

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50236697 (5-L-isoleucineangiotensin II | 5-isoleucine-angiot...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50236697 (5-L-isoleucineangiotensin II | 5-isoleucine-angiot...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50159153 (CHEMBL3787243) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50159153 (CHEMBL3787243) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50159151 (CHEMBL3786145) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50159151 (CHEMBL3786145) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50159152 (CHEMBL3786986) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50159152 (CHEMBL3786986) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50159154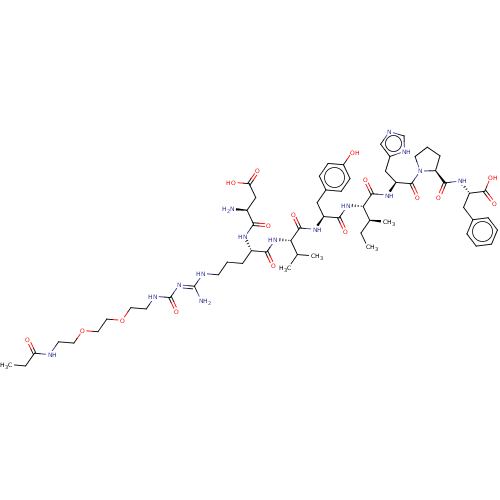 (CHEMBL3787379) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50159154 (CHEMBL3787379) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor transfected in CHO cells co-expressing Galpha16-mtAEQ assessed as induction of intracellular Ca2+ mobilization... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50518037 (CHEMBL4542269) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor assessed as increase in beta-arrestin recruitment by chemiluminescent assay | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50518053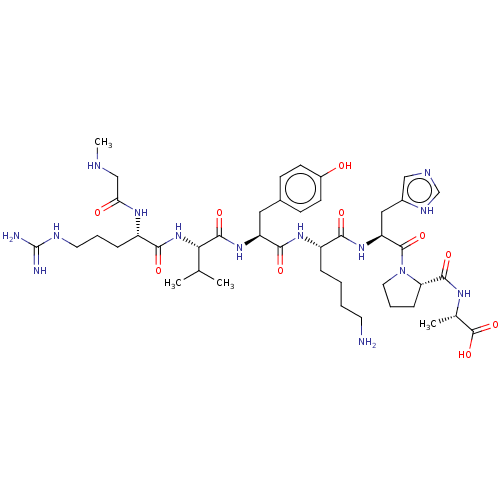 (CHEMBL4438122) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human AT1 receptor assessed as increase in beta-arrestin recruitment by chemiluminescent assay | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM25761 (Anapriline | Avlocardyl | CHEMBL27 | PROPANOLOL(-)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | <5.01E+3 | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica N.V. Curated by ChEMBL | Assay Description Agonist activity at human wild-type AGTR1 receptor expressed in CHO-K1 cells assessed as calcium level by FDSS assay | J Med Chem 58: 9287-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b01088 BindingDB Entry DOI: 10.7270/Q21C20VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50499311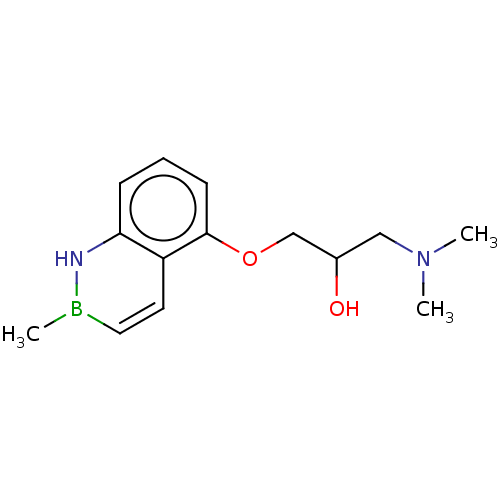 (CHEMBL3736156) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | <5.01E+3 | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica N.V. Curated by ChEMBL | Assay Description Agonist activity at human wild-type AGTR1 receptor expressed in CHO-K1 cells assessed as calcium level by FDSS assay | J Med Chem 58: 9287-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b01088 BindingDB Entry DOI: 10.7270/Q21C20VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50499310 (CHEMBL3736433) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | <5.01E+3 | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica N.V. Curated by ChEMBL | Assay Description Agonist activity at human wild-type AGTR1 receptor expressed in CHO-K1 cells assessed as calcium level by FDSS assay | J Med Chem 58: 9287-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b01088 BindingDB Entry DOI: 10.7270/Q21C20VP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50028423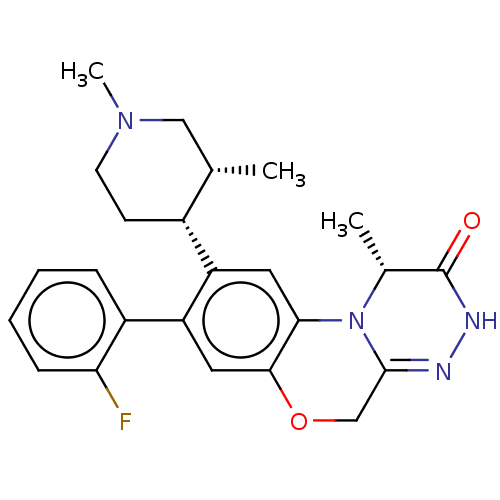 (CHEMBL3355127) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Agonist activity at AT1 receptor (unknown origin) | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50028420 (CHEMBL3355137) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Agonist activity at AT1 receptor (unknown origin) | J Med Chem 58: 333-46 (2015) Article DOI: 10.1021/jm5013006 BindingDB Entry DOI: 10.7270/Q2B859P2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM51796 ((5Z)-5-[(2Z)-2-(3-ethyl-4,5-diphenyl-1,3-thiazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 7.44E+3 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50183341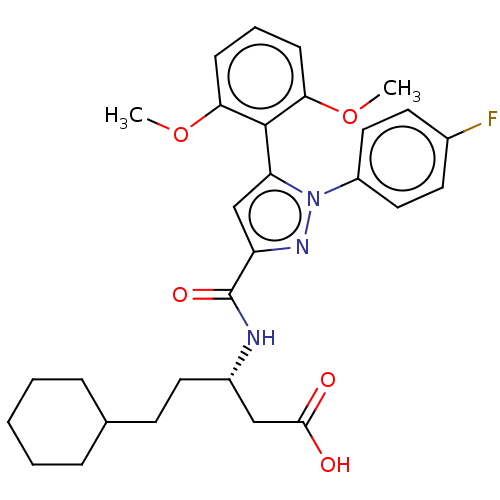 (CHEMBL3817879) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544720 (CHEMBL4632564) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544728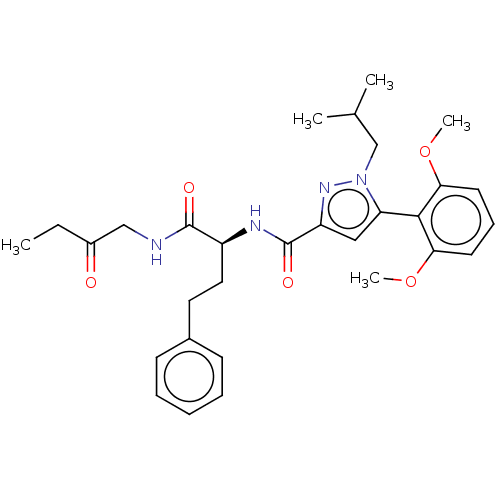 (CHEMBL4641474) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544736 (CHEMBL4647853) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544735 (CHEMBL4640815) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544729 (CHEMBL4647391) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544744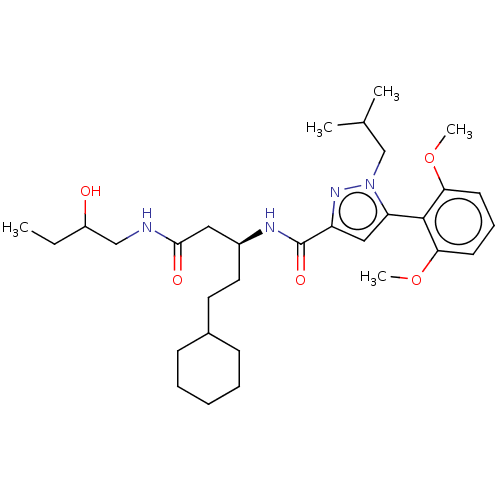 (CHEMBL4647996) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544742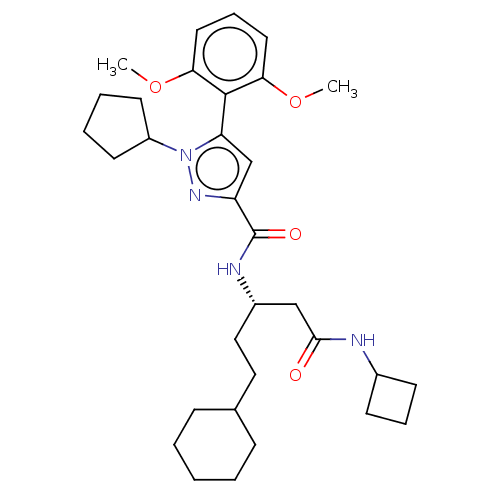 (CHEMBL4647910) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544721 (CHEMBL4644803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544749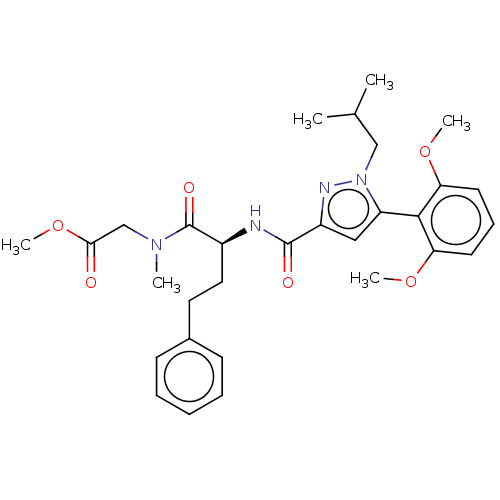 (CHEMBL4640653) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50544733 (CHEMBL4640390) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
RTI International Curated by ChEMBL | Assay Description Agonist activity at human AGTR1 stably expressed in CHO cells assessed as increase in intracellular calcium mobilization measured at 1 sec intervals ... | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115237 BindingDB Entry DOI: 10.7270/Q2J67MJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50571385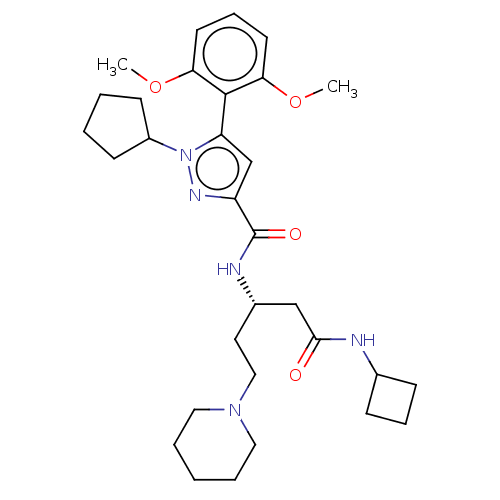 (CHEMBL4875417) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at Galphaq16-fused human AGTR1 expressed in CHO cells assessed as stimulation of Ca2+ mobilization incubated for 1 hr by Calcium 5-d... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01448 BindingDB Entry DOI: 10.7270/Q2TH8RGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM66953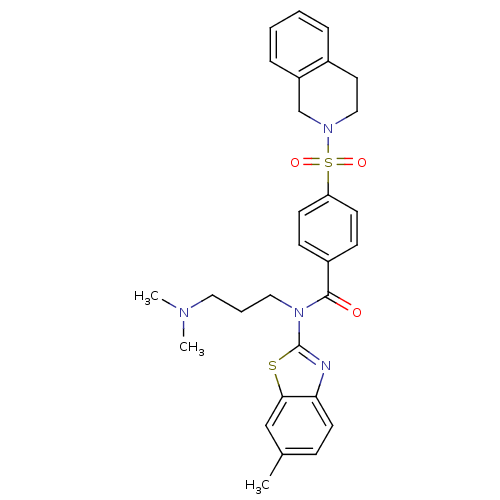 (4-(3,4-dihydro-1H-isoquinolin-2-ylsulfonyl)-N-[3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | 5.78E+4 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76097 (2-(3-Cyclohexyl-3H-imidazo[4,5-b]pyridin-2-ylsulfa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76098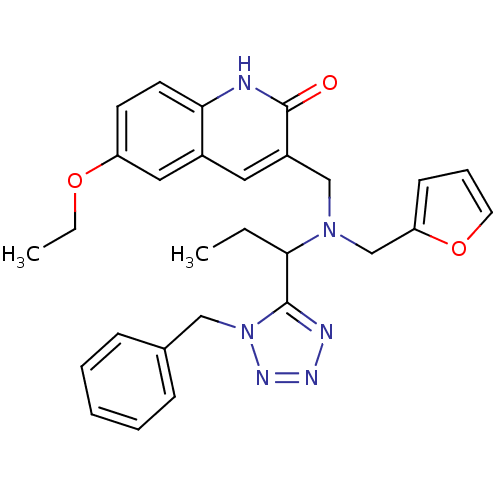 (3-({[1-(1-Benzyl-1H-tetrazol-5-yl)-propyl]-furan-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76099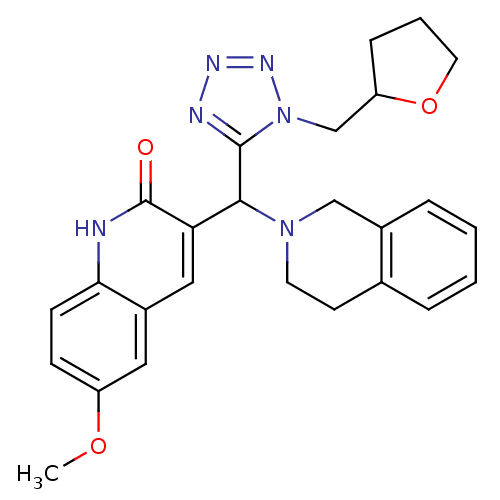 (3-[3,4-dihydro-1H-isoquinolin-2-yl-[1-(2-oxolanylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76100 (3-[[[1-(1-cyclohexyl-1,2,3,4-tetrazol-5-yl)-2-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76101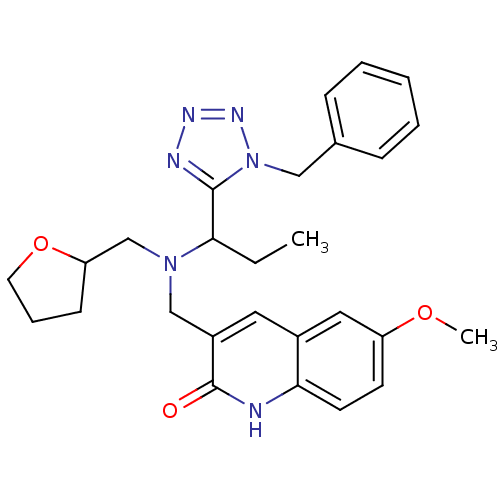 (3-[[1-(1-benzyltetrazol-5-yl)propyl-(oxolan-2-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76102 (6-Methoxy-3-{[{1-[1-(2-methoxy-ethyl)-1H-tetrazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM38416 (4-(2-furyl)-6,8-dimethyl-2-piperidin-1-ylpyrimido[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76103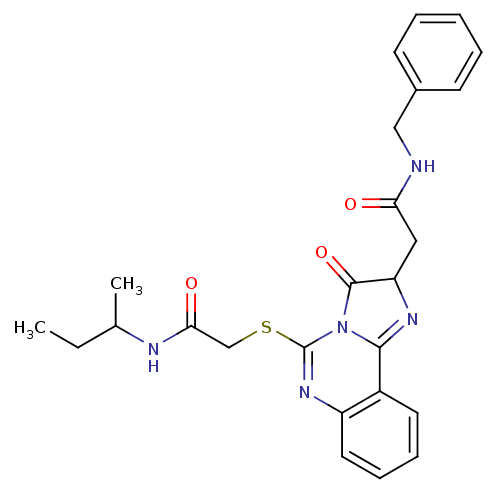 (2-({2-[2-(benzylamino)-2-oxoethyl]-3-oxo-2,3-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76104 (6,7-diethoxy-3-[(1-ethyl-2-pyrrolidinyl)methyl]-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76105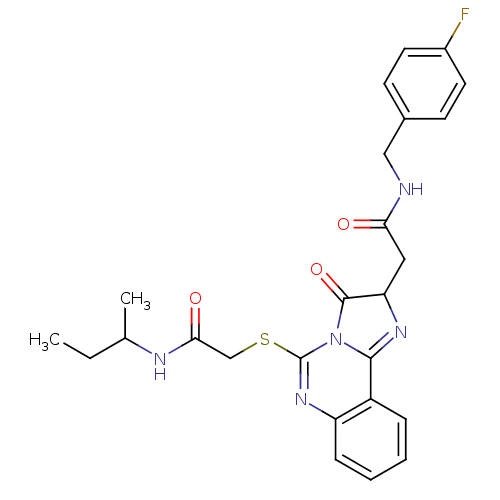 (2-(5-{[2-(sec-butylamino)-2-oxoethyl]thio}-3-oxo-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76106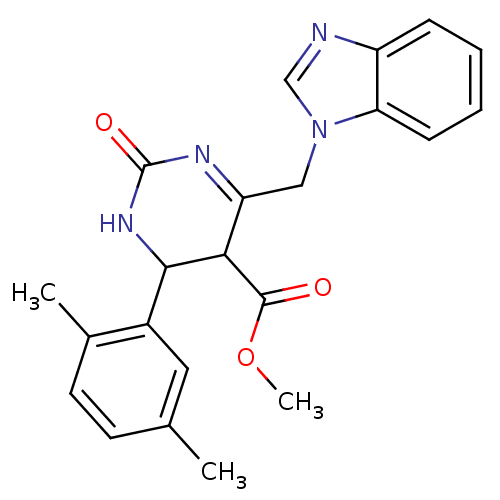 (6-(1-benzimidazolylmethyl)-4-(2,5-dimethylphenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76107 (1-butan-2-yl-5-ethyl-6-hydroxy-2-(propylthio)-4-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM39035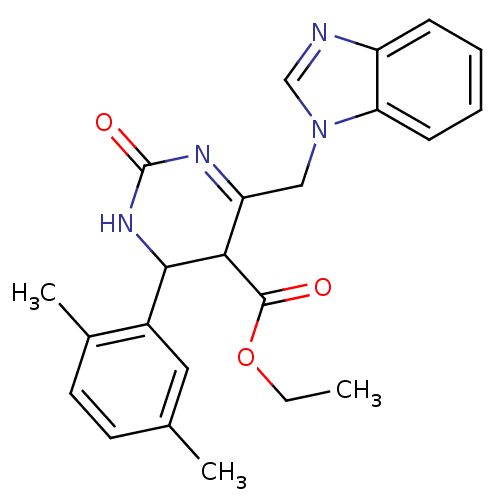 (6-(1-benzimidazolylmethyl)-4-(2,5-dimethylphenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76108 (1-(2-methoxyphenyl)-2-methylsulfanyl-6-oxidanyl-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76109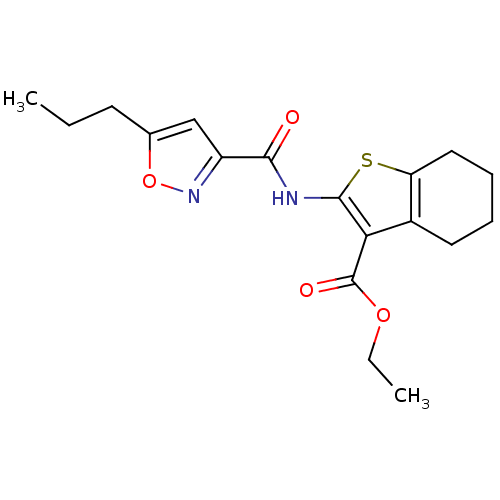 (2-[(5-propylisoxazole-3-carbonyl)amino]-4,5,6,7-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76110 (1-butan-2-yl-5-ethyl-2-(ethylthio)-6-hydroxy-4-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76111 (1-butan-2-yl-5-ethyl-6-hydroxy-2-(prop-2-enylthio)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76112 ((3E)-6-hexyl-3-[5-(3,4,5-trimethoxyphenyl)-3H-1,3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM76113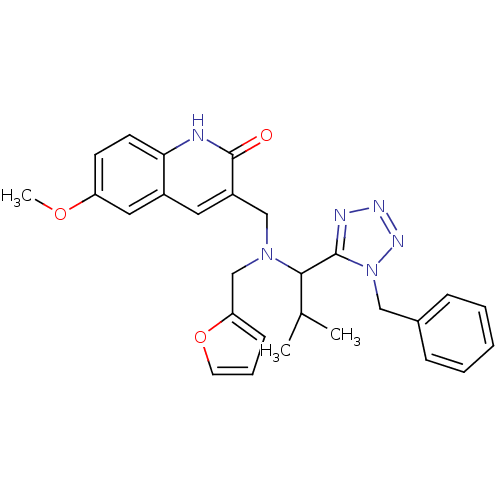 (3-({[1-(1-Benzyl-1H-tetrazol-5-yl)-2-methyl-propyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego) Ne... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2PR7TFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 106 total ) | Next | Last >> |