

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM23927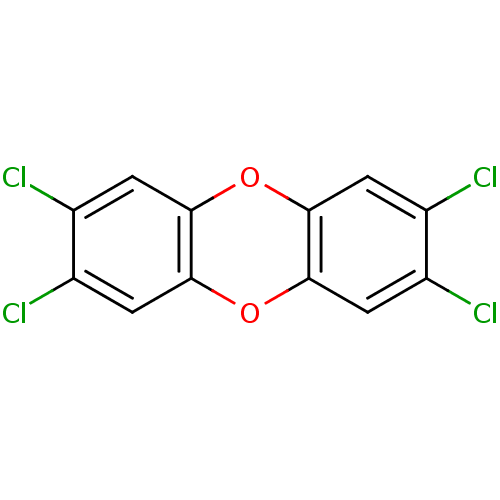 (2,3,7,8-Tetrachlorodibenzo-p-dioxin | 2,3,7,8-tetr...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at Ah receptor in genetically engineered mouse cells expressing firefly luciferase gene by CALUX transactivational assay | Bioorg Med Chem Lett 21: 6258-63 (2011) Article DOI: 10.1016/j.bmcl.2011.09.008 BindingDB Entry DOI: 10.7270/Q21836XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50602335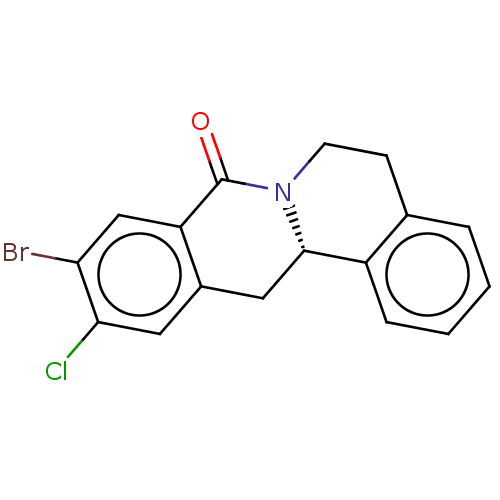 (CHEMBL5186309) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00956 BindingDB Entry DOI: 10.7270/Q2HM5DH2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50506039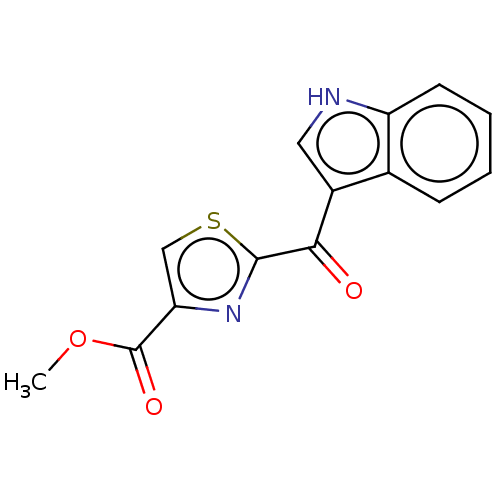 (CHEMBL1324296) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00208 BindingDB Entry DOI: 10.7270/Q27D3076 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50602335 (CHEMBL5186309) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00956 BindingDB Entry DOI: 10.7270/Q2HM5DH2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50015301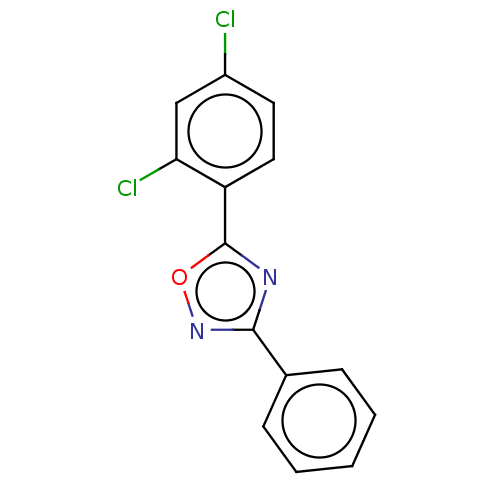 (CHEMBL3263111) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Agonist activity at AHR in FVB/N mouse primary mammary epithelial cells assessed as blockade of mammary branching morphogenesis after 144 hrs by micr... | Bioorg Med Chem Lett 24: 2473-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.013 BindingDB Entry DOI: 10.7270/Q2C24Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50015302 (CHEMBL3263112) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Agonist activity at AHR in FVB/N mouse primary mammary epithelial cells assessed as blockade of mammary branching morphogenesis after 144 hrs by micr... | Bioorg Med Chem Lett 24: 2473-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.013 BindingDB Entry DOI: 10.7270/Q2C24Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50015303 (CHEMBL3263118) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Agonist activity at AHR in FVB/N mouse primary mammary epithelial cells assessed as blockade of mammary branching morphogenesis after 144 hrs by micr... | Bioorg Med Chem Lett 24: 2473-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.013 BindingDB Entry DOI: 10.7270/Q2C24Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50015304 (CHEMBL3263119) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Agonist activity at AHR in FVB/N mouse primary mammary epithelial cells assessed as blockade of mammary branching morphogenesis after 144 hrs by micr... | Bioorg Med Chem Lett 24: 2473-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.013 BindingDB Entry DOI: 10.7270/Q2C24Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50357344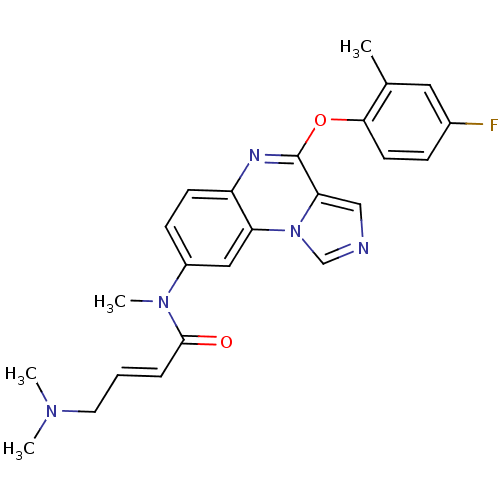 (CHEMBL1916902) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at Ah receptor in genetically engineered mouse cells expressing firefly luciferase gene by CALUX transactivational assay | Bioorg Med Chem Lett 21: 6258-63 (2011) Article DOI: 10.1016/j.bmcl.2011.09.008 BindingDB Entry DOI: 10.7270/Q21836XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50357339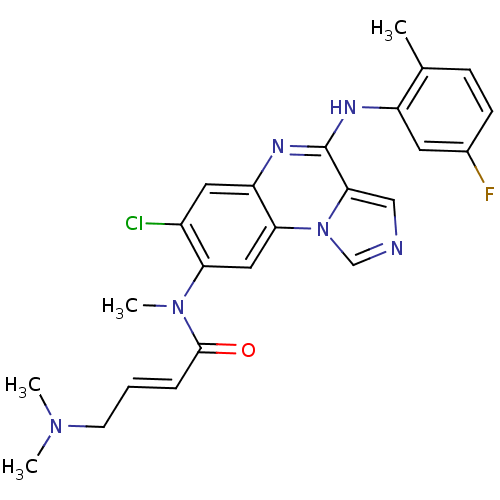 (CHEMBL1916897) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at Ah receptor in genetically engineered mouse cells expressing firefly luciferase gene by CALUX transactivational assay | Bioorg Med Chem Lett 21: 6258-63 (2011) Article DOI: 10.1016/j.bmcl.2011.09.008 BindingDB Entry DOI: 10.7270/Q21836XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50357322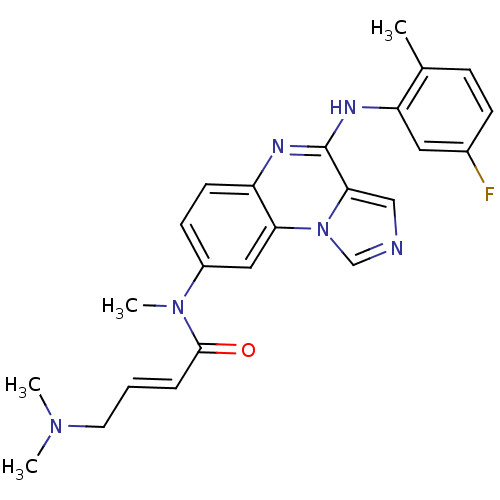 (CHEMBL1916880) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at Ah receptor in genetically engineered mouse cells expressing firefly luciferase gene by CALUX transactivational assay | Bioorg Med Chem Lett 21: 6258-63 (2011) Article DOI: 10.1016/j.bmcl.2011.09.008 BindingDB Entry DOI: 10.7270/Q21836XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50015305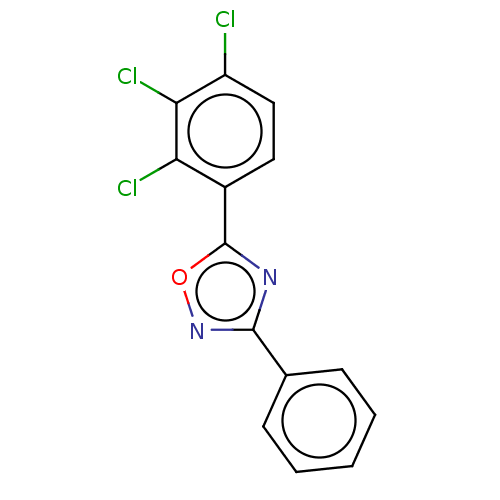 (CHEMBL3263120) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Agonist activity at AHR in FVB/N mouse primary mammary epithelial cells assessed as blockade of mammary branching morphogenesis after 144 hrs by micr... | Bioorg Med Chem Lett 24: 2473-6 (2014) Article DOI: 10.1016/j.bmcl.2014.04.013 BindingDB Entry DOI: 10.7270/Q2C24Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50357333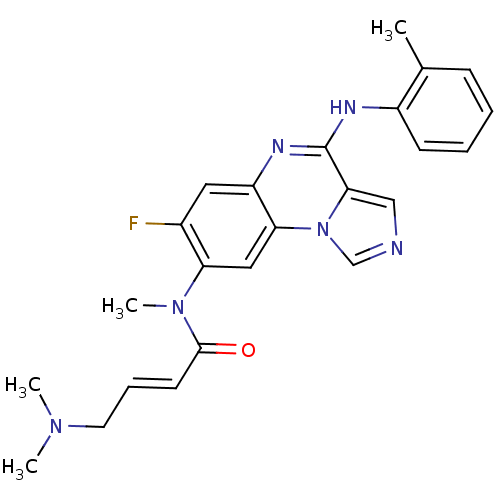 (CHEMBL1916891) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at Ah receptor in genetically engineered mouse cells expressing firefly luciferase gene by CALUX transactivational assay | Bioorg Med Chem Lett 21: 6258-63 (2011) Article DOI: 10.1016/j.bmcl.2011.09.008 BindingDB Entry DOI: 10.7270/Q21836XD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aryl hydrocarbon receptor (Mus musculus (Mouse)) | BDBM50239186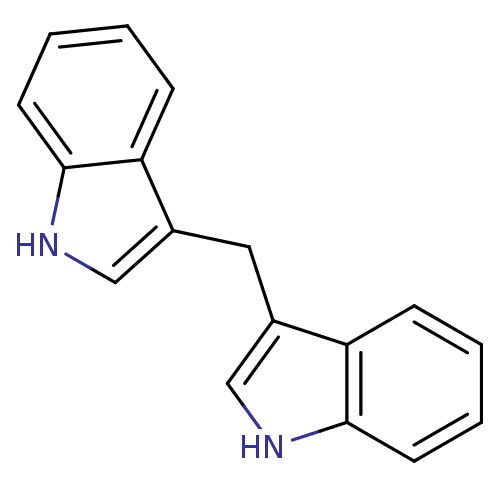 (1,1-Bis(3''-Indolyl)Methane | 3,3''-Diindolylmetha...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.80E+4 | n/a | n/a | n/a | n/a |
University of Perugia Curated by ChEMBL | Assay Description Displacement of 2-azido-3-[125I]7,8-dibromodibenzo-pdioxin from C57BL/6J mouse liver AhR relative to TCDD | Eur J Med Chem 185: (2020) Article DOI: 10.1016/j.ejmech.2019.111842 BindingDB Entry DOI: 10.7270/Q2N019SX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||