

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000900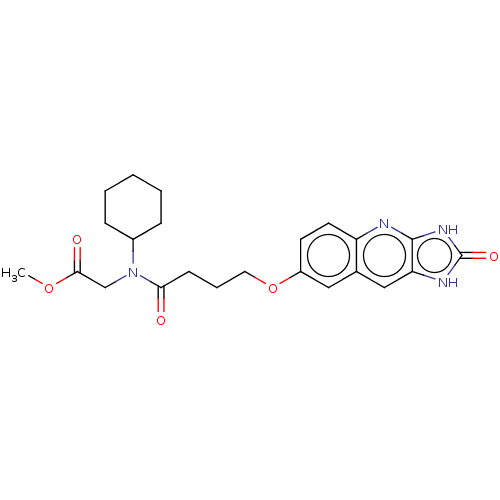 (CHEMBL90804 | {Cyclohexyl-[4-(2-oxo-2,3-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000915 (5-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50297387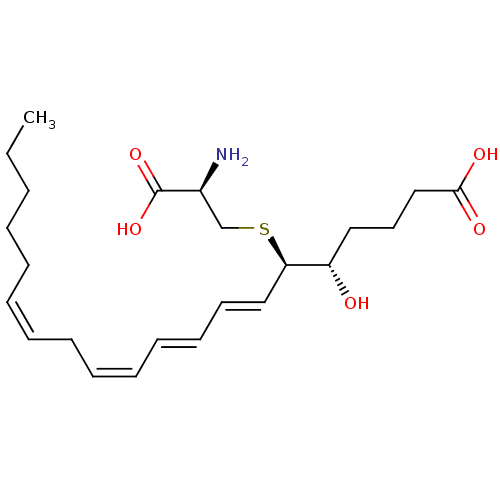 ((5S,6R,7E,9E,11Z,14Z)-6-(cystein-S-yl)-5-hydroxyic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human P2Y12 receptor | J Med Chem 52: 5803-15 (2009) Article DOI: 10.1021/jm900945d BindingDB Entry DOI: 10.7270/Q2G44QB1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50118223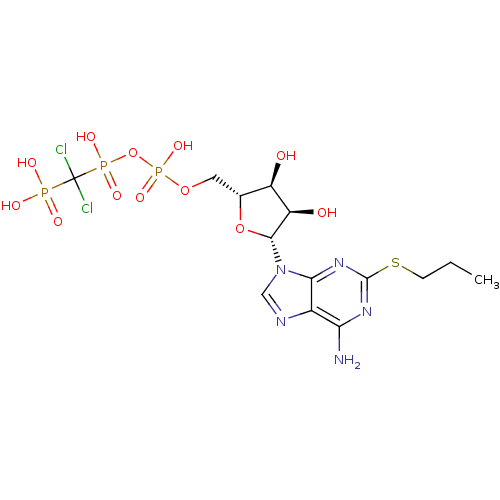 (AR-C67085 | Adenosine triphosphate derivative | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description The compound was evaluated for antagonist activity against platelet P2Y purinoceptor 12 (P2Y12) | J Med Chem 45: 4057-93 (2002) BindingDB Entry DOI: 10.7270/Q2VX0H71 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000915 (5-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000900 (CHEMBL90804 | {Cyclohexyl-[4-(2-oxo-2,3-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000931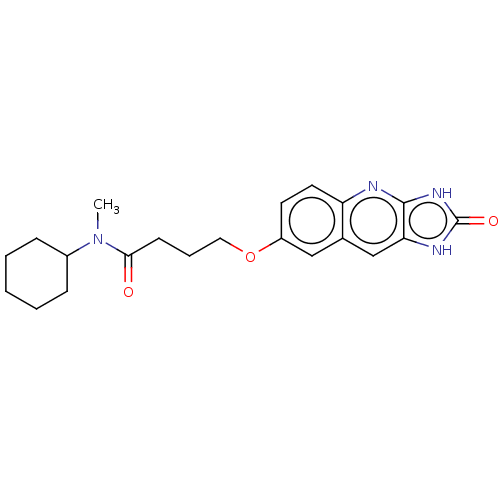 (CHEMBL91368 | N-Cyclohexyl-N-methyl-4-(2-oxo-2,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000931 (CHEMBL91368 | N-Cyclohexyl-N-methyl-4-(2-oxo-2,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000892 (5-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000892 (5-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000909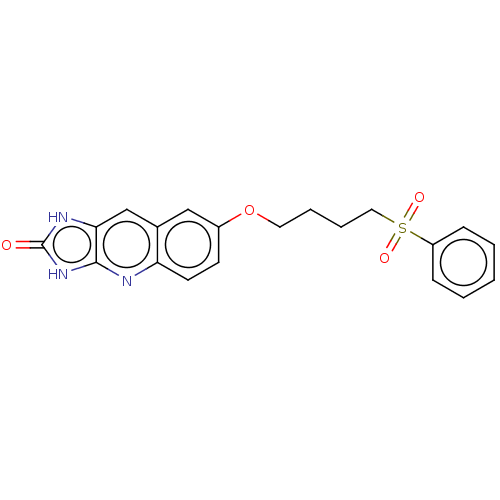 (7-(4-Benzenesulfonyl-butoxy)-1,3-dihydro-imidazo[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000899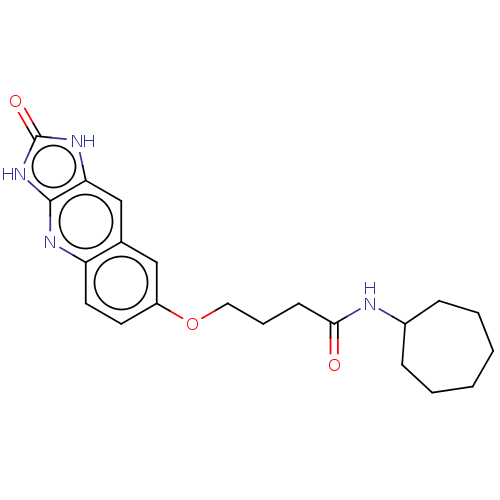 (CHEMBL93688 | N-Cycloheptyl-4-(2-oxo-2,3-dihydro-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000868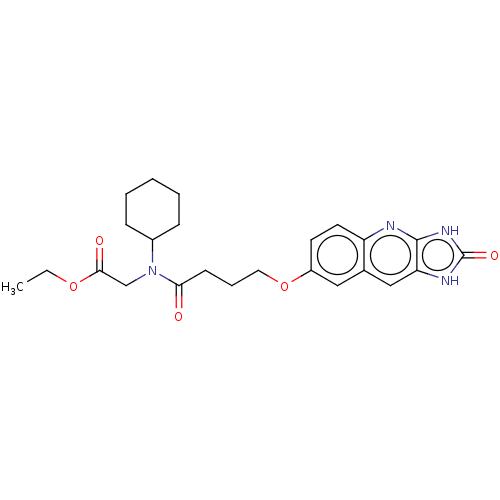 (CHEMBL88444 | {Cyclohexyl-[4-(2-oxo-2,3-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000880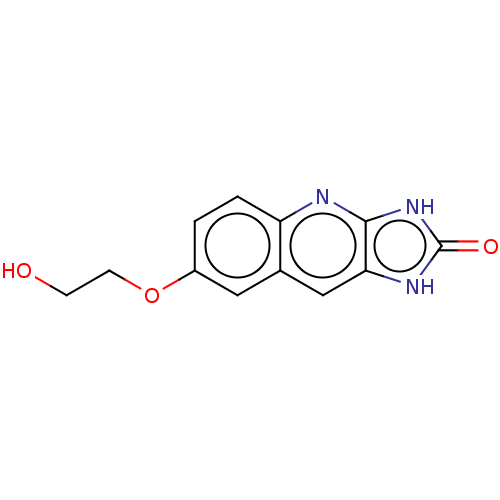 (7-(2-Hydroxy-ethoxy)-1,3-dihydro-imidazo[4,5-b]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000880 (7-(2-Hydroxy-ethoxy)-1,3-dihydro-imidazo[4,5-b]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000910 (6-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000868 (CHEMBL88444 | {Cyclohexyl-[4-(2-oxo-2,3-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000933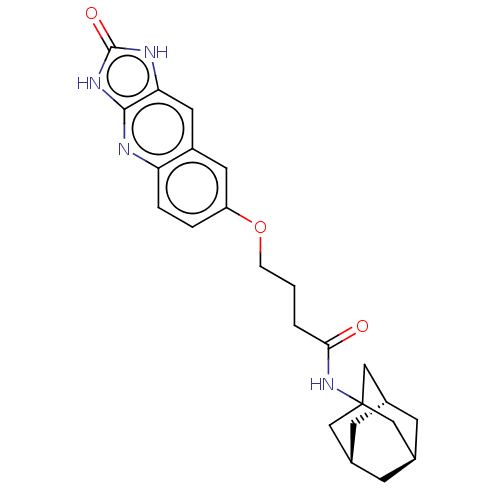 (CHEMBL92264 | N-Adamantan-1-yl-4-(2-oxo-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000932 (4-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 38 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000920 (CHEMBL88218 | N-Cyclohexyl-4-(2-oxo-2,3-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000908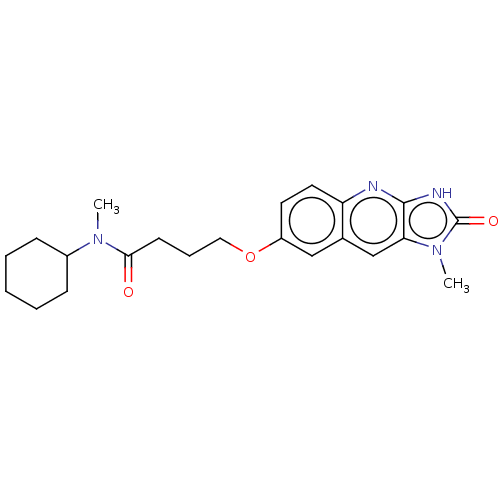 (CHEMBL92363 | N-Cyclohexyl-N-methyl-4-(1-methyl-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000918 (7-(4-Hydroxy-butoxy)-1,3-dihydro-imidazo[4,5-b]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000918 (7-(4-Hydroxy-butoxy)-1,3-dihydro-imidazo[4,5-b]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000877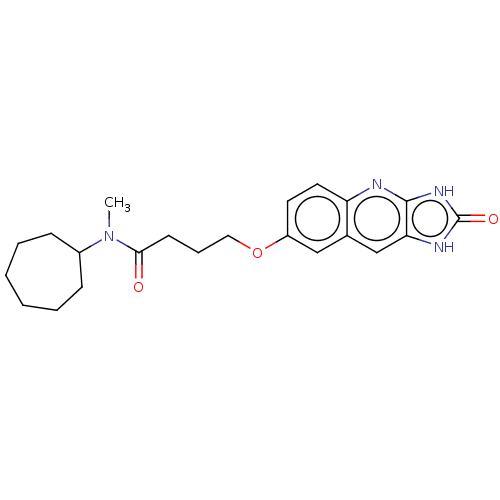 (CHEMBL88955 | N-Cycloheptyl-N-methyl-4-(2-oxo-2,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50601863 (CHEMBL5179763) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01571 BindingDB Entry DOI: 10.7270/Q25H7MBX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000869 (4-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000903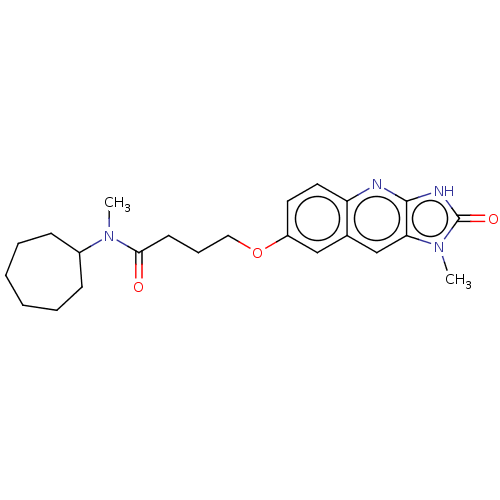 (CHEMBL91422 | N-Cycloheptyl-N-methyl-4-(1-methyl-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 70 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000869 (4-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 83 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma PRP | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000890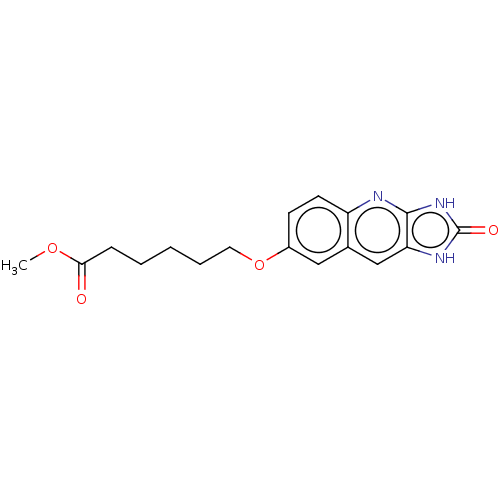 (6-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000875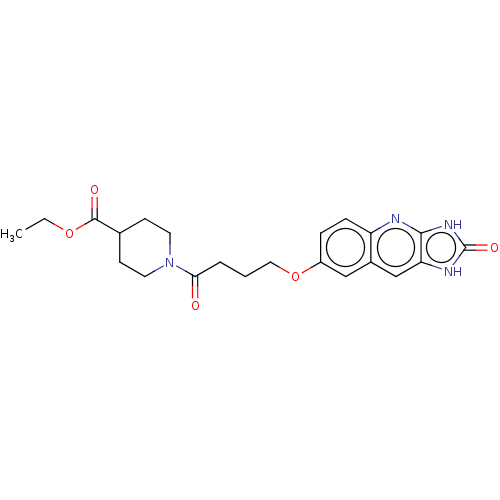 (1-[4-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000867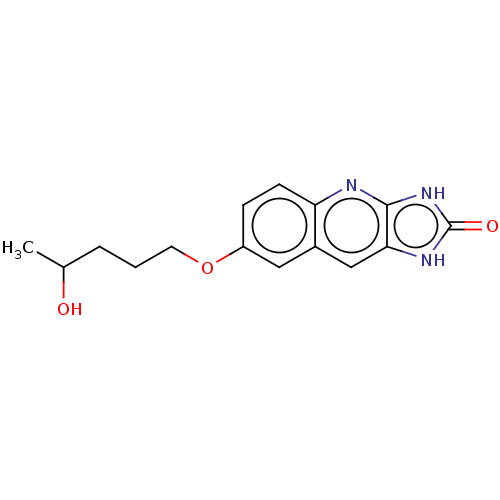 (7-(4-Hydroxy-pentyloxy)-1,3-dihydro-imidazo[4,5-b]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000871 (CHEMBL69139 | N-Cyclohexyl-N-methyl-4-(2-oxo-1,2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000933 (CHEMBL92264 | N-Adamantan-1-yl-4-(2-oxo-2,3-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000897 (7-(4-Oxo-4-piperidin-1-yl-butoxy)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000897 (7-(4-Oxo-4-piperidin-1-yl-butoxy)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000891 (5-(1-Methyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000928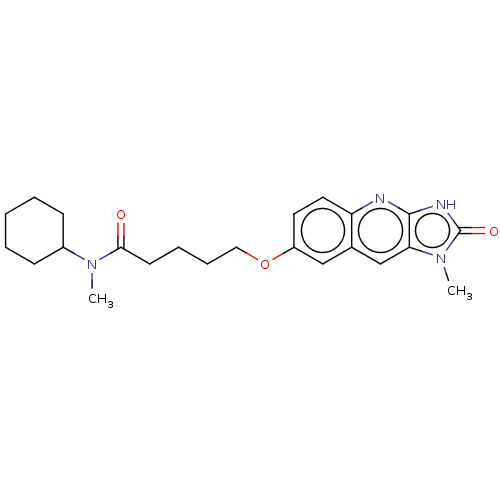 (5-(1-Methyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000909 (7-(4-Benzenesulfonyl-butoxy)-1,3-dihydro-imidazo[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000907 (4-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000893 (CHEMBL91356 | N-Cyclohexyl-4-(1-methyl-2-oxo-2,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000912 (CHEMBL90958 | N,N-Dimethyl-4-(2-oxo-2,3-dihydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using adenosine diphosphate (ADP) as activating agent in human platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000875 (1-[4-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000872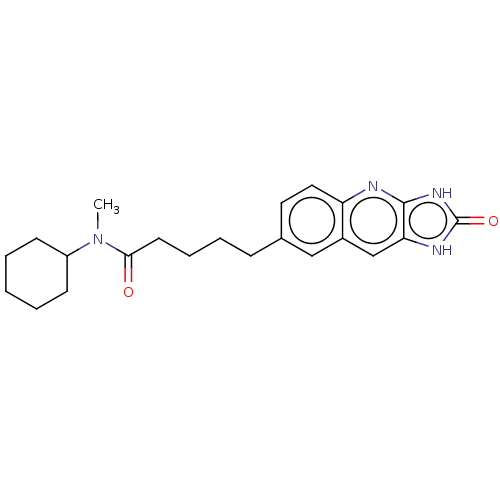 (5-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-7-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000879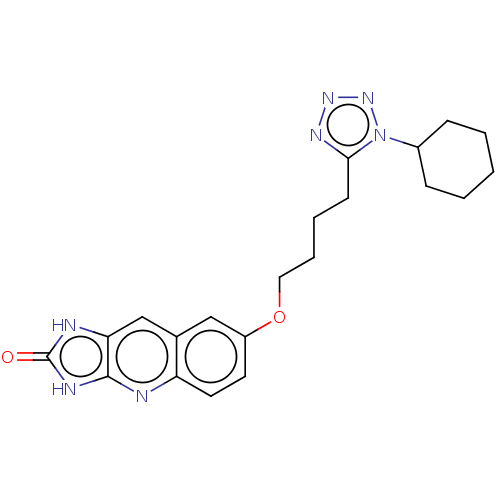 (7-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)-butoxy]-1,3-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000913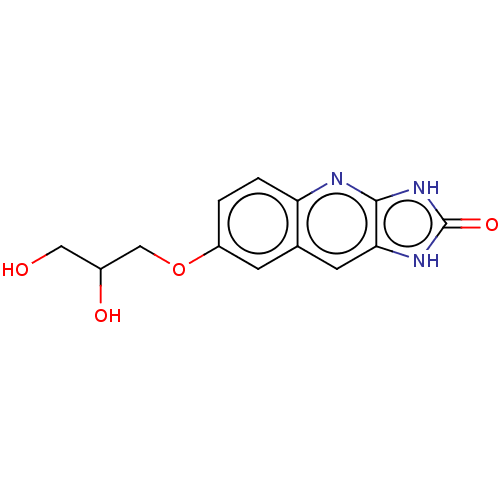 (7-(2,3-Dihydroxy-propoxy)-1,3-dihydro-imidazo[4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000913 (7-(2,3-Dihydroxy-propoxy)-1,3-dihydro-imidazo[4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 260 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000906 (7-(4-Morpholin-4-yl-4-oxo-butoxy)-1,3-dihydro-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000941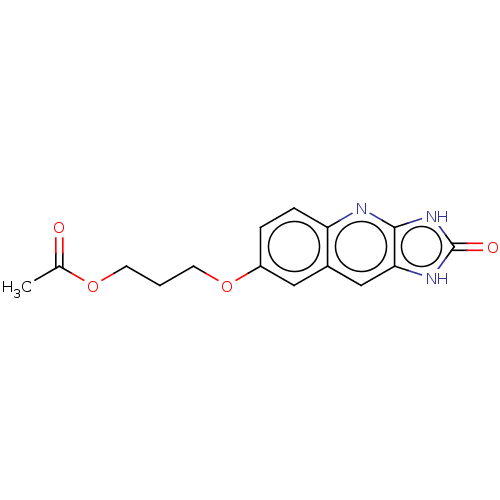 (Acetic acid 3-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000916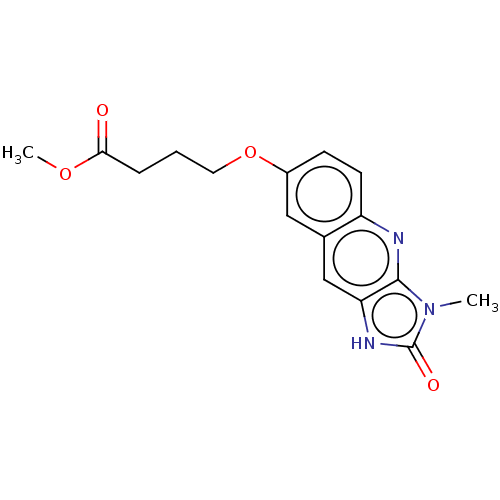 (4-(3-Methyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-b]qui...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50000939 (1-[4-(2-Oxo-2,3-dihydro-1H-imidazo[4,5-b]quinolin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of platelet aggregation using Adenosine diphosphate (ADP) as activating agent in rabbit platelet rich plasma (PRP) | J Med Chem 35: 2672-87 (1992) BindingDB Entry DOI: 10.7270/Q2513X52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 124 total ) | Next | Last >> |