 Found 29 hits of ec50 data for polymerid = 50006512,5066
Found 29 hits of ec50 data for polymerid = 50006512,5066 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50100662

(CHEMBL553371 | CHEMBL77971 | methyl 2-(4-aminophen...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O7/c1-38-26-15-19(16-27(39-2)30(26)40-3)28-24-13-12-23(42-18-21-7-5-6-14-34-21)17-25(24)31(36)35(29(28)32(37)41-4)22-10-8-20(33)9-11-22/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Relaxant effect on isolated rabbit corpus cavernosum |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Relaxant effect on isolated rabbit corpus cavernosum |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50100659
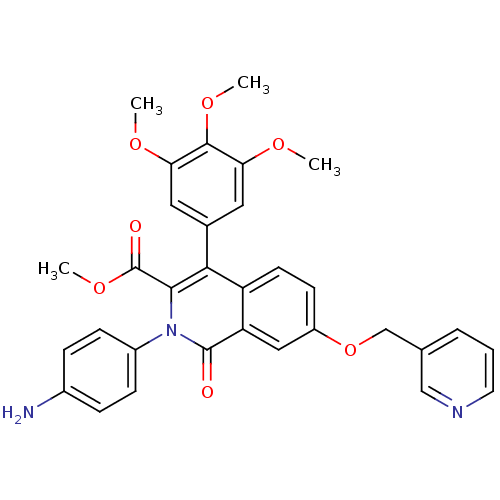
(CHEMBL544289 | methyl 2-(4-aminophenyl)-1-oxo-7-(3...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3cccnc3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O7/c1-38-26-14-20(15-27(39-2)30(26)40-3)28-24-12-11-23(42-18-19-6-5-13-34-17-19)16-25(24)31(36)35(29(28)32(37)41-4)22-9-7-21(33)8-10-22/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Relaxant effect on isolated rabbit corpus cavernosum |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559974
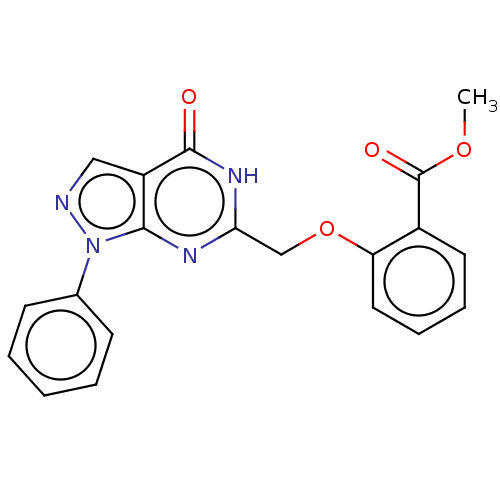
(CHEMBL4790850)Show SMILES COC(=O)c1ccccc1OCc1nc2n(ncc2c(=O)[nH]1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50100670
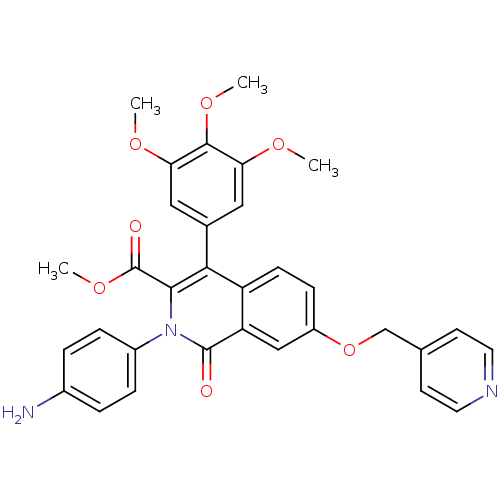
(CHEMBL553786 | methyl 2-(4-aminophenyl)-1-oxo-7-(4...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccncc3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O7/c1-38-26-15-20(16-27(39-2)30(26)40-3)28-24-10-9-23(42-18-19-11-13-34-14-12-19)17-25(24)31(36)35(29(28)32(37)41-4)22-7-5-21(33)6-8-22/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Relaxant effect on isolated rabbit corpus cavernosum |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50100674
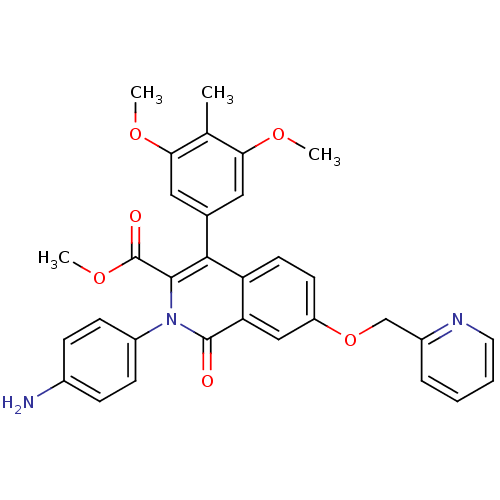
(CHEMBL541818 | methyl 2-(4-aminophenyl)-4-(3,5-dim...)Show SMILES COC(=O)c1c(-c2cc(OC)c(C)c(OC)c2)c2ccc(OCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C32H29N3O6/c1-19-27(38-2)15-20(16-28(19)39-3)29-25-13-12-24(41-18-22-7-5-6-14-34-22)17-26(25)31(36)35(30(29)32(37)40-4)23-10-8-21(33)9-11-23/h5-17H,18,33H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Relaxant effect on isolated rabbit corpus cavernosum |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559973
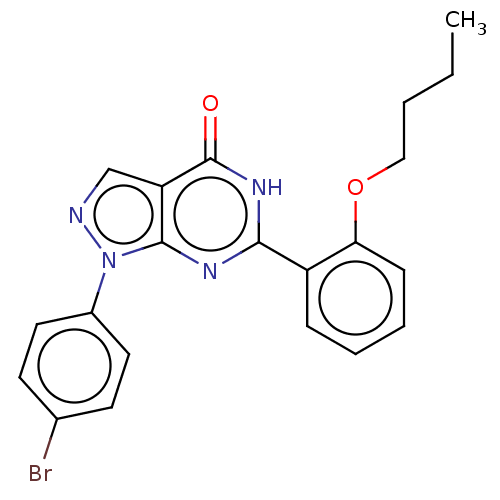
(CHEMBL4790858)Show SMILES CCCCOc1ccccc1-c1nc2n(ncc2c(=O)[nH]1)-c1ccc(Br)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Relaxant effect on rabbit corpus cavernosal tissue strips |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50100673

(CHEMBL538498 | methyl 2-(4-aminophenyl)-4-(4-bromo...)Show SMILES COC(=O)c1c(-c2cc(OC)c(Br)c(OC)c2)c2ccc(OCc3ccccn3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C31H26BrN3O6/c1-38-25-14-18(15-26(39-2)28(25)32)27-23-12-11-22(41-17-20-6-4-5-13-34-20)16-24(23)30(36)35(29(27)31(37)40-3)21-9-7-19(33)8-10-21/h4-16H,17,33H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Relaxant effect on isolated rabbit corpus cavernosum |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50098220
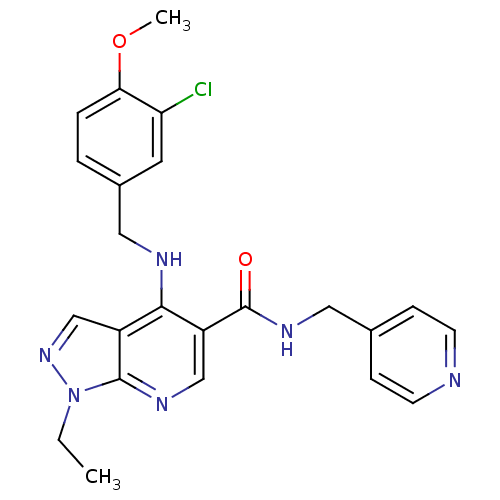
(4-(3-Chloro-4-methoxy-benzylamino)-1-ethyl-1H-pyra...)Show SMILES CCn1ncc2c(NCc3ccc(OC)c(Cl)c3)c(cnc12)C(=O)NCc1ccncc1 Show InChI InChI=1S/C23H23ClN6O2/c1-3-30-22-17(14-29-30)21(26-12-16-4-5-20(32-2)19(24)10-16)18(13-27-22)23(31)28-11-15-6-8-25-9-7-15/h4-10,13-14H,3,11-12H2,1-2H3,(H,26,27)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Relaxant effect on rabbit corpus cavernosal tissue strips |
J Med Chem 44: 1025-7 (2001)
BindingDB Entry DOI: 10.7270/Q2154G8J |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50100656

(2-(4-Amino-phenyl)-7-benzyloxy-1-oxo-4-(3,4,5-trim...)Show SMILES COC(=O)c1c(-c2cc(OC)c(OC)c(OC)c2)c2ccc(OCc3ccccc3)cc2c(=O)n1-c1ccc(N)cc1 Show InChI InChI=1S/C33H30N2O7/c1-38-27-16-21(17-28(39-2)31(27)40-3)29-25-15-14-24(42-19-20-8-6-5-7-9-20)18-26(25)32(36)35(30(29)33(37)41-4)23-12-10-22(34)11-13-23/h5-18H,19,34H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Tanabe Seiyaku Co., Ltd.
Curated by ChEMBL
| Assay Description
Relaxant effect on isolated rabbit corpus cavernosum |
J Med Chem 44: 2204-18 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4XT5 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559975
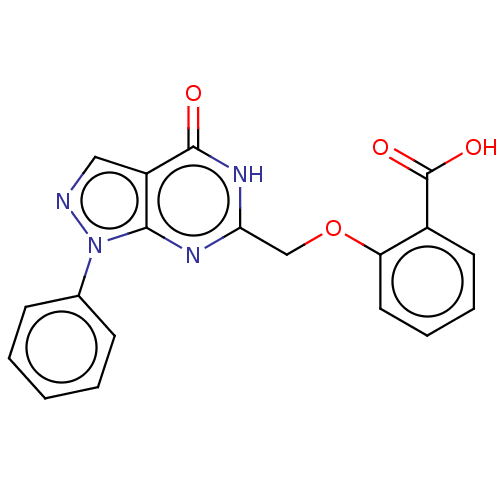
(CHEMBL4753374)Show SMILES OC(=O)c1ccccc1OCc1nc2n(ncc2c(=O)[nH]1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559983
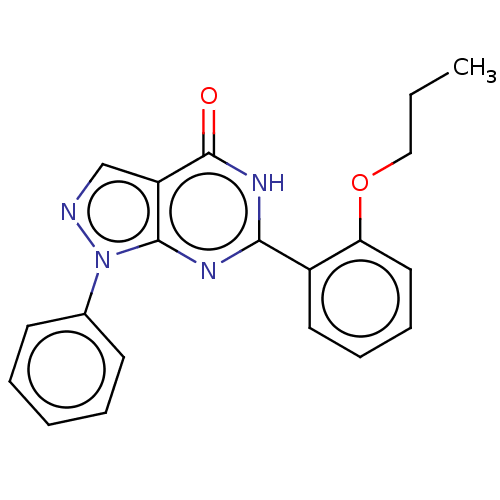
(CHEMBL4800568)Show SMILES CCCOc1ccccc1-c1nc2n(ncc2c(=O)[nH]1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559976
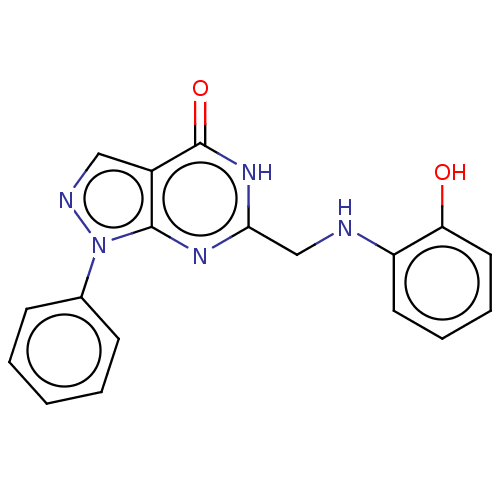
(CHEMBL4785159) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559977
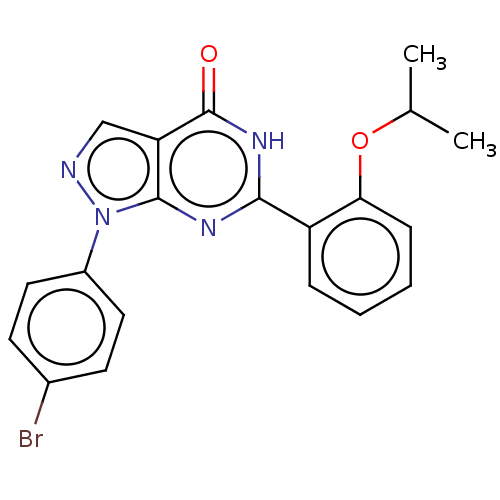
(CHEMBL4777918)Show SMILES CC(C)Oc1ccccc1-c1nc2n(ncc2c(=O)[nH]1)-c1ccc(Br)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559978
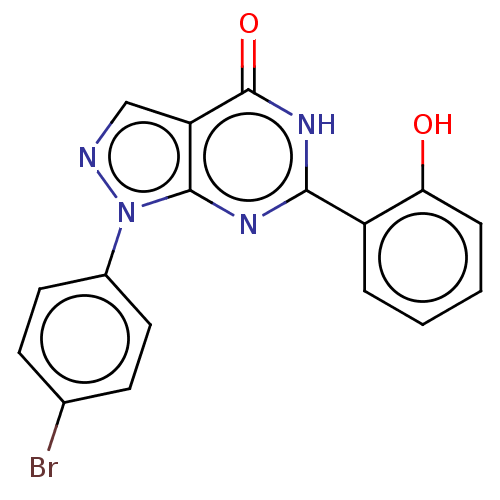
(CHEMBL4745690)Show SMILES Oc1ccccc1-c1nc2n(ncc2c(=O)[nH]1)-c1ccc(Br)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559984

(CHEMBL4788014)Show SMILES CC(C)Oc1ccccc1-c1nc2n(ncc2c(=O)[nH]1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559985
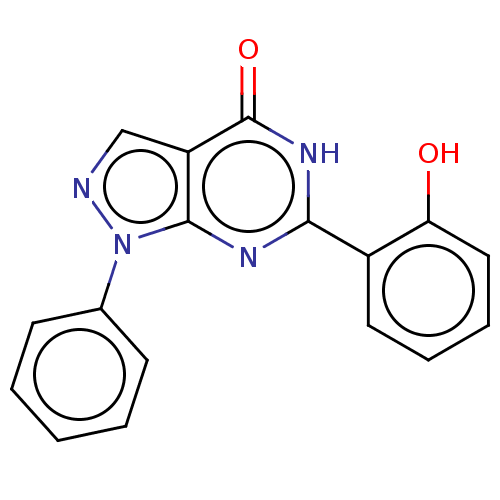
(CHEMBL4799568) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559986

(CHEMBL4749840)Show SMILES CCCCOc1ccccc1-c1nc2n(ncc2c(=O)[nH]1)-c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559979
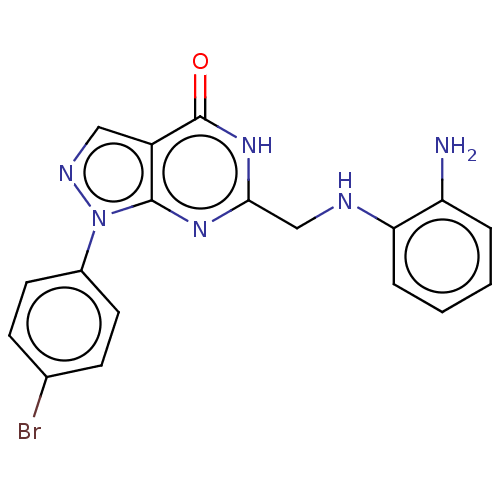
(CHEMBL4798704)Show SMILES Nc1ccccc1NCc1nc2n(ncc2c(=O)[nH]1)-c1ccc(Br)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559980
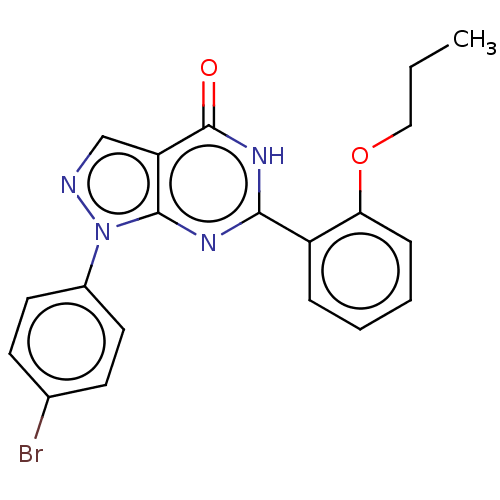
(CHEMBL4763502)Show SMILES CCCOc1ccccc1-c1nc2n(ncc2c(=O)[nH]1)-c1ccc(Br)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 830 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14390

(5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...)Show SMILES CCCc1nn(C)c2c1nc([nH]c2=O)-c1cc(ccc1OCC)S(=O)(=O)N1CCN(C)CC1 Show InChI InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50566443

(CHEMBL4879176)Show SMILES COc1ccc(C2CC(=NN2c2ccc(cc2)C(=O)N2CCN(C)CC2)C(C)(C)C)c(F)c1F |c:8| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5A in HEK293 cells stably transfected with cGMP biosensor assessed as increase in SNP-induced intracellular cGMP level measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01120
BindingDB Entry DOI: 10.7270/Q2765K34 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559981
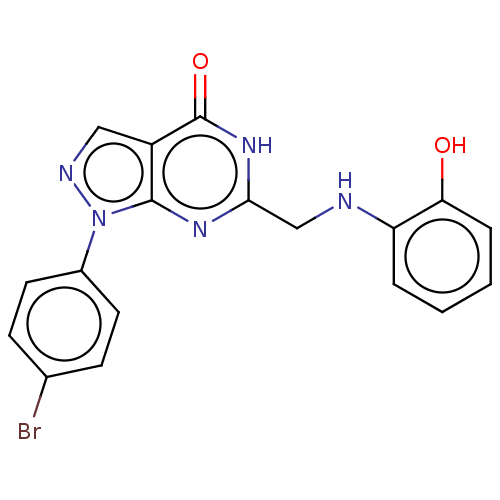
(CHEMBL4791250)Show SMILES Oc1ccccc1NCc1nc2n(ncc2c(=O)[nH]1)-c1ccc(Br)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.05E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14777

((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...)Show SMILES [H][C@]12Cc3c([nH]c4ccccc34)[C@H](N1C(=O)CN(C)C2=O)c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C22H19N3O4/c1-24-10-19(26)25-16(22(24)27)9-14-13-4-2-3-5-15(13)23-20(14)21(25)12-6-7-17-18(8-12)29-11-28-17/h2-8,16,21,23H,9-11H2,1H3/t16-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 5.81E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5A in HEK293 cells stably transfected with cGMP biosensor assessed as increase in SNP-induced intracellular cGMP level measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01120
BindingDB Entry DOI: 10.7270/Q2765K34 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50566435
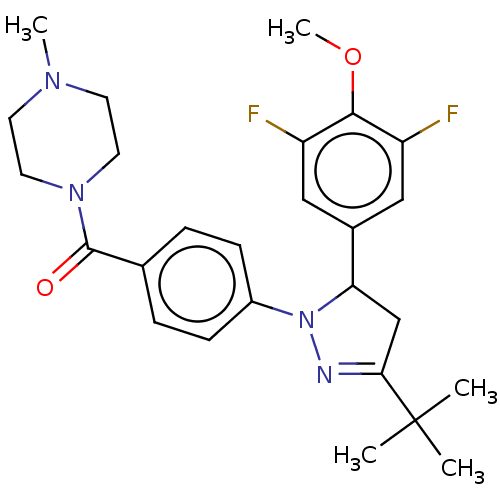
(CHEMBL4872902)Show SMILES COc1c(F)cc(cc1F)C1CC(=NN1c1ccc(cc1)C(=O)N1CCN(C)CC1)C(C)(C)C |c:13| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5A in HEK293 cells stably transfected with cGMP biosensor assessed as increase in SNP-induced intracellular cGMP level measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01120
BindingDB Entry DOI: 10.7270/Q2765K34 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50559982

(CHEMBL4747542) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5 in human platelets incubated for 30 mins using cGMP as substrate by HPLC analysis relative to sildenafil |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127337
BindingDB Entry DOI: 10.7270/Q2X35250 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50566439
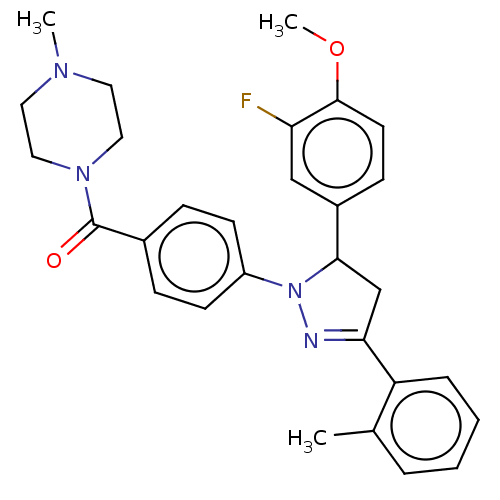
(CHEMBL4869439)Show SMILES COc1ccc(cc1F)C1CC(=NN1c1ccc(cc1)C(=O)N1CCN(C)CC1)c1ccccc1C |c:12| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5A in HEK293 cells stably transfected with cGMP biosensor assessed as increase in SNP-induced intracellular cGMP level measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01120
BindingDB Entry DOI: 10.7270/Q2765K34 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50566438
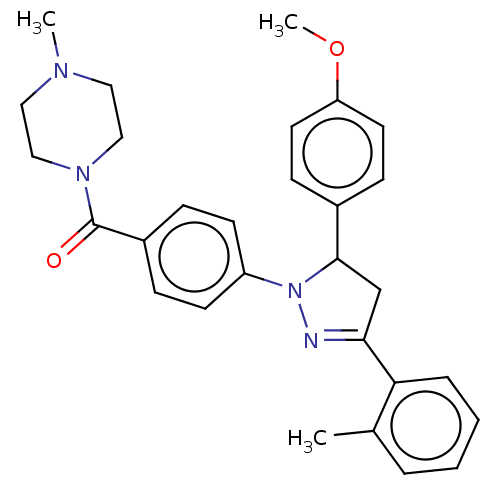
(CHEMBL4852714)Show SMILES COc1ccc(cc1)C1CC(=NN1c1ccc(cc1)C(=O)N1CCN(C)CC1)c1ccccc1C |c:11| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE5A in HEK293 cells stably transfected with cGMP biosensor assessed as increase in SNP-induced intracellular cGMP level measured afte... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01120
BindingDB Entry DOI: 10.7270/Q2765K34 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data