

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538220 (CHEMBL4638964) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538202 (CHEMBL4633816) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538203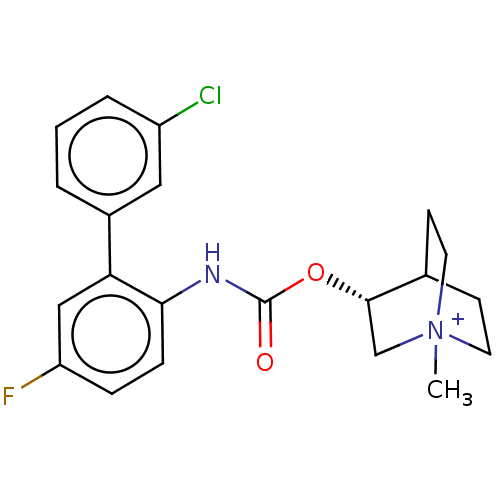 (CHEMBL4647437) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in BHK-21 cells assessed as increase of acetylcholine-induced calcium flux by FLIPR assay | J Med Chem 53: 6386-97 (2010) Article DOI: 10.1021/jm100697g BindingDB Entry DOI: 10.7270/Q2765G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in BHK-21 cells assessed as calcium mobilization for 6 mins by Calcium4-based staining | Bioorg Med Chem Lett 22: 5134-40 (2012) Article DOI: 10.1016/j.bmcl.2012.05.048 BindingDB Entry DOI: 10.7270/Q24M95MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50079583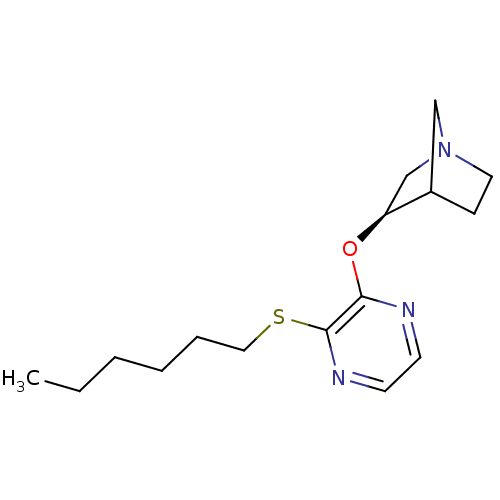 ((R)-3-(3-Hexylsulfanyl-pyrazin-2-yloxy)-1-aza-bicy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro functional agonism against M3 muscarinic receptor (PI) | Bioorg Med Chem Lett 9: 1895-900 (1999) BindingDB Entry DOI: 10.7270/Q2G161CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538205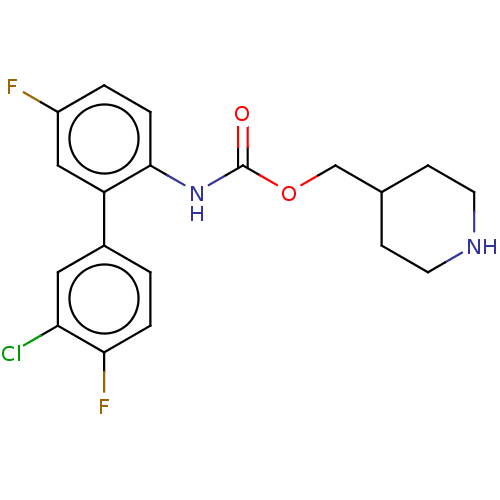 (CHEMBL4636528) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in HEK293 cells assessed as increase in IP1 accumulation incubated for 90 min by TR-HTRF a... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538205 (CHEMBL4636528) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538220 (CHEMBL4638964) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538203 (CHEMBL4647437) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538202 (CHEMBL4633816) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538206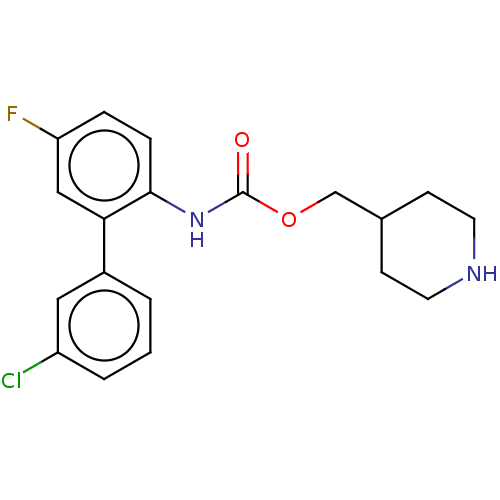 (CHEMBL4640703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in HEK293 cells assessed as increase in IP1 accumulation incubated for 90 min by TR-HTRF a... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538205 (CHEMBL4636528) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50109647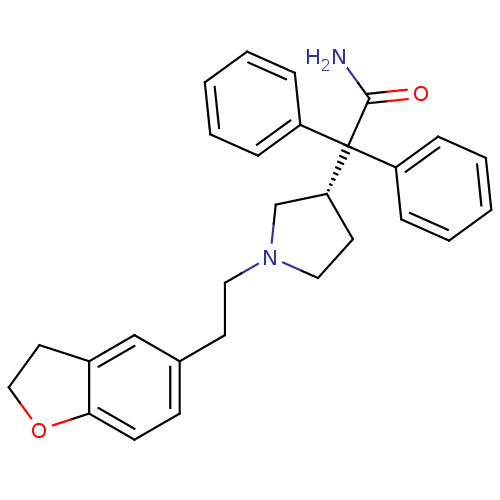 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538206 (CHEMBL4640703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538208 (CHEMBL4648071) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538207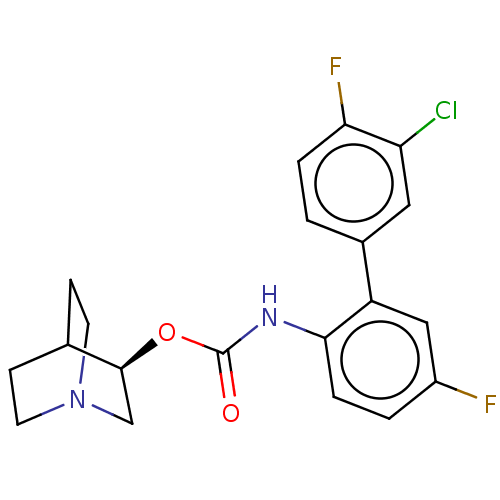 (CHEMBL4640324) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | n/a | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as beta-arrestin... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at recombinant human muscarinic M3 receptor expressed in CHO cells assessed as upregulation in ERK1/2 phosphorylation after 5 mins b... | J Med Chem 60: 9239-9250 (2017) Article DOI: 10.1021/acs.jmedchem.7b01113 BindingDB Entry DOI: 10.7270/Q2445PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at recombinant human muscarinic M3 receptor expressed in CHO cells assessed as upregulation in ERK1/2 phosphorylation after 5 mins b... | J Med Chem 60: 9239-9250 (2017) Article DOI: 10.1021/acs.jmedchem.7b01113 BindingDB Entry DOI: 10.7270/Q2445PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50370682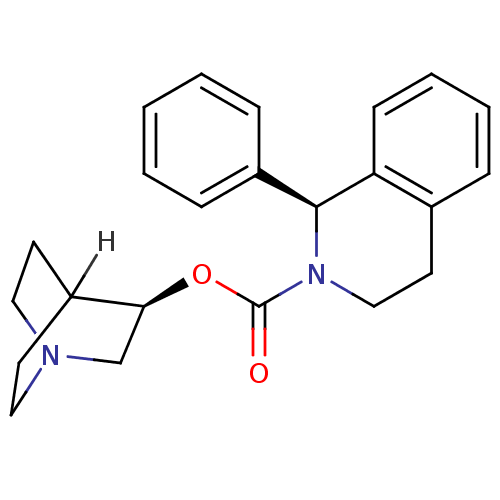 (SOLIFENACIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | DrugBank | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in HEK293 cells assessed as increase in IP1 accumulation incubated for 90 min by TR-HTRF a... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538207 (CHEMBL4640324) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538206 (CHEMBL4640703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50538208 (CHEMBL4648071) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at muscarinic M3 receptor (unknown origin) expressed in HEK293 cells coexpressing EA tagged beta-arrestin2 assessed as inhibition... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50109647 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in HEK293 cells assessed as increase in IP1 accumulation incubated for 90 min by TR-HTRF a... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM193787 (US9670209, Compound 304 1-((R)-3-(3-(Cyclopropylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM193787 (US9670209, Compound 304 1-((R)-3-(3-(Cyclopropylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50109647 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Friedrich-Alexander-Universit£t Erlangen-N£rnberg Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M3 receptor expressed in HEK293 assessed as inhibition of carbachol-induced IP1 accumulation preincubated for... | J Med Chem 63: 4349-4369 (2020) Article DOI: 10.1021/acs.jmedchem.0c00297 BindingDB Entry DOI: 10.7270/Q2CR5XWB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM193789 (US9670209, Compound 307 1-((R)-3-(3-(Cyclopropylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50416733 (CHEMBL1223803) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50004656 ((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine | J Med Chem 29: 1004-9 (1986) BindingDB Entry DOI: 10.7270/Q2XP73XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM193834 (US9670209, Comparative compound 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 79 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50416732 (CHEMBL1223754) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM46858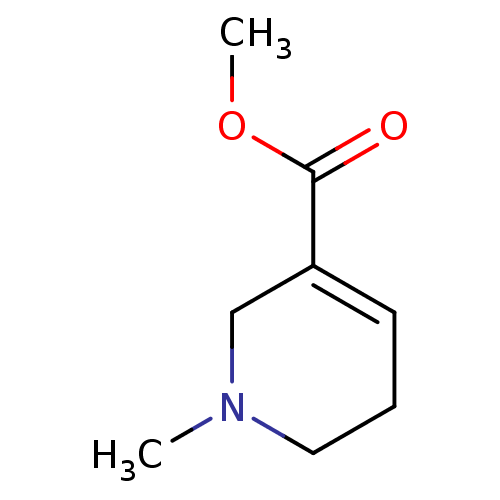 (1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Contraction of guinea pig ileum by muscarinic AChR activation, which could be inhibited by application of atropine | J Med Chem 29: 1004-9 (1986) BindingDB Entry DOI: 10.7270/Q2XP73XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50263888 (CHEMBL491209 | Ethyl 4-(2-oxo-2,3-dihydro-1Hbenzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Agonist activity at muscarinic M3 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5439-42 (2008) Article DOI: 10.1016/j.bmcl.2008.09.023 BindingDB Entry DOI: 10.7270/Q2708198 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50275588 (2-(4-(2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 113 | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Agonist activity at muscarinic M3 receptor (unknown origin) | Bioorg Med Chem Lett 18: 5443-7 (2008) Article DOI: 10.1016/j.bmcl.2008.09.032 BindingDB Entry DOI: 10.7270/Q29K4B1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50061705 ((R)-1-Aza-bicyclo[2.2.2]oct-3-yl-[(Z)-methoxyimino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 117 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay | Bioorg Med Chem Lett 25: 4158-63 (2015) Article DOI: 10.1016/j.bmcl.2015.08.011 BindingDB Entry DOI: 10.7270/Q2GM8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50003359 (5-(4-Hexyloxy-[1,2,5]thiadiazol-3-yl)-1-methyl-1,2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in BHK-21 cells assessed as increase of acetylcholine-induced calcium flux by FLIPR assay | J Med Chem 53: 6386-97 (2010) Article DOI: 10.1021/jm100697g BindingDB Entry DOI: 10.7270/Q2765G9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50416734 (CHEMBL1223804) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 126 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in CHO cells assessed as effect on calcium mobilization by FLIPR assay | Bioorg Med Chem Lett 20: 5434-8 (2010) Article DOI: 10.1016/j.bmcl.2010.07.097 BindingDB Entry DOI: 10.7270/Q2MG7QR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM193791 (US9670209, Compound 309 1-((R)-3-(3-(2-Methoxyethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50035277 (3-Prop-2-ynyloxy-5,6,7,8-tetrahydro-4H-isoxazolo[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description In Vitro activity at the cloned Human Muscarinic acetylcholine receptor M3 determined by receptor selection and amplification technology (R-SAT) | J Med Chem 38: 2188-95 (1995) BindingDB Entry DOI: 10.7270/Q28K7838 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50035280 (3-Isopropoxy-5,6,7,8-tetrahydro-4H-isoxazolo[4,5-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description In Vitro activity at the cloned Human Muscarinic acetylcholine receptor M3 determined by receptor selection and amplification technology (R-SAT) | J Med Chem 38: 2188-95 (1995) BindingDB Entry DOI: 10.7270/Q28K7838 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM193784 (US9670209, Compound 302 1-((R)-3-((1R,3R,5S)-3-(cy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
ACADIA PHARMACEUTICALS INC.; ALLERGAN, INC. US Patent | Assay Description NIH-3T3 cells were grown in 96-well tissue culture plates to 70% to 80% confluence. Cells were transfected with plasmid DNAs using Superfect Reagent ... | US Patent US9670209 (2017) BindingDB Entry DOI: 10.7270/Q2MS3QX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 205 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human muscarinic M3 receptor expressed in CHO cells after 30 mins by GTPgamma35S binding assay | Bioorg Med Chem Lett 25: 4158-63 (2015) Article DOI: 10.1016/j.bmcl.2015.08.011 BindingDB Entry DOI: 10.7270/Q2GM8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50004665 ((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description In Vitro activity at the cloned Human Muscarinic acetylcholine receptor M3 determined by receptor selection and amplification technology (R-SAT) | J Med Chem 38: 2188-95 (1995) BindingDB Entry DOI: 10.7270/Q28K7838 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
University of Wuerzburg Curated by ChEMBL | Assay Description Activation of M3-ACh receptor-FLASH/CFP expressed in HEK293 cells assessed as FRET signal | Bioorg Med Chem 19: 1048-54 (2011) Article DOI: 10.1016/j.bmc.2010.07.060 BindingDB Entry DOI: 10.7270/Q2SF2WFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM10759 (2-acetoxyethyl(trimethyl)ammonium;bromide | 2-acet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Agonist activity at Gq/11 coupled recombinant human muscarinic M3 AChR expressed in CHO-FlpIn cells assessed as IP-one accumulation after 40 mins by ... | J Med Chem 60: 9239-9250 (2017) Article DOI: 10.1021/acs.jmedchem.7b01113 BindingDB Entry DOI: 10.7270/Q2445PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 195 total ) | Next | Last >> |