

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50229897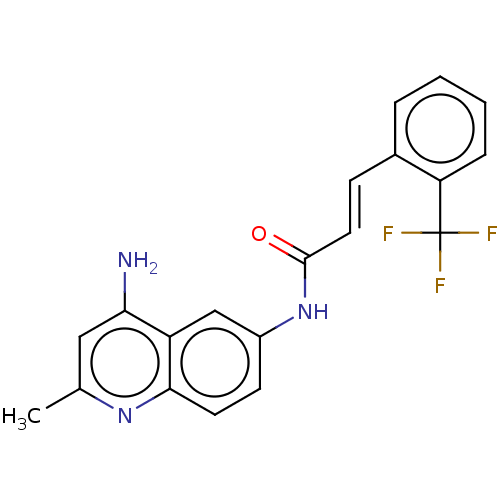 (CHEMBL314007) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | <3.00E+3 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for the induction of nonspecific myeloperoxidase (MPO) release | J Med Chem 35: 252-8 (1992) BindingDB Entry DOI: 10.7270/Q2X63Q54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50229904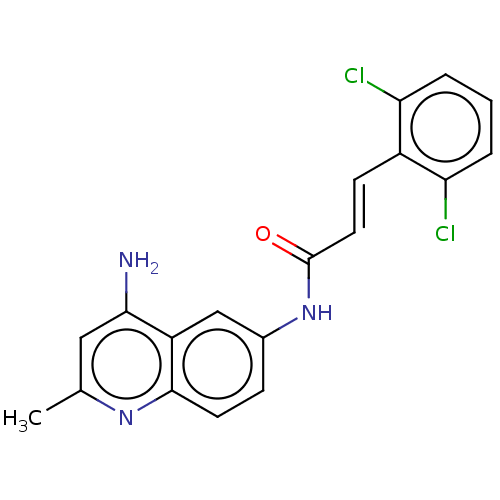 (CHEMBL87465) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | <3.00E+3 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for the induction of nonspecific myeloperoxidase (MPO) release | J Med Chem 35: 252-8 (1992) BindingDB Entry DOI: 10.7270/Q2X63Q54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM55880 ((E)-N-(4-amino-2-methyl-6-quinolinyl)-3-(2-chlorop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | <3.00E+3 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for the induction of nonspecific myeloperoxidase (MPO) release | J Med Chem 35: 252-8 (1992) BindingDB Entry DOI: 10.7270/Q2X63Q54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50229902 (CHEMBL313562) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | <3.00E+3 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for the induction of nonspecific myeloperoxidase (MPO) release | J Med Chem 35: 252-8 (1992) BindingDB Entry DOI: 10.7270/Q2X63Q54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50444748 (Aminoquinuride) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | <3.00E+3 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for the induction of nonspecific myeloperoxidase (MPO) release | J Med Chem 35: 252-8 (1992) BindingDB Entry DOI: 10.7270/Q2X63Q54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50229909 (CHEMBL83403) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 15.00E+3 | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for the induction of nonspecific myeloperoxidase (MPO) release | J Med Chem 35: 252-8 (1992) BindingDB Entry DOI: 10.7270/Q2X63Q54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008382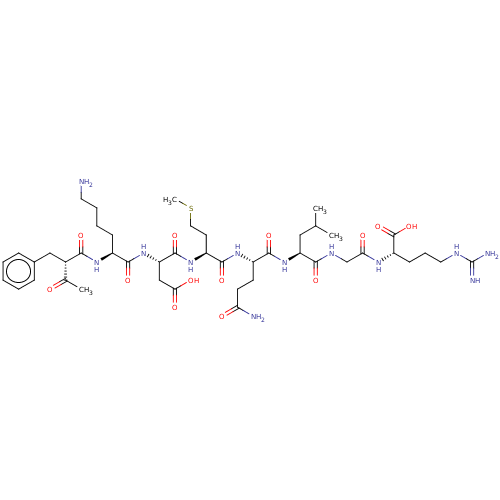 (Ac-Phe-Lys-AspMet-GIn-Leu-Gly-Arg-OH | CHEMBL23706...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008381 (CHEMBL441393 | H-Ile-Ser-Phe-Lys-AspMet-GIn-Leu-Gl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008373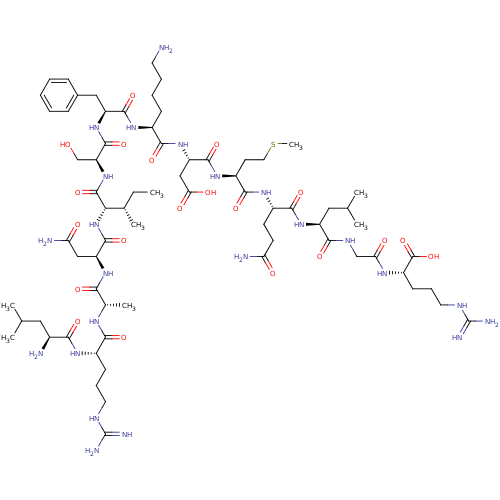 (CHEMBL439468 | H-Leu-Arg-Ala-Asn-Ile-Ser-Phe-Lys-A...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008371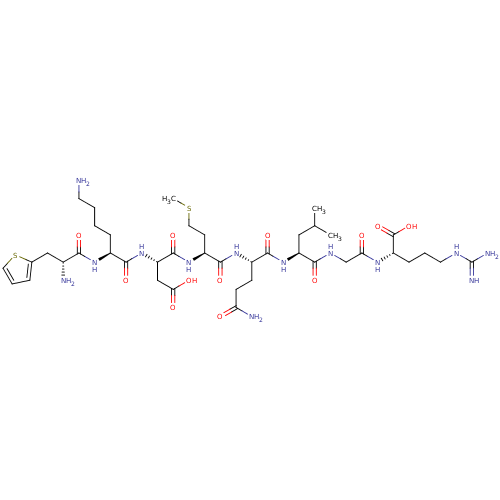 (CHEMBL127385 | H-Thi-Lys-AspMet-GIn-Leu-Gly-Arg-OH) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50008383 (CHEMBL408929 | H-Ala-Asn-Ile-Ser-Phe-Lys-AspMet-GI...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.30E+5 | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vivo potency of the compound was determined as the maximal lysosomal myeloperoxidase (MPO) release in PMNL assay | J Med Chem 35: 402-6 (1992) BindingDB Entry DOI: 10.7270/Q2HH6J1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||