.51,5.98,;25.46,1.36,;25.44,-.18,;26.8,2.12,;28.12,1.33,;29.46,2.09,;30.79,1.3,;29.47,3.63,)| Show InChI InChI=1S/C76H99N19O16/c1-5-6-22-53(89-74(110)65(43(2)3)94-72(108)56(86-44(4)96)33-47-26-28-50(97)29-27-47)66(102)84-41-63(99)87-58(36-49-39-80-42-85-49)70(106)93-60(34-46-19-11-8-12-20-46)75(111)95-31-16-25-61(95)73(109)92-57(35-48-38-82-52-23-14-13-21-51(48)52)69(105)91-59(37-64(100)101)71(107)88-54(24-15-30-81-76(78)79)68(104)90-55(67(103)83-40-62(77)98)32-45-17-9-7-10-18-45/h7-14,17-21,23,26-29,38-39,42-43,53-61,65,82,97H,5-6,15-16,22,24-25,30-37,40-41H2,1-4H3,(H2,77,98)(H,80,85)(H,83,103)(H,84,102)(H,86,96)(H,87,99)(H,88,107)(H,89,110)(H,90,104)(H,91,105)(H,92,109)(H,93,106)(H,94,108)(H,100,101)(H4,78,79,81)/t53-,54-,55-,56-,57-,58-,59-,60+,61-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC5R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50268798
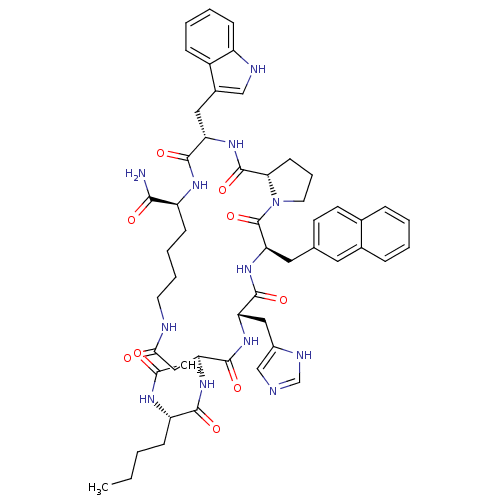
(Ac-Nle-c[Asp-His-DNaI(2')-Pro-Trp-Lys]-NH2 | CHEMB...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C53H66N12O9/c1-3-4-15-40(59-31(2)66)48(69)62-43-27-46(67)56-21-10-9-17-39(47(54)68)60-49(70)41(25-35-28-57-38-16-8-7-14-37(35)38)63-52(73)45-18-11-22-65(45)53(74)44(24-32-19-20-33-12-5-6-13-34(33)23-32)64-50(71)42(61-51(43)72)26-36-29-55-30-58-36/h5-8,12-14,16,19-20,23,28-30,39-45,57H,3-4,9-11,15,17-18,21-22,24-27H2,1-2H3,(H2,54,68)(H,55,58)(H,56,67)(H,59,66)(H,60,70)(H,61,72)(H,62,69)(H,63,73)(H,64,71)/t39-,40-,41-,42-,43-,44+,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC5R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50141027
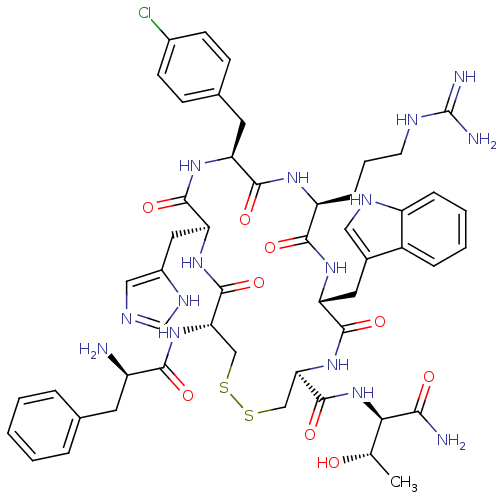
(1N-(1-carbamoyl-2-hydroxypropyl)-15-[3-amino(imino...)Show SMILES C[C@H](O)[C@@H](NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](Cc2ccc(Cl)cc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N1)C(N)=O Show InChI InChI=1S/C51H64ClN15O9S2/c1-27(68)42(43(54)69)67-50(76)41-25-78-77-24-40(65-44(70)34(53)18-28-8-3-2-4-9-28)49(75)64-39(21-32-23-57-26-60-32)48(74)62-37(19-29-13-15-31(52)16-14-29)46(72)61-36(12-7-17-58-51(55)56)45(71)63-38(47(73)66-41)20-30-22-59-35-11-6-5-10-33(30)35/h2-6,8-11,13-16,22-23,26-27,34,36-42,59,68H,7,12,17-21,24-25,53H2,1H3,(H2,54,69)(H,57,60)(H,61,72)(H,62,74)(H,63,71)(H,64,75)(H,65,70)(H,66,73)(H,67,76)(H4,55,56,58)/t27-,34+,36-,37-,38+,39-,40-,41+,42+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Maximum agonist response for mouse melanocortin 5 receptor (MC5R) |
J Med Chem 47: 1514-26 (2004)
Article DOI: 10.1021/jm030452x
BindingDB Entry DOI: 10.7270/Q2XP75PK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5R expressed in HEK293 cells assessed as increase in forskolin-induced cAMP accumulation preincubated for 2 hrs followed ... |
J Med Chem 60: 805-813 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01707
BindingDB Entry DOI: 10.7270/Q2R213MT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at mouse MC5R by alphascreen cAMP assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00641
BindingDB Entry DOI: 10.7270/Q2VQ36C5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50033130
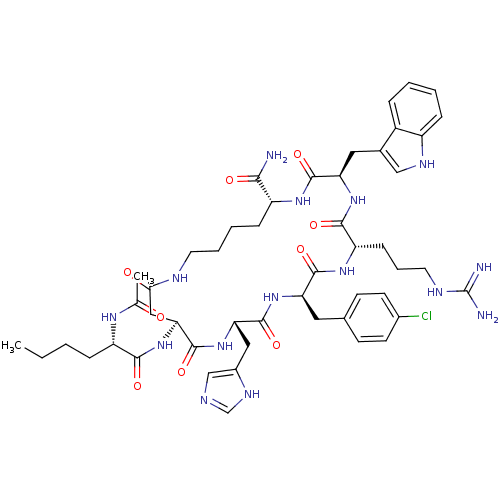
((3R,6S,9R,12S,15S,23R)-15-((S)-2-Acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C50H68ClN15O9/c1-3-4-11-36(60-28(2)67)44(70)66-41-24-42(68)56-19-8-7-13-35(43(52)69)61-47(73)39(22-30-25-58-34-12-6-5-10-33(30)34)64-45(71)37(14-9-20-57-50(53)54)62-46(72)38(21-29-15-17-31(51)18-16-29)63-48(74)40(65-49(41)75)23-32-26-55-27-59-32/h5-6,10,12,15-18,25-27,35-41,58H,3-4,7-9,11,13-14,19-24H2,1-2H3,(H2,52,69)(H,55,59)(H,56,68)(H,60,67)(H,61,73)(H,62,72)(H,63,74)(H,64,71)(H,65,75)(H,66,70)(H4,53,54,57)/t35-,36+,37+,38-,39-,40+,41+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.117 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Evaluated for agonist activity at cloned mammalian mouse Melanocortin 5 receptor |
J Med Chem 38: 3454-61 (1995)
BindingDB Entry DOI: 10.7270/Q2Z32094 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
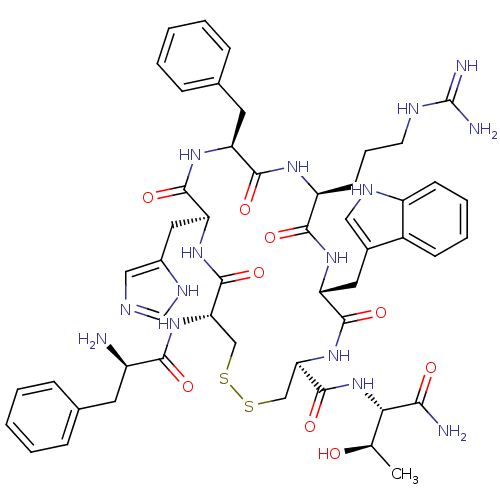
(Mus musculus (Mouse)) | BDBM50141026
(1N-(1-carbamoyl-2-hydroxypropyl)-15-[3-amino(imino...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N1)C(N)=O Show InChI InChI=1S/C51H65N15O9S2/c1-28(67)42(43(53)68)66-50(75)41-26-77-76-25-40(64-44(69)34(52)19-29-11-4-2-5-12-29)49(74)63-39(22-32-24-56-27-59-32)48(73)61-37(20-30-13-6-3-7-14-30)46(71)60-36(17-10-18-57-51(54)55)45(70)62-38(47(72)65-41)21-31-23-58-35-16-9-8-15-33(31)35/h2-9,11-16,23-24,27-28,34,36-42,58,67H,10,17-22,25-26,52H2,1H3,(H2,53,68)(H,56,59)(H,60,71)(H,61,73)(H,62,70)(H,63,74)(H,64,69)(H,65,72)(H,66,75)(H4,54,55,57)/t28-,34-,36+,37+,38-,39+,40+,41-,42+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Maximum agonist response for mouse melanocortin 5 receptor (MC5R) |
J Med Chem 47: 1514-26 (2004)
Article DOI: 10.1021/jm030452x
BindingDB Entry DOI: 10.7270/Q2XP75PK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50121268
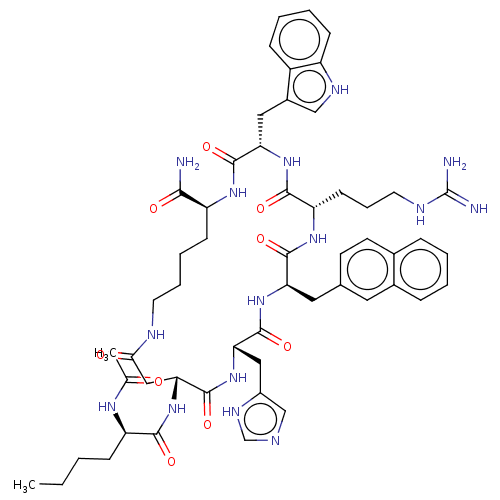
(21-(2-Acetylamino-hexanoylamino)-7-[3-(diaminometh...)Show SMILES CCCC[C@@H](NC(C)=O)C(=O)N[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O Show InChI InChI=1S/C54H71N15O9/c1-3-4-15-40(63-31(2)70)48(73)69-45-27-46(71)59-21-10-9-17-39(47(55)72)64-51(76)43(25-35-28-61-38-16-8-7-14-37(35)38)67-49(74)41(18-11-22-60-54(56)57)65-50(75)42(24-32-19-20-33-12-5-6-13-34(33)23-32)66-52(77)44(68-53(45)78)26-36-29-58-30-62-36/h5-8,12-14,16,19-20,23,28-30,39-45,61H,3-4,9-11,15,17-18,21-22,24-27H2,1-2H3,(H2,55,72)(H,58,62)(H,59,71)(H,63,70)(H,64,76)(H,65,75)(H,66,77)(H,67,74)(H,68,78)(H,69,73)(H4,56,57,60)/t39-,40+,41-,42+,43-,44-,45+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity against human Melanocortin 5 receptor |
J Med Chem 45: 5287-94 (2002)
BindingDB Entry DOI: 10.7270/Q20G3KW8 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Homo sapiens (Human)) | BDBM50105879
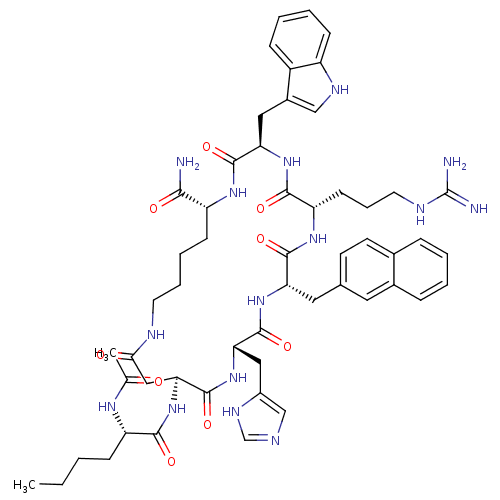
(15-(2-Acetylamino-hexanoylamino)-6-(3-guanidino-pr...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C54H71N15O9/c1-3-4-15-40(63-31(2)70)48(73)69-45-27-46(71)59-21-10-9-17-39(47(55)72)64-51(76)43(25-35-28-61-38-16-8-7-14-37(35)38)67-49(74)41(18-11-22-60-54(56)57)65-50(75)42(24-32-19-20-33-12-5-6-13-34(33)23-32)66-52(77)44(68-53(45)78)26-36-29-58-30-62-36/h5-8,12-14,16,19-20,23,28-30,39-45,61H,3-4,9-11,15,17-18,21-22,24-27H2,1-2H3,(H2,55,72)(H,58,62)(H,59,71)(H,63,70)(H,64,76)(H,65,75)(H,66,77)(H,67,74)(H,68,78)(H,69,73)(H4,56,57,60)/t39-,40+,41+,42+,43-,44+,45+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Signal transduction efficacy in cAMP assay in CHO cells expressing human melanocortin receptor 5 |
J Med Chem 44: 3665-72 (2001)
BindingDB Entry DOI: 10.7270/Q2RF5T9S |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Agonist activity at mouse melanocortin receptor 5 expressed in HEK293 cells assessed as stimulation of cAMP accumulation incubated for 2 hrs by cAMP ... |
J Med Chem 63: 2194-2208 (2020)
Article DOI: 10.1021/acs.jmedchem.9b00860
BindingDB Entry DOI: 10.7270/Q25142KG |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031496
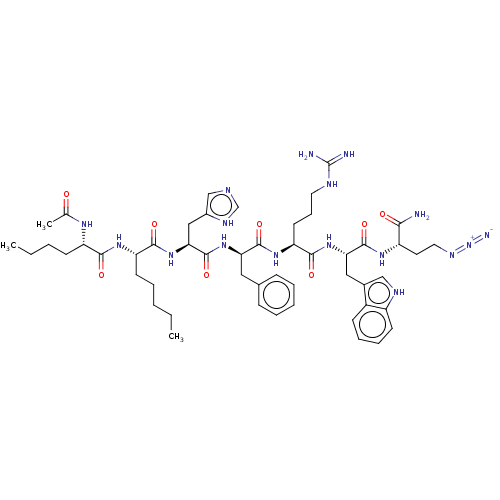
(CHEMBL3358535)Show SMILES CCCCC[C@H](NC(=O)[C@H](CCCC)NC(C)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCN=[N+]=[N-])C(N)=O |r| Show InChI InChI=1S/C51H73N17O8/c1-4-6-9-20-39(63-45(71)38(18-7-5-2)61-31(3)69)46(72)67-43(27-34-29-56-30-59-34)50(76)65-41(25-32-15-10-8-11-16-32)48(74)64-40(21-14-23-57-51(53)54)47(73)66-42(26-33-28-58-36-19-13-12-17-35(33)36)49(75)62-37(44(52)70)22-24-60-68-55/h8,10-13,15-17,19,28-30,37-43,58H,4-7,9,14,18,20-27H2,1-3H3,(H2,52,70)(H,56,59)(H,61,69)(H,62,75)(H,63,71)(H,64,74)(H,65,76)(H,66,73)(H,67,72)(H4,53,54,57)/t37-,38-,39-,40-,41+,42-,43-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031490
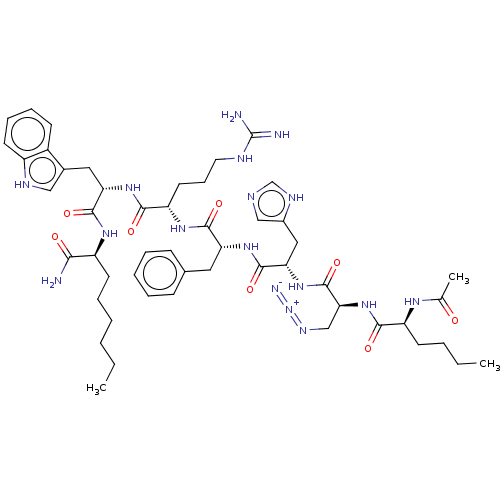
(CHEMBL3358540)Show SMILES CCCCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CN=[N+]=[N-])NC(=O)[C@H](CCCC)NC(C)=O)C(N)=O |r| Show InChI InChI=1S/C51H73N17O8/c1-4-6-8-12-21-37(44(52)70)62-48(74)41(25-33-27-58-36-20-14-13-18-35(33)36)65-46(72)39(22-15-23-57-51(53)54)63-47(73)40(24-32-16-10-9-11-17-32)64-49(75)42(26-34-28-56-30-59-34)66-50(76)43(29-60-68-55)67-45(71)38(19-7-5-2)61-31(3)69/h9-11,13-14,16-18,20,27-28,30,37-43,58H,4-8,12,15,19,21-26,29H2,1-3H3,(H2,52,70)(H,56,59)(H,61,69)(H,62,74)(H,63,73)(H,64,75)(H,65,72)(H,66,76)(H,67,71)(H4,53,54,57)/t37-,38-,39-,40+,41-,42-,43-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031478
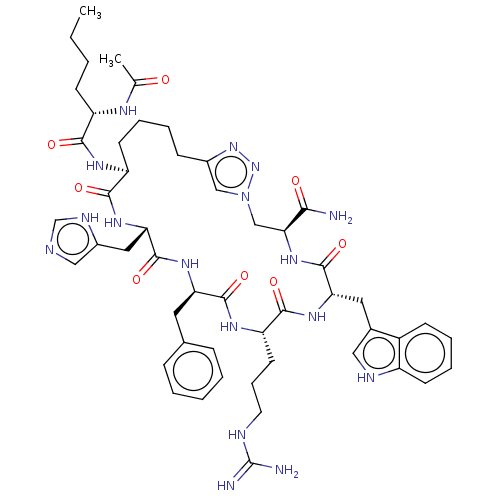
(CHEMBL3358546)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CCCCc2cn(C[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC1=O)C(N)=O)nn2 |r| Show InChI InChI=1S/C51H69N17O8/c1-3-4-17-37(59-30(2)69)45(71)60-38-19-10-8-15-33-27-68(67-66-33)28-43(44(52)70)65-49(75)41(23-32-25-57-36-18-11-9-16-35(32)36)63-47(73)39(20-12-21-56-51(53)54)61-48(74)40(22-31-13-6-5-7-14-31)62-50(76)42(64-46(38)72)24-34-26-55-29-58-34/h5-7,9,11,13-14,16,18,25-27,29,37-43,57H,3-4,8,10,12,15,17,19-24,28H2,1-2H3,(H2,52,70)(H,55,58)(H,59,69)(H,60,71)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,53,54,56)/t37-,38-,39-,40+,41-,42-,43-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50141019
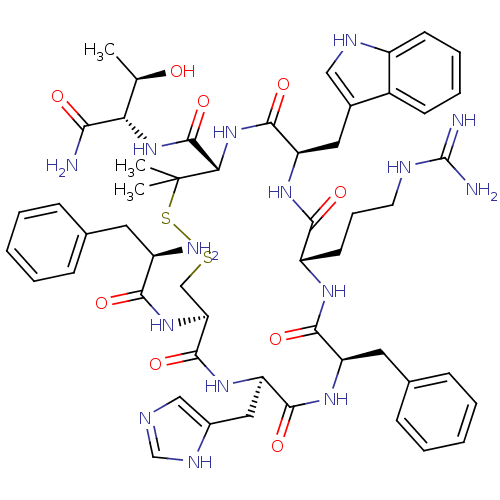
(CHEMBL411400 | H-D-Phe-c[Cys-His-D-Phe-Arg-Trp-Pen...)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H69N15O9S2/c1-29(69)42(44(55)70)67-51(77)43-53(2,3)79-78-27-41(66-45(71)35(54)21-30-13-6-4-7-14-30)50(76)65-40(24-33-26-58-28-61-33)48(74)63-38(22-31-15-8-5-9-16-31)47(73)62-37(19-12-20-59-52(56)57)46(72)64-39(49(75)68-43)23-32-25-60-36-18-11-10-17-34(32)36/h4-11,13-18,25-26,28-29,35,37-43,60,69H,12,19-24,27,54H2,1-3H3,(H2,55,70)(H,58,61)(H,62,73)(H,63,74)(H,64,72)(H,65,76)(H,66,71)(H,67,77)(H,68,75)(H4,56,57,59)/t29-,35-,37+,38-,39-,40-,41+,42+,43+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Maximum agonist response for mouse melanocortin 5 receptor (MC5R) |
J Med Chem 47: 1514-26 (2004)
Article DOI: 10.1021/jm030452x
BindingDB Entry DOI: 10.7270/Q2XP75PK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50499283

(CHEMBL3735690)Show SMILES CCCCC(NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36?,37-,38+,39-,40-,41-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5 receptor expressed in HEK293 cells after 2 hrs by cAMP alpha screen assay |
Bioorg Med Chem Lett 25: 5708-11 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.095
BindingDB Entry DOI: 10.7270/Q2DN482M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50499283

(CHEMBL3735690)Show SMILES CCCCC(NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36?,37-,38+,39-,40-,41-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Agonist activity at mouse MC5 receptor expressed in HEK293 cells after 2 hrs by cAMP alpha screen assay |
Bioorg Med Chem Lett 25: 5708-11 (2015)
Article DOI: 10.1016/j.bmcl.2015.10.095
BindingDB Entry DOI: 10.7270/Q2DN482M |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inverse agonist activity at mouse MC5R expressed in HEK293 cells incubated for 2 hrs by AlphaScreen cAMP assay |
J Med Chem 61: 7729-7740 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00684
BindingDB Entry DOI: 10.7270/Q2X63QHM |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031471
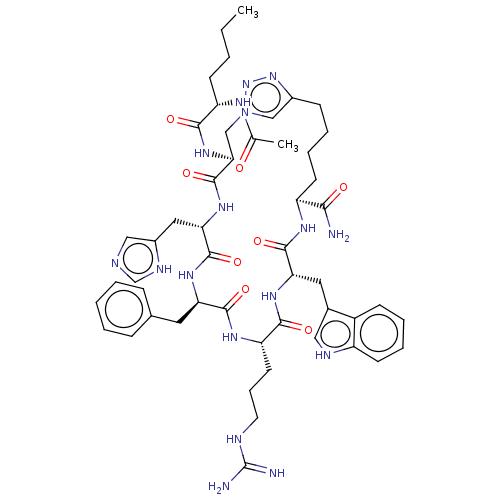
(CHEMBL3358550)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1Cn2cc(CCCC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC1=O)C(N)=O)nn2 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50031470
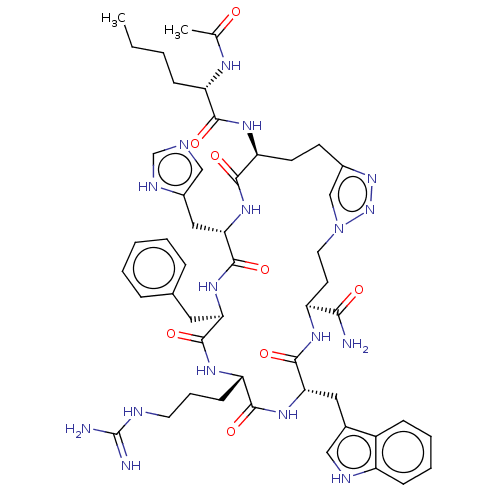
(CHEMBL3358551)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CCc2cn(CC[C@H](NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC1=O)C(N)=O)nn2 |r| | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a |
University of Cergy-Pontoise
Curated by ChEMBL
| Assay Description
Activity at mouse MC5R expressed in HEK293 cells assessed as cAMP accumulation by CRE/beta-galactosidase reporter gene assay |
J Med Chem 57: 9424-34 (2014)
Article DOI: 10.1021/jm501027w
BindingDB Entry DOI: 10.7270/Q21G0NVT |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50114736
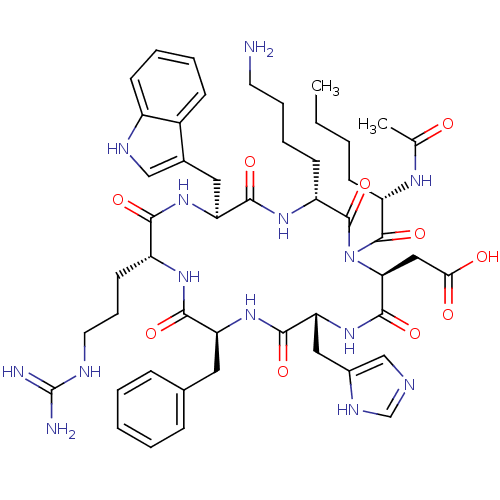
(CHEMBL386583 | [1-(2-Acetylamino-hexanoyl)-17-(4-a...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N1[C@@H](CC(O)=O)C(=O)N[C@H](Cc2cnc[nH]2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N[C@H](CCCCN)C1=O Show InChI InChI=1S/C50H68N14O10/c1-3-4-16-36(58-29(2)65)48(73)64-41(25-42(66)67)47(72)63-40(24-32-27-54-28-57-32)46(71)61-38(22-30-13-6-5-7-14-30)44(69)59-35(19-12-21-55-50(52)53)43(68)62-39(23-31-26-56-34-17-9-8-15-33(31)34)45(70)60-37(49(64)74)18-10-11-20-51/h5-9,13-15,17,26-28,35-41,56H,3-4,10-12,16,18-25,51H2,1-2H3,(H,54,57)(H,58,65)(H,59,69)(H,60,70)(H,61,71)(H,62,68)(H,63,72)(H,66,67)(H4,52,53,55)/t35-,36+,37-,38+,39+,40-,41+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
In vitro activaction of mouse recombinant Melanocortin-5 receptor. |
J Med Chem 45: 2801-10 (2002)
BindingDB Entry DOI: 10.7270/Q27945DV |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 5
(Mus musculus (Mouse)) | BDBM50144873
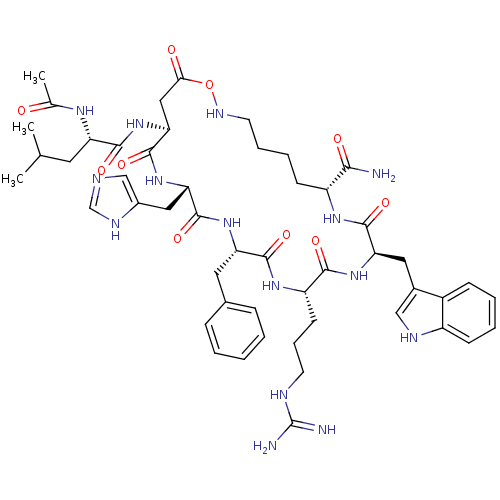
(Ac-Nle-c[Asp-His-DPhe-Arg-Trp-Gly-Lys]-NH2 | CHEMB...)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)ONCCCC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C50H69N15O10/c1-28(2)20-37(59-29(3)66)45(70)65-41-24-42(67)75-58-19-10-9-16-35(43(51)68)60-47(72)39(22-31-25-56-34-15-8-7-14-33(31)34)63-44(69)36(17-11-18-55-50(52)53)61-46(71)38(21-30-12-5-4-6-13-30)62-48(73)40(64-49(41)74)23-32-26-54-27-57-32/h4-8,12-15,25-28,35-41,56,58H,9-11,16-24H2,1-3H3,(H2,51,68)(H,54,57)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,69)(H,64,74)(H,65,70)(H4,52,53,55)/t35-,36+,37+,38+,39-,40+,41+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Effective concentration against mouse Melanocortin 5 receptor |
J Med Chem 47: 2194-207 (2004)
Article DOI: 10.1021/jm0303608
BindingDB Entry DOI: 10.7270/Q2474BM0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































