

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50587205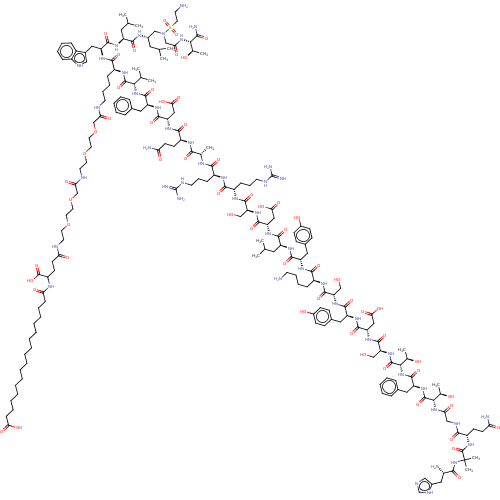 (CHEMBL5089850) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human GCCR expressed in human HEK293 cells assessed as luminescence signal incubated for 16 hrs at 37C measured by CRE-driven luciferas... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01289 BindingDB Entry DOI: 10.7270/Q2Q81J0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50266693 (CHEBI:5391 | Glucagen Hypokit | Glucagon | Glucago...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human GCCR expressed in human CHO-K1 cell lines assessed as intracellular cAMP generation incubated for 30 mins measured by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01289 BindingDB Entry DOI: 10.7270/Q2Q81J0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50266693 (CHEBI:5391 | Glucagen Hypokit | Glucagon | Glucago...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human GCCR expressed in human HEK293 cells assessed as luminescence signal incubated for 16 hrs at 37C measured by CRE-driven luciferas... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01289 BindingDB Entry DOI: 10.7270/Q2Q81J0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50206899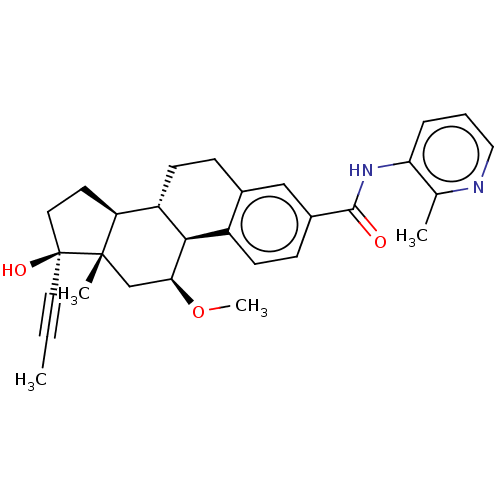 (CHEMBL3964460) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at GR in human HepG2 cells assessed as protein mediated-transcriptional activity by MMTV-promoter driven luciferase reporter gene as... | Bioorg Med Chem Lett 27: 347-353 (2017) Article DOI: 10.1016/j.bmcl.2016.11.007 BindingDB Entry DOI: 10.7270/Q24B339Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50587188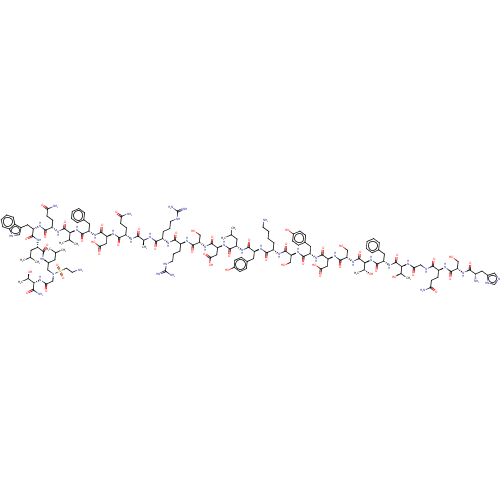 (CHEMBL5080959) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human GCCR expressed in human CHO-K1 cell lines assessed as intracellular cAMP generation incubated for 30 mins measured by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01289 BindingDB Entry DOI: 10.7270/Q2Q81J0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50206940 (CHEMBL3981243) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at GR in human HepG2 cells assessed as protein mediated-transcriptional activity by MMTV-promoter driven luciferase reporter gene as... | Bioorg Med Chem Lett 27: 347-353 (2017) Article DOI: 10.1016/j.bmcl.2016.11.007 BindingDB Entry DOI: 10.7270/Q24B339Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18623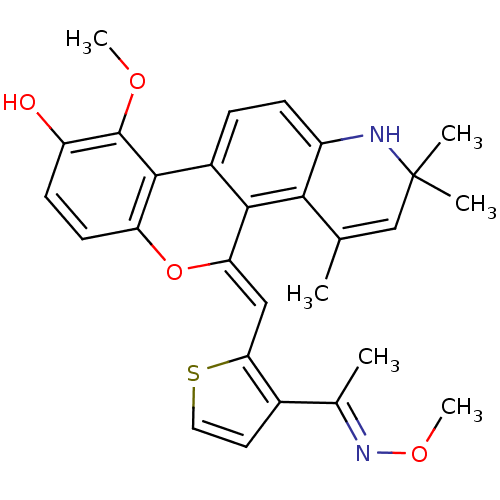 ((18Z)-12-methoxy-18-({3-[(1E)-1-(methoxyimino)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | -47.5 | 0.200 | n/a | 0.100 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440220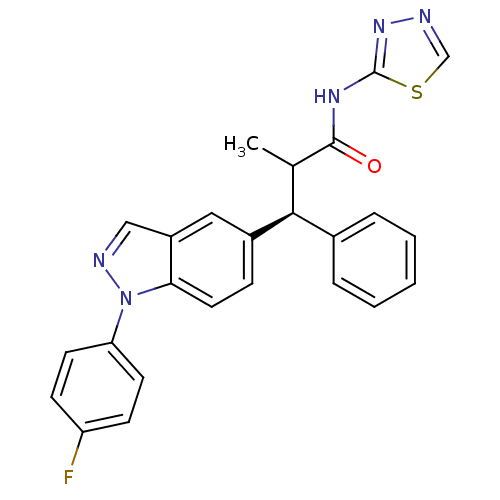 (CHEMBL2426624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals Curated by ChEMBL | Assay Description Transactivation of glucocorticoid receptor ligand binding domain (unknown origin) transfected in human HeLa cells assessed as activation of NP-1 by G... | Bioorg Med Chem Lett 23: 5442-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.089 BindingDB Entry DOI: 10.7270/Q29888FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440220 (CHEMBL2426624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals Curated by ChEMBL | Assay Description Transactivation of glucocorticoid receptor ligand binding domain (unknown origin) transfected in human HeLa cells assessed as activation of NP-1 by G... | Bioorg Med Chem Lett 23: 5442-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.089 BindingDB Entry DOI: 10.7270/Q29888FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Guangzhou University of Chinese Medicine Curated by ChEMBL | Assay Description Agonist activity at recombinant human glucocorticoid receptor expressed in African green monkey CV1 cells by MMTV luciferase reporter gene assay | Eur J Med Chem 161: 192-204 (2019) Article DOI: 10.1016/j.ejmech.2018.10.020 BindingDB Entry DOI: 10.7270/Q2F19313 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50206900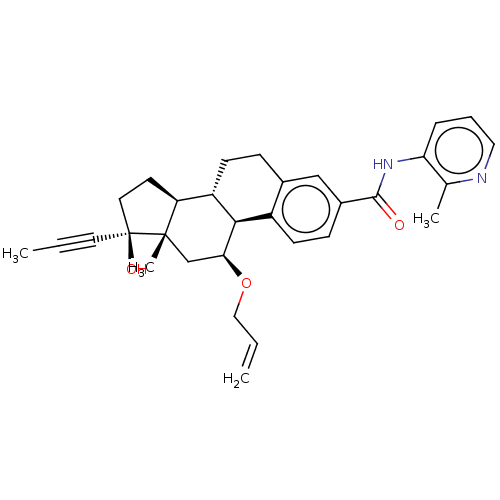 (CHEMBL3895181) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Agonist activity at GR in human HepG2 cells assessed as protein mediated-transcriptional activity by MMTV-promoter driven luciferase reporter gene as... | Bioorg Med Chem Lett 27: 347-353 (2017) Article DOI: 10.1016/j.bmcl.2016.11.007 BindingDB Entry DOI: 10.7270/Q24B339Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18617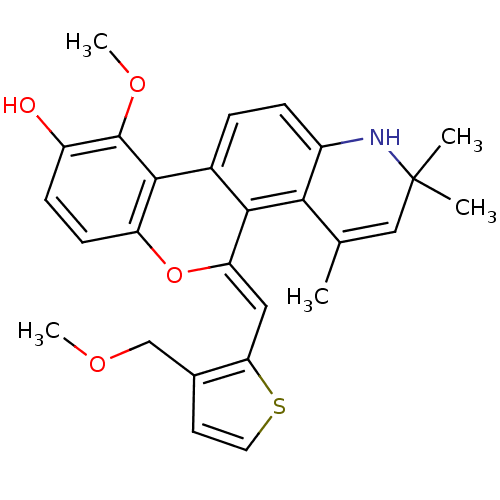 ((18Z)-12-methoxy-18-{[3-(methoxymethyl)thiophen-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | -51.5 | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18616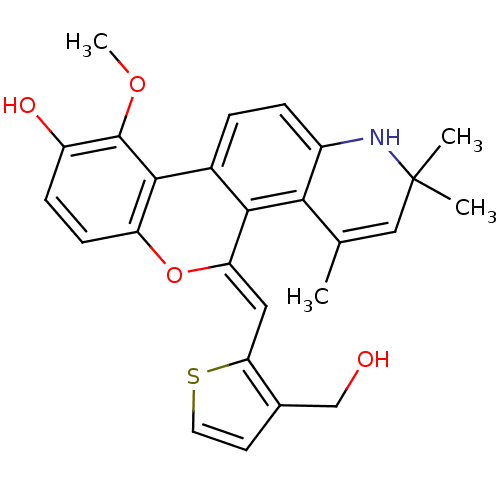 ((18Z)-18-{[3-(hydroxymethyl)thiophen-2-yl]methylid...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -48.6 | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.10 | -46.0 | 1.40 | n/a | 0.200 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18629 ((18Z)-12-methoxy-3,5,5-trimethyl-18-({3-[(1S)-2,2,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | 0.200 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Antagonist Activity Assay- GR-mediated antagonist activity is measured in the presence of a concentration of dexamethasone empirically de... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338317 ((+/-)-3-hydroxy-6-(1H-indol-7-yl)-2,2,4,8-tetramet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR expressed in african green monkey CV1 cells transfected with luciferase gene linked to MMTV promoter assessed as induction of ... | Bioorg Med Chem Lett 21: 1697-700 (2011) Article DOI: 10.1016/j.bmcl.2011.01.093 BindingDB Entry DOI: 10.7270/Q2639Q12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214932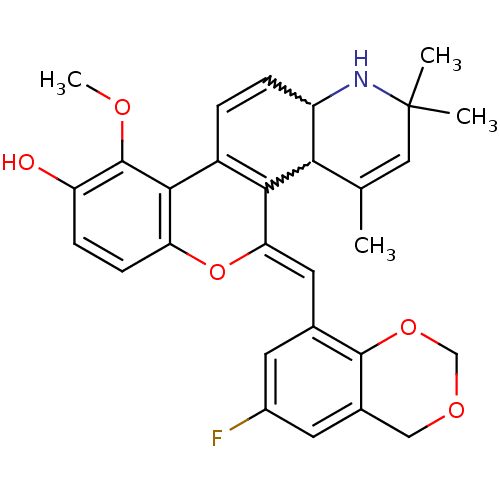 ((Z)-5-((6-fluoro-4H-benzo[d][1,3]dioxin-8-yl)methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR by GRE activation assay | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214928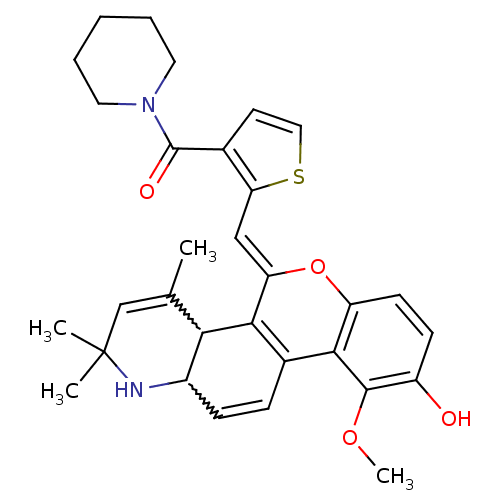 ((Z)-(2-((9-hydroxy-10-methoxy-2,2,4-trimethyl-1,2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR by GRE activation assay | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18620 (2-{[(18Z)-13-hydroxy-12-methoxy-3,5,5-trimethyl-17...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | 0.200 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50587188 (CHEMBL5080959) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human GCCR expressed in human HEK293 cells assessed as luminescence signal incubated for 16 hrs at 37C measured by CRE-driven luciferas... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01289 BindingDB Entry DOI: 10.7270/Q2Q81J0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18628 ((18Z)-12-methoxy-3,5,5-trimethyl-18-{[3-(2,2,2-tri...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -47.0 | 0.400 | n/a | 0.300 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440220 (CHEMBL2426624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human A549 cells assessed as inhibition of PMA-induced AP-1 activation by luciferase reporter gene ass... | Bioorg Med Chem Lett 23: 5442-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.089 BindingDB Entry DOI: 10.7270/Q29888FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18615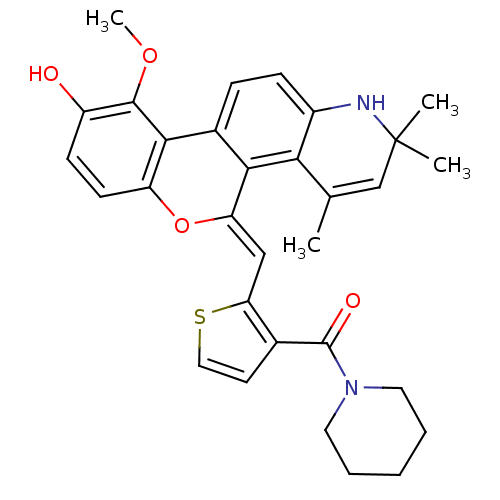 ((18Z)-12-methoxy-3,5,5-trimethyl-18-{[3-(piperidin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | 0.300 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440220 (CHEMBL2426624) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human A549 cells assessed as inhibition of PMA-induced AP-1 activation by luciferase reporter gene ass... | Bioorg Med Chem Lett 23: 5442-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.089 BindingDB Entry DOI: 10.7270/Q29888FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354857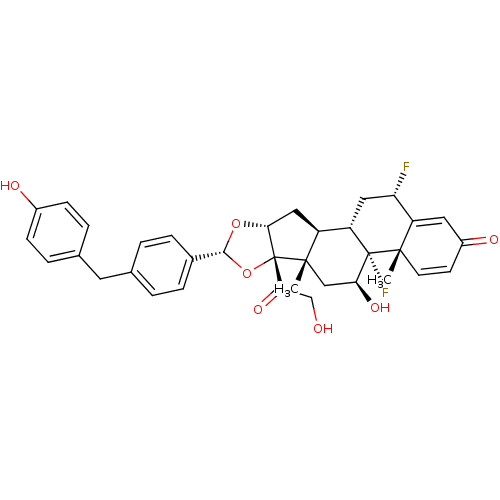 (CHEMBL1834538) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18624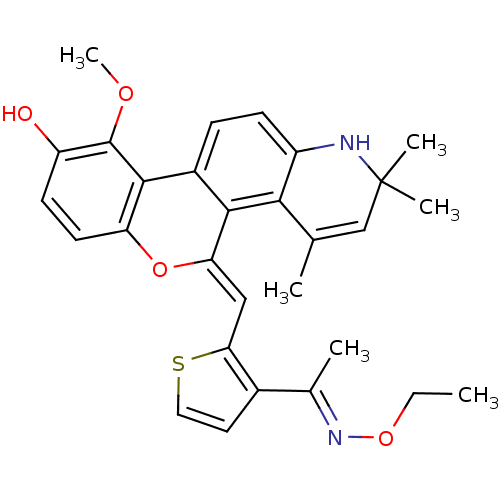 ((18Z)-18-({3-[(1E)-1-(ethoxyimino)ethyl]thiophen-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | 0.400 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18631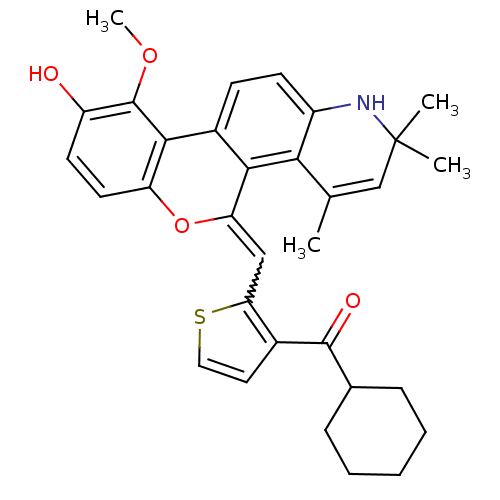 ((18Z)-18-[(3-cyclohexanecarbonylthiophen-2-yl)meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | 0.400 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Antagonist Activity Assay- GR-mediated antagonist activity is measured in the presence of a concentration of dexamethasone empirically de... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338318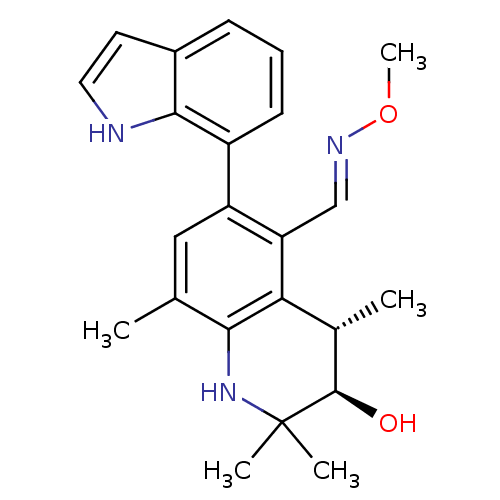 ((+/-)-3-hydroxy-6-(1H-indol-7-yl)-2,2,4,8-tetramet...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR expressed in african green monkey CV1 cells transfected with luciferase gene linked to MMTV promoter assessed as induction of ... | Bioorg Med Chem Lett 21: 1697-700 (2011) Article DOI: 10.1016/j.bmcl.2011.01.093 BindingDB Entry DOI: 10.7270/Q2639Q12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50587189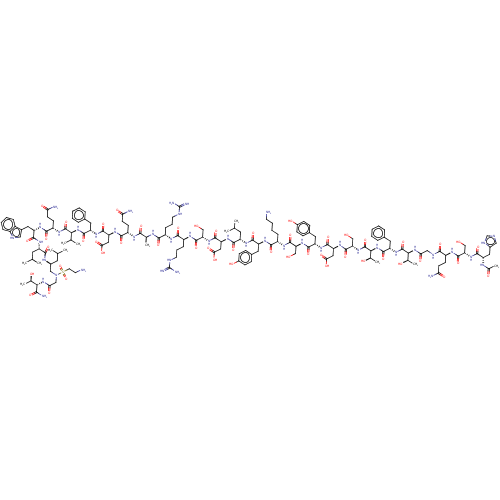 (CHEMBL5091611) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human GCCR expressed in human CHO-K1 cell lines assessed as intracellular cAMP generation incubated for 30 mins measured by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01289 BindingDB Entry DOI: 10.7270/Q2Q81J0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354851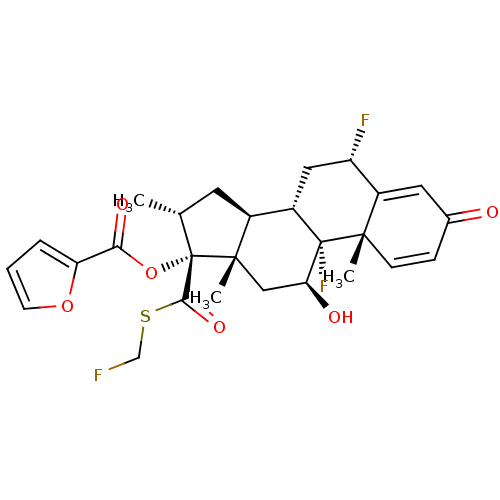 (FLUTICASONE FUROATE | Veramyst) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Induction of nuclear translocation of human recombinant ProLabel-tagged glucocorticoid receptor expressed in CHO-K1 cells after 3 hrs by luminescence... | J Med Chem 61: 4757-4773 (2018) Article DOI: 10.1021/acs.jmedchem.7b01873 BindingDB Entry DOI: 10.7270/Q2JS9T19 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50587202 (CHEMBL5090987) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human GCCR expressed in human CHO-K1 cell lines assessed as intracellular cAMP generation incubated for 30 mins measured by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01289 BindingDB Entry DOI: 10.7270/Q2Q81J0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354853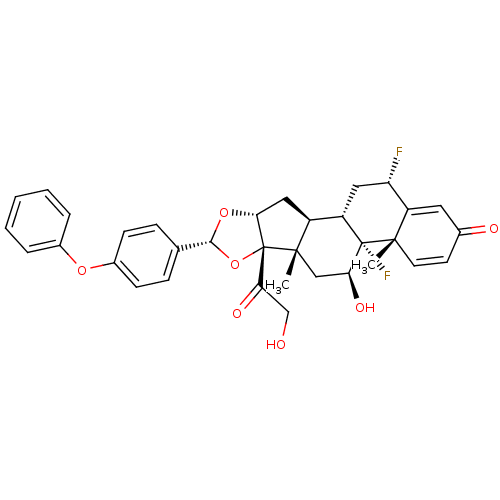 (CHEMBL1834534) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50587187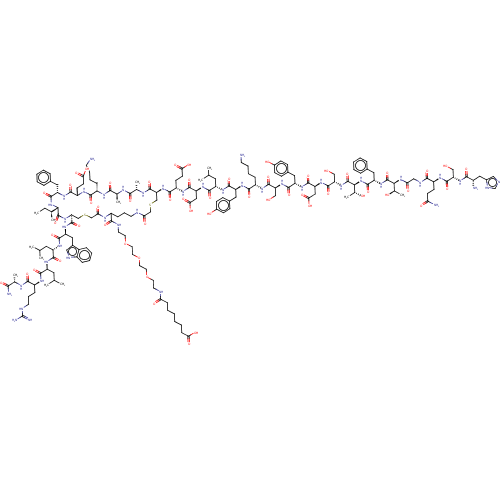 (CHEMBL5093620) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human GCCR expressed in human HEK293 cells assessed as luminescence signal incubated for 16 hrs at 37C measured by CRE-driven luciferas... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01289 BindingDB Entry DOI: 10.7270/Q2Q81J0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354851 (FLUTICASONE FUROATE | Veramyst) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GR in human SW1353 cells transfected with luciferase gene linked to MMTV promoter assessed as luciferase transactivation activity | Bioorg Med Chem Lett 21: 5826-30 (2011) Article DOI: 10.1016/j.bmcl.2011.07.106 BindingDB Entry DOI: 10.7270/Q22V2GH4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18626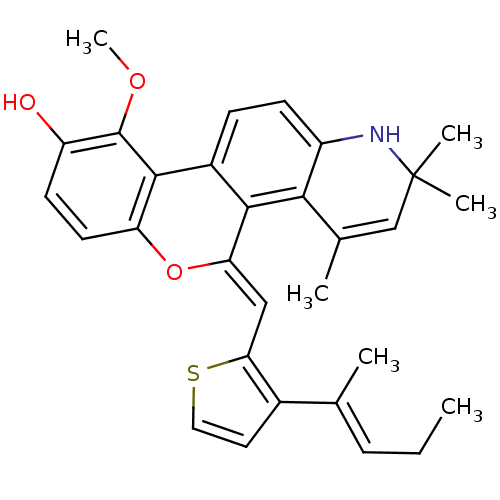 ((18Z)-12-methoxy-3,5,5-trimethyl-18-({3-[(2E)-pent...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40 | -44.3 | 6.10 | n/a | 0.5 | n/a | n/a | 7.5 | 4 |
Ligand Pharmaceuticals Inc. | Assay Description Competitive Ligand Binding Assay- The Ki values were determined by the application of the Cheng-Prusoff equation: Ki=IC50/(1+[L]/Kd) where [L] is the... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440219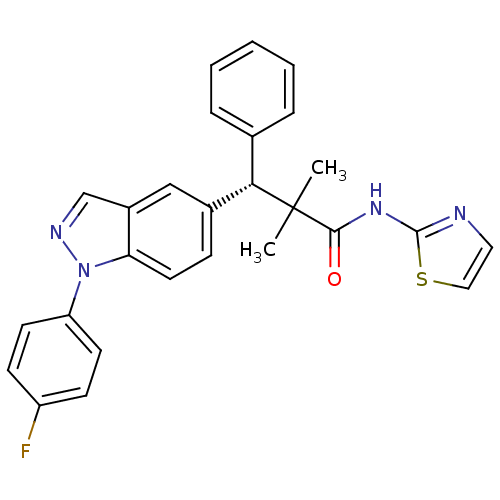 (CHEMBL2426665) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Ironwood Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human A549 cells assessed as inhibition of IL-1beta-induced NF-kappaB dependent E-selectin activation ... | Bioorg Med Chem Lett 23: 5442-7 (2013) Article DOI: 10.1016/j.bmcl.2013.06.089 BindingDB Entry DOI: 10.7270/Q29888FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18619 ((18Z)-12-methoxy-3,5,5-trimethyl-18-[(3-{[(2,2,2-t...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | 0.5 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338324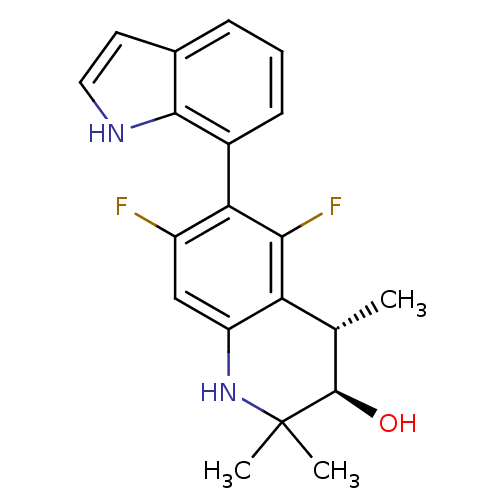 ((+/-)-(3R,4S)-5,7-difluoro-6-(1H-indol-7-yl)-2,2,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR expressed in african green monkey CV1 cells transfected with luciferase gene linked to MMTV promoter assessed as induction of ... | Bioorg Med Chem Lett 21: 1658-62 (2011) Article DOI: 10.1016/j.bmcl.2011.01.106 BindingDB Entry DOI: 10.7270/Q2VQ32Z4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50340663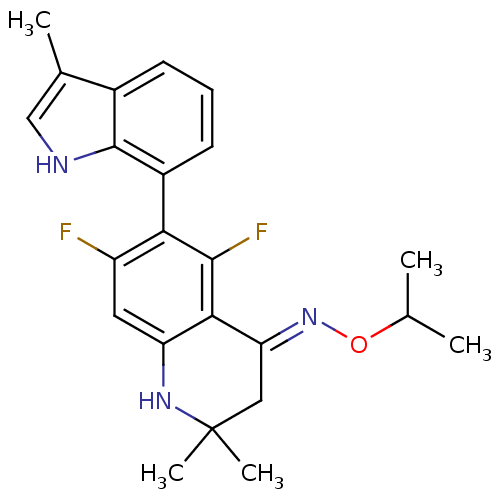 (5,7-difluoro-2,2-dimethyl-6-(3-methyl-1H-indol-7-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human HepG2 cells co-transfected with GRE assessed as GRE activation by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 1654-7 (2011) Article DOI: 10.1016/j.bmcl.2011.01.104 BindingDB Entry DOI: 10.7270/Q21V5F8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338324 ((+/-)-(3R,4S)-5,7-difluoro-6-(1H-indol-7-yl)-2,2,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human HepG2 cells co-transfected with GRE assessed as GRE activation by luciferase reporter gene assay | Bioorg Med Chem Lett 21: 1654-7 (2011) Article DOI: 10.1016/j.bmcl.2011.01.104 BindingDB Entry DOI: 10.7270/Q21V5F8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Antagonist Activity Assay- GR-mediated antagonist activity is measured in the presence of a concentration of dexamethasone empirically de... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18633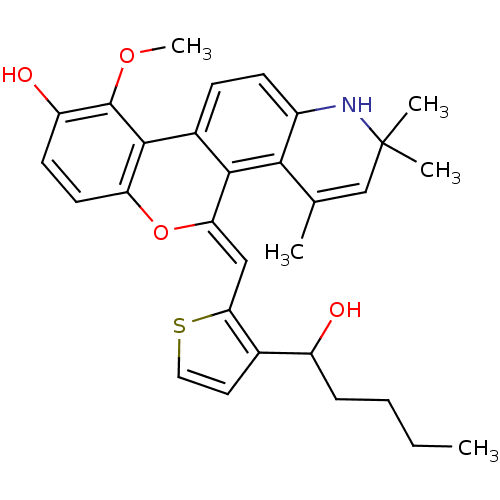 ((18Z)-18-{[3-(1-hydroxypentyl)thiophen-2-yl]methyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | 0.600 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Antagonist Activity Assay- GR-mediated antagonist activity is measured in the presence of a concentration of dexamethasone empirically de... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18621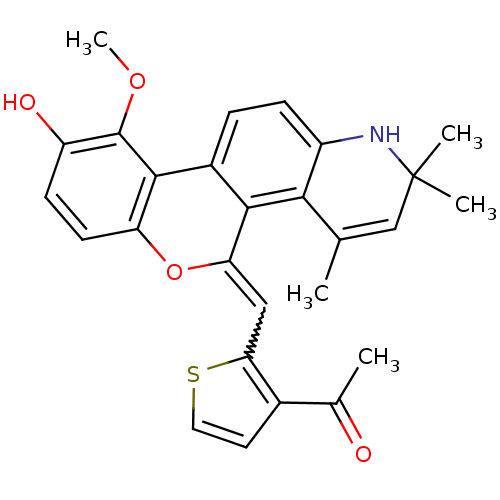 (1-(2-{[(18Z)-13-hydroxy-12-methoxy-3,5,5-trimethyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | 0.600 | n/a | n/a | 7.5 | n/a |
Ligand Pharmaceuticals Inc. | Assay Description GR-Mediated Agonist Activity Assay- GR-mediated activation by compounds is measured against maximal activation of dexamethasone in the same experimen... | J Med Chem 50: 4699-709 (2007) Article DOI: 10.1021/jm070370z BindingDB Entry DOI: 10.7270/Q2T151XT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50048344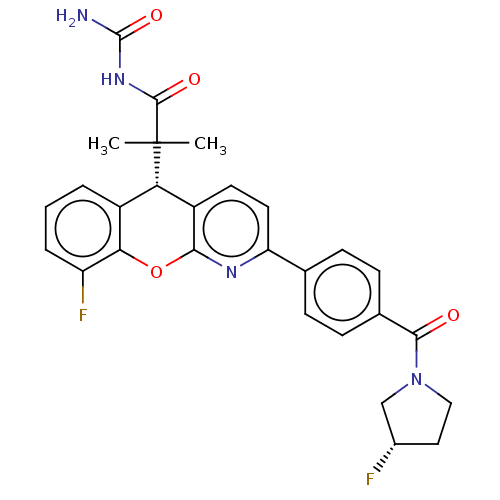 (CHEMBL3315066) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human A549 cells assessed as E-selectin transrepression by luciferase reporter gene assay | Bioorg Med Chem Lett 24: 3268-73 (2014) Article DOI: 10.1016/j.bmcl.2014.06.010 BindingDB Entry DOI: 10.7270/Q21N82SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50338324 ((+/-)-(3R,4S)-5,7-difluoro-6-(1H-indol-7-yl)-2,2,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR expressed in african green monkey CV1 cells transfected with luciferase gene linked to MMTV promoter assessed as induction of ... | Bioorg Med Chem Lett 21: 1697-700 (2011) Article DOI: 10.1016/j.bmcl.2011.01.093 BindingDB Entry DOI: 10.7270/Q2639Q12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214949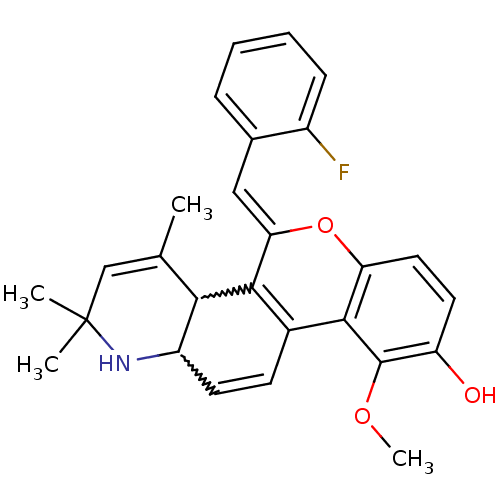 ((Z)-5-(2-fluorobenzylidene)-10-methoxy-2,2,4-trime...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR by GRE activation assay | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50587203 (CHEMBL5088610) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a |
TBA | Assay Description Activation of human GCCR expressed in human CHO-K1 cell lines assessed as intracellular cAMP generation incubated for 30 mins measured by HTRF assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01289 BindingDB Entry DOI: 10.7270/Q2Q81J0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50440101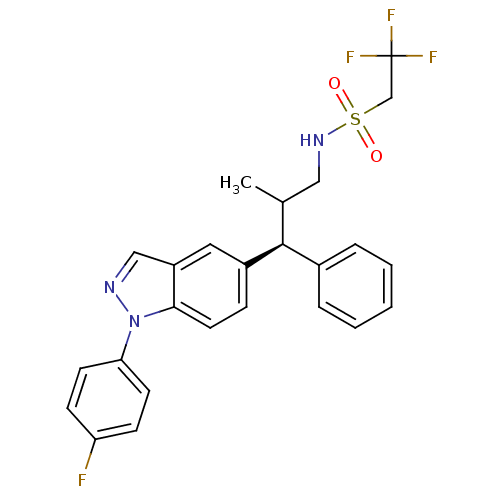 (CHEMBL2426119) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human A549 cells assessed as inhibition of IL1beta-stimulated NFkappaB-dependent E-selectin tr... | Bioorg Med Chem Lett 23: 5448-51 (2013) Article DOI: 10.1016/j.bmcl.2013.06.085 BindingDB Entry DOI: 10.7270/Q22R3T2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50214933 ((Z)-10-methoxy-2,2,4-trimethyl-5-((6-methylpyridin...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at GR by GRE activation assay | Bioorg Med Chem Lett 17: 4158-62 (2007) Article DOI: 10.1016/j.bmcl.2007.05.062 BindingDB Entry DOI: 10.7270/Q2DF6QXW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50354849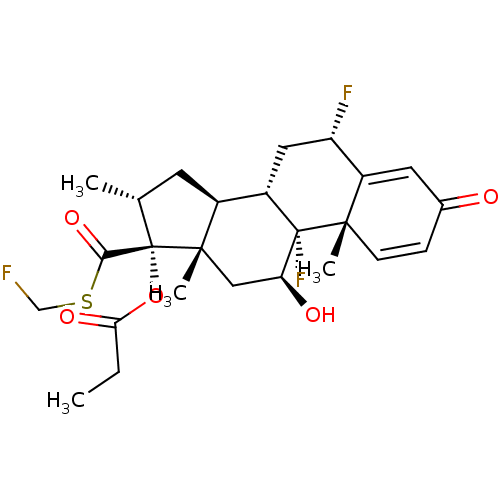 (CCI-18781 | Cutivate | FLUTICASONE PROPIONATE | Fl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity at glucocorticoid receptor in human HepG2 cells assessed as induction of tyrosine amino transferase | Bioorg Med Chem Lett 21: 6343-7 (2011) Article DOI: 10.1016/j.bmcl.2011.08.108 BindingDB Entry DOI: 10.7270/Q2FF3SRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1509 total ) | Next | Last >> |