 Found 629 hits of ec50 data for polymerid = 9852
Found 629 hits of ec50 data for polymerid = 9852 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0617 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human melanocortin receptor 3 expressed in human T-REx-293 cells assessed as stimulation of intracellular cAMP accumulation incub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0617 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50033130
((3R,6S,9R,12S,15S,23R)-15-((S)-2-Acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc(Cl)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C50H68ClN15O9/c1-3-4-11-36(60-28(2)67)44(70)66-41-24-42(68)56-19-8-7-13-35(43(52)69)61-47(73)39(22-30-25-58-34-12-6-5-10-33(30)34)64-45(71)37(14-9-20-57-50(53)54)62-46(72)38(21-29-15-17-31(51)18-16-29)63-48(74)40(65-49(41)75)23-32-26-55-27-59-32/h5-6,10,12,15-18,25-27,35-41,58H,3-4,7-9,11,13-14,19-24H2,1-2H3,(H2,52,69)(H,55,59)(H,56,68)(H,60,67)(H,61,73)(H,62,72)(H,63,74)(H,64,71)(H,65,75)(H,66,70)(H4,53,54,57)/t35-,36+,37+,38-,39-,40+,41+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Evaluated for agonist activity at cloned Melanocortin 3 receptor |
J Med Chem 38: 3454-61 (1995)
BindingDB Entry DOI: 10.7270/Q2Z32094 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50268805
(Ac-Tyr-Val-Nle-Gly-His-DPhe-trans-Xaa-Trp-Asp-Arg-...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@@H](C[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O)N=C(N)N |r,wU:8.8,45.46,59.63,96.101,35.35,77.82,wD:4.4,57.117,12.21,63.66,85.90,(3.89,-26.77,;2.57,-27.55,;1.22,-26.79,;-.1,-27.57,;-1.45,-26.81,;-2.78,-27.6,;-2.76,-29.14,;-1.41,-29.89,;-4.09,-29.92,;-4.07,-31.46,;-5.4,-32.25,;-6.73,-31.49,;-5.38,-33.78,;-4.04,-34.54,;-2.72,-33.75,;-2.73,-32.22,;-1.4,-31.43,;-.06,-32.19,;1.27,-31.4,;-.04,-33.74,;-1.37,-34.52,;-6.71,-34.57,;-6.71,-36.11,;-8.02,-36.89,;-5.35,-36.86,;-5.43,-29.17,;-5.44,-27.63,;-6.74,-29.95,;-1.46,-25.28,;-2.8,-24.52,;-.14,-24.49,;-.16,-22.95,;1.17,-22.16,;2.52,-22.92,;1.16,-20.62,;2.48,-19.84,;2.46,-18.3,;1.13,-17.55,;.96,-16.02,;-.56,-15.71,;-1.31,-17.05,;-.27,-18.18,;3.82,-20.6,;3.85,-22.14,;5.15,-19.82,;6.5,-20.57,;6.51,-22.12,;7.85,-22.87,;7.87,-24.42,;9.21,-25.18,;10.53,-24.39,;10.51,-22.85,;9.17,-22.09,;7.86,-19.86,;9.11,-20.76,;7.93,-18.32,;6.9,-17.17,;7.69,-15.84,;9.19,-16.18,;9.34,-17.71,;10.66,-18.5,;10.65,-20.04,;12,-17.74,;13.32,-18.54,;13.31,-20.08,;14.59,-20.94,;16.03,-20.4,;16.98,-21.61,;16.14,-22.89,;16.51,-24.38,;15.41,-25.47,;13.93,-25.05,;13.55,-23.56,;14.65,-22.48,;14.67,-17.78,;14.68,-16.24,;15.99,-18.57,;17.33,-17.82,;17.34,-16.27,;18.68,-15.52,;20.02,-16.29,;18.7,-13.97,;18.66,-18.59,;18.64,-20.14,;20,-17.84,;21.32,-18.63,;21.3,-20.18,;22.62,-20.95,;22.61,-22.5,;23.94,-23.27,;23.91,-24.81,;22.59,-25.56,;25.26,-25.58,;22.66,-17.88,;22.67,-16.34,;23.99,-18.65,;25.33,-17.89,;25.35,-16.35,;26.69,-15.6,;28.02,-16.38,;29.36,-15.63,;29.38,-14.09,;28.05,-13.3,;26.71,-14.06,;26.66,-18.67,;26.64,-20.22,;28,-17.92,;29.32,-18.7,;30.66,-17.95,;31.99,-18.73,;30.68,-16.41,;7.08,-14.43,;7.99,-13.19,;7.38,-11.78,;9.52,-13.37,)| Show InChI InChI=1S/C77H102N22O16/c1-5-6-21-53(93-74(114)65(42(2)3)98-72(112)56(89-43(4)100)30-46-24-26-50(101)27-25-46)66(106)87-39-63(103)91-58(33-48-37-83-41-88-48)70(110)97-60(31-45-18-11-8-12-19-45)75(115)99-40-49(90-77(81)82)34-61(99)73(113)96-57(32-47-36-85-52-22-14-13-20-51(47)52)69(109)95-59(35-64(104)105)71(111)92-54(23-15-28-84-76(79)80)68(108)94-55(67(107)86-38-62(78)102)29-44-16-9-7-10-17-44/h7-14,16-20,22,24-27,36-37,41-42,49,53-61,65,85,101H,5-6,15,21,23,28-35,38-40H2,1-4H3,(H2,78,102)(H,83,88)(H,86,107)(H,87,106)(H,89,100)(H,91,103)(H,92,111)(H,93,114)(H,94,108)(H,95,109)(H,96,113)(H,97,110)(H,98,112)(H,104,105)(H4,79,80,84)(H4,81,82,90)/t49-,53+,54+,55+,56+,57+,58+,59+,60-,61+,65+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50268792
(AC-Nle-c[Asp-His-DPhe-Pro-Trp-Lys]-NH2 | CHEMBL501...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C49H64N12O9/c1-3-4-16-36(55-29(2)62)44(65)58-39-25-42(63)52-20-11-10-18-35(43(50)64)56-45(66)37(23-31-26-53-34-17-9-8-15-33(31)34)59-48(69)41-19-12-21-61(41)49(70)40(22-30-13-6-5-7-14-30)60-46(67)38(57-47(39)68)24-32-27-51-28-54-32/h5-9,13-15,17,26-28,35-41,53H,3-4,10-12,16,18-25H2,1-2H3,(H2,50,64)(H,51,54)(H,52,63)(H,55,62)(H,56,66)(H,57,68)(H,58,65)(H,59,69)(H,60,67)/t35-,36-,37-,38-,39-,40+,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50121268
(21-(2-Acetylamino-hexanoylamino)-7-[3-(diaminometh...)Show SMILES CCCC[C@@H](NC(C)=O)C(=O)N[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O Show InChI InChI=1S/C54H71N15O9/c1-3-4-15-40(63-31(2)70)48(73)69-45-27-46(71)59-21-10-9-17-39(47(55)72)64-51(76)43(25-35-28-61-38-16-8-7-14-37(35)38)67-49(74)41(18-11-22-60-54(56)57)65-50(75)42(24-32-19-20-33-12-5-6-13-34(33)23-32)66-52(77)44(68-53(45)78)26-36-29-58-30-62-36/h5-8,12-14,16,19-20,23,28-30,39-45,61H,3-4,9-11,15,17-18,21-22,24-27H2,1-2H3,(H2,55,72)(H,58,62)(H,59,71)(H,63,70)(H,64,76)(H,65,75)(H,66,77)(H,67,74)(H,68,78)(H,69,73)(H4,56,57,60)/t39-,40+,41-,42+,43-,44-,45+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50268794
(Ac-Nle-c[Asp-His-DPhe-cis-4-guanidinyl-Pro-Trp-Lys...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H]2C[C@@H](CN2C(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)N=C(N)N)C(N)=O |r,wU:46.49,21.76,12.11,41.72,4.4,wD:57.61,25.25,39.45,(20.33,-40.8,;18.97,-40.08,;18.92,-38.54,;17.56,-37.81,;17.51,-36.27,;18.82,-35.46,;18.77,-33.92,;20.08,-33.11,;17.41,-33.19,;16.15,-35.55,;16.1,-34.01,;14.85,-36.36,;13.49,-35.64,;12.18,-36.45,;12.23,-37.99,;13.59,-38.71,;10.92,-38.8,;9.56,-38.08,;8.26,-38.89,;6.9,-38.17,;5.59,-38.98,;4.23,-38.25,;4.18,-36.71,;2.82,-35.99,;1.52,-36.8,;2.77,-34.45,;1.41,-33.72,;1.36,-32.19,;2.59,-31.24,;2.06,-29.79,;.52,-29.85,;-.55,-28.75,;-2.04,-29.13,;-2.45,-30.61,;-1.37,-31.7,;.1,-31.32,;4.08,-33.64,;4.74,-34.72,;5.06,-36.27,;5.44,-34.36,;6.06,-35.94,;7.6,-35.89,;8.02,-34.41,;6.75,-33.55,;6.7,-32.01,;5.34,-31.28,;8,-31.2,;7.95,-29.66,;6.59,-28.93,;6.55,-27.39,;5.19,-26.67,;3.88,-27.48,;3.94,-29.03,;5.29,-29.75,;9.36,-31.92,;10.67,-31.11,;10.62,-29.57,;12.03,-31.83,;13.33,-31.02,;13.29,-29.48,;14.51,-28.54,;14,-27.09,;12.46,-27.13,;12.02,-28.61,;12.08,-33.37,;13.44,-34.1,;14.74,-33.28,;8.54,-37.11,;10.04,-36.73,;10.8,-37.54,;10.65,-35.47,;2.93,-39.07,;1.57,-38.34,;2.98,-40.61,)| Show InChI InChI=1S/C50H67N15O9/c1-3-4-15-36(58-28(2)66)44(69)62-39-23-42(67)55-18-11-10-17-35(43(51)68)60-45(70)37(20-30-24-56-34-16-9-8-14-33(30)34)63-48(73)41-22-32(59-50(52)53)26-65(41)49(74)40(19-29-12-6-5-7-13-29)64-46(71)38(61-47(39)72)21-31-25-54-27-57-31/h5-9,12-14,16,24-25,27,32,35-41,56H,3-4,10-11,15,17-23,26H2,1-2H3,(H2,51,68)(H,54,57)(H,55,67)(H,58,66)(H,60,70)(H,61,72)(H,62,69)(H,63,73)(H,64,71)(H4,52,53,59)/t32-,35-,36-,37-,38-,39-,40+,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50268798
(Ac-Nle-c[Asp-His-DNaI(2')-Pro-Trp-Lys]-NH2 | CHEMB...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C53H66N12O9/c1-3-4-15-40(59-31(2)66)48(69)62-43-27-46(67)56-21-10-9-17-39(47(54)68)60-49(70)41(25-35-28-57-38-16-8-7-14-37(35)38)63-52(73)45-18-11-22-65(45)53(74)44(24-32-19-20-33-12-5-6-13-34(33)23-32)64-50(71)42(61-51(43)72)26-36-29-55-30-58-36/h5-8,12-14,16,19-20,23,28-30,39-45,57H,3-4,9-11,15,17-18,21-22,24-27H2,1-2H3,(H2,54,68)(H,55,58)(H,56,67)(H,59,66)(H,60,70)(H,61,72)(H,62,69)(H,63,73)(H,64,71)/t39-,40-,41-,42-,43-,44+,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50268800
(Ac-Nle-c[Asp-His-DNal(2')-cis-4-guanidinyl-Pro-Trp...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2C[C@H](CN2C(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)N=C(N)N)C(N)=O |r,wU:46.49,21.81,12.11,4.4,39.45,wD:61.66,25.25,41.77,(17.08,-28.2,;15.72,-27.49,;15.65,-25.95,;14.29,-25.23,;14.22,-23.7,;15.52,-22.87,;15.46,-21.33,;16.76,-20.51,;14.09,-20.62,;12.85,-22.98,;12.79,-21.44,;11.56,-23.81,;10.19,-23.09,;8.89,-23.93,;8.95,-25.47,;10.32,-26.17,;7.66,-26.29,;6.29,-25.58,;4.99,-26.4,;3.62,-25.69,;2.33,-26.52,;.96,-25.8,;.9,-24.26,;-.47,-23.55,;-1.77,-24.38,;-.53,-22.01,;-1.9,-21.3,;-1.97,-19.76,;-.75,-18.81,;-1.29,-17.36,;-2.83,-17.43,;-3.91,-16.34,;-5.39,-16.73,;-5.79,-18.22,;-4.71,-19.3,;-3.24,-18.91,;.77,-21.19,;1.43,-22.26,;1.77,-23.81,;2.13,-21.9,;2.76,-23.47,;4.3,-23.41,;4.72,-21.93,;3.43,-21.07,;3.37,-19.54,;2,-18.82,;4.67,-18.71,;4.6,-17.17,;3.24,-16.46,;1.94,-17.29,;.57,-16.58,;.5,-15.04,;-.85,-14.32,;-.92,-12.79,;.39,-11.96,;1.75,-12.68,;1.81,-14.22,;3.17,-14.92,;6.03,-19.42,;7.33,-18.6,;7.26,-17.05,;8.7,-19.3,;10,-18.48,;9.94,-16.94,;11.15,-15.99,;10.63,-14.55,;9.09,-14.6,;8.66,-16.08,;8.76,-20.84,;10.13,-21.56,;11.43,-20.73,;5.25,-24.61,;6.76,-24.38,;7.52,-24.99,;7.32,-22.97,;-.34,-26.63,;-1.71,-25.91,;-.27,-28.17,)| Show InChI InChI=1S/C54H69N15O9/c1-3-4-14-40(62-30(2)70)48(73)66-43-25-46(71)59-19-10-9-16-39(47(55)72)64-49(74)41(22-34-26-60-38-15-8-7-13-37(34)38)67-52(77)45-24-36(63-54(56)57)28-69(45)53(78)44(21-31-17-18-32-11-5-6-12-33(32)20-31)68-50(75)42(65-51(43)76)23-35-27-58-29-61-35/h5-8,11-13,15,17-18,20,26-27,29,36,39-45,60H,3-4,9-10,14,16,19,21-25,28H2,1-2H3,(H2,55,72)(H,58,61)(H,59,71)(H,62,70)(H,64,74)(H,65,76)(H,66,73)(H,67,77)(H,68,75)(H4,56,57,63)/t36-,39+,40+,41+,42+,43+,44-,45+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50268802
(Ac-Nle-c[Asp-cis-4-guanidinyl-Pro-DNal(2')-Arg-Trp...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H]2C[C@@H](CN2C1=O)N=C(N)N)C(N)=O |r,wU:4.4,12.11,21.81,50.52,wD:25.25,39.41,65.73,67.77,(39.57,-47.74,;38.21,-47.02,;38.16,-45.48,;36.8,-44.75,;36.75,-43.21,;38.06,-42.4,;38,-40.86,;39.31,-40.04,;36.65,-40.13,;35.39,-42.49,;35.34,-40.95,;34.08,-43.3,;32.72,-42.58,;31.42,-43.39,;31.47,-44.93,;32.83,-45.65,;30.16,-45.74,;28.8,-45.02,;27.49,-45.83,;26.13,-45.1,;24.82,-45.91,;23.47,-45.19,;23.42,-43.65,;22.06,-42.92,;20.75,-43.74,;22.01,-41.38,;20.63,-40.65,;19.18,-41.16,;17.92,-40.26,;16.69,-41.18,;17.19,-42.65,;16.46,-44,;17.25,-45.3,;18.8,-45.26,;19.52,-43.91,;18.73,-42.62,;23.31,-40.57,;24.63,-40.8,;25.96,-41.59,;23.26,-39.03,;21.9,-38.31,;21.85,-36.77,;20.5,-36.04,;20.44,-34.5,;19.08,-33.78,;19.04,-32.24,;17.77,-34.59,;24.57,-38.22,;25.93,-38.94,;25.98,-40.48,;27.24,-38.13,;27.19,-36.59,;25.83,-35.86,;24.52,-36.68,;23.17,-35.96,;23.11,-34.42,;21.76,-33.69,;21.71,-32.15,;23.02,-31.33,;24.38,-32.07,;24.43,-33.61,;25.78,-34.33,;28.59,-38.85,;29.91,-38.05,;29.86,-36.5,;31.27,-38.78,;32.35,-37.67,;33.85,-38.07,;33.84,-39.61,;31.31,-40.3,;32.67,-41.04,;33.98,-40.22,;35.07,-37.14,;34.87,-35.61,;33.45,-35.01,;36.1,-34.67,;22.16,-46,;20.8,-45.28,;22.21,-47.54,)| Show InChI InChI=1S/C54H74N16O9/c1-3-4-15-39(63-30(2)71)47(74)69-43-27-45(72)60-21-10-9-17-38(46(55)73)65-50(77)42(25-34-28-62-37-16-8-7-14-36(34)37)67-48(75)40(18-11-22-61-53(56)57)66-49(76)41(24-31-19-20-32-12-5-6-13-33(32)23-31)68-51(78)44-26-35(64-54(58)59)29-70(44)52(43)79/h5-8,12-14,16,19-20,23,28,35,38-44,62H,3-4,9-11,15,17-18,21-22,24-27,29H2,1-2H3,(H2,55,73)(H,60,72)(H,63,71)(H,65,77)(H,66,76)(H,67,75)(H,68,78)(H,69,74)(H4,56,57,61)(H4,58,59,64)/t35-,38-,39-,40-,41+,42-,43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50268803
(Ac-Tyr-Val-Nle-Gly-His-DPhe-cis-Xaa-Trp-Asp-Arg-Ph...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1C[C@H](C[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O)N=C(N)N |r,wU:8.8,45.46,59.63,57.117,96.101,35.35,77.82,wD:4.4,12.21,63.66,85.90,(2.53,-50.05,;1.21,-50.83,;-.13,-50.07,;-1.45,-50.85,;-2.8,-50.09,;-4.13,-50.88,;-4.11,-52.42,;-2.77,-53.17,;-5.44,-53.2,;-5.43,-54.75,;-6.75,-55.53,;-8.09,-54.77,;-6.74,-57.06,;-5.4,-57.82,;-4.07,-57.04,;-4.09,-55.5,;-2.76,-54.71,;-1.41,-55.47,;-.08,-54.68,;-1.39,-57.02,;-2.73,-57.8,;-8.07,-57.85,;-8.06,-59.39,;-9.38,-60.18,;-6.71,-60.14,;-6.78,-52.45,;-6.8,-50.91,;-8.1,-53.23,;-2.82,-48.56,;-4.16,-47.8,;-1.49,-47.77,;-1.51,-46.23,;-.18,-45.44,;1.17,-46.2,;-.2,-43.9,;1.13,-43.12,;1.11,-41.58,;-.23,-40.83,;-.4,-39.3,;-1.91,-38.99,;-2.66,-40.33,;-1.63,-41.47,;2.47,-43.88,;2.49,-45.42,;3.8,-43.1,;5.14,-43.85,;5.16,-45.4,;6.5,-46.15,;6.51,-47.7,;7.85,-48.46,;9.17,-47.68,;9.16,-46.14,;7.81,-45.37,;6.5,-43.14,;7.76,-44.04,;6.57,-41.61,;5.55,-40.45,;6.33,-39.12,;7.84,-39.46,;7.99,-40.99,;9.31,-41.78,;9.3,-43.32,;10.65,-41.02,;11.97,-41.82,;11.95,-43.36,;13.23,-44.22,;14.68,-43.68,;15.63,-44.89,;14.78,-46.18,;15.16,-47.67,;14.06,-48.75,;12.58,-48.33,;12.19,-46.84,;13.3,-45.76,;13.31,-41.06,;13.32,-39.52,;14.64,-41.85,;15.98,-41.1,;15.99,-39.56,;17.33,-38.8,;18.67,-39.57,;17.35,-37.26,;17.3,-41.87,;17.29,-43.42,;18.65,-41.12,;19.96,-41.91,;19.94,-43.46,;21.27,-44.24,;21.26,-45.78,;22.59,-46.55,;22.56,-48.09,;21.24,-48.84,;23.9,-48.86,;21.3,-41.16,;21.31,-39.62,;22.64,-41.93,;23.98,-41.17,;23.99,-39.64,;25.33,-38.88,;26.66,-39.66,;28.01,-38.91,;28.02,-37.37,;26.7,-36.58,;25.36,-37.34,;25.31,-41.95,;25.29,-43.5,;26.65,-41.2,;27.97,-41.99,;29.31,-41.23,;30.64,-42.01,;29.32,-39.69,;5.72,-37.71,;6.64,-36.47,;6.03,-35.06,;8.17,-36.65,)| Show InChI InChI=1S/C77H102N22O16/c1-5-6-21-53(93-74(114)65(42(2)3)98-72(112)56(89-43(4)100)30-46-24-26-50(101)27-25-46)66(106)87-39-63(103)91-58(33-48-37-83-41-88-48)70(110)97-60(31-45-18-11-8-12-19-45)75(115)99-40-49(90-77(81)82)34-61(99)73(113)96-57(32-47-36-85-52-22-14-13-20-51(47)52)69(109)95-59(35-64(104)105)71(111)92-54(23-15-28-84-76(79)80)68(108)94-55(67(107)86-38-62(78)102)29-44-16-9-7-10-17-44/h7-14,16-20,22,24-27,36-37,41-42,49,53-61,65,85,101H,5-6,15,21,23,28-35,38-40H2,1-4H3,(H2,78,102)(H,83,88)(H,86,107)(H,87,106)(H,89,100)(H,91,103)(H,92,111)(H,93,114)(H,94,108)(H,95,109)(H,96,113)(H,97,110)(H,98,112)(H,104,105)(H4,79,80,84)(H4,81,82,90)/t49-,53-,54-,55-,56-,57-,58-,59-,60+,61-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50268793
(Ac-Nle-c[Asp-His-DPhe-trans-4-guanidinyl-Pro-Trp-L...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2C[C@@H](CN2C(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)N=C(N)N)C(N)=O |r,wU:46.49,21.76,12.11,41.72,4.4,39.45,wD:57.61,25.25,(17.82,-24,;16.46,-23.28,;16.41,-21.74,;15.05,-21.01,;15,-19.47,;16.31,-18.66,;16.26,-17.12,;17.56,-16.31,;14.9,-16.39,;13.64,-18.75,;13.59,-17.21,;12.33,-19.56,;10.98,-18.84,;9.67,-19.65,;9.72,-21.19,;11.08,-21.91,;8.41,-22,;7.05,-21.28,;5.75,-22.09,;4.39,-21.37,;3.08,-22.18,;1.72,-21.45,;1.67,-19.91,;.31,-19.19,;-1,-20,;.26,-17.65,;-1.1,-16.92,;-1.15,-15.39,;.08,-14.44,;-.45,-12.99,;-2,-13.05,;-3.06,-11.95,;-4.55,-12.33,;-4.96,-13.81,;-3.88,-14.9,;-2.41,-14.52,;1.57,-16.84,;2.22,-17.92,;2.54,-19.47,;2.93,-17.56,;3.55,-19.14,;5.08,-19.09,;5.51,-17.61,;4.23,-16.75,;4.18,-15.21,;2.82,-14.48,;5.49,-14.4,;5.44,-12.86,;4.08,-12.13,;4.03,-10.59,;2.68,-9.87,;1.37,-10.68,;1.42,-12.23,;2.78,-12.95,;6.85,-15.12,;8.16,-14.31,;8.11,-12.77,;9.51,-15.03,;10.82,-14.22,;10.78,-12.68,;12,-11.74,;11.49,-10.29,;9.95,-10.33,;9.51,-11.81,;9.57,-16.57,;10.92,-17.3,;12.23,-16.48,;6.03,-20.31,;7.52,-19.93,;8.28,-20.74,;8.13,-18.67,;.41,-22.27,;-.95,-21.54,;.46,-23.81,)| Show InChI InChI=1S/C50H67N15O9/c1-3-4-15-36(58-28(2)66)44(69)62-39-23-42(67)55-18-11-10-17-35(43(51)68)60-45(70)37(20-30-24-56-34-16-9-8-14-33(30)34)63-48(73)41-22-32(59-50(52)53)26-65(41)49(74)40(19-29-12-6-5-7-13-29)64-46(71)38(61-47(39)72)21-31-25-54-27-57-31/h5-9,12-14,16,24-25,27,32,35-41,56H,3-4,10-11,15,17-23,26H2,1-2H3,(H2,51,68)(H,54,57)(H,55,67)(H,58,66)(H,60,70)(H,61,72)(H,62,69)(H,63,73)(H,64,71)(H4,52,53,59)/t32-,35-,36-,37-,38-,39-,40+,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50268804
(Ac-Tyr-Val-Nle-Gly-His-DPhe-Pro-Trp-Asp-Arg-Phe-Gl...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |r,wU:8.8,45.46,59.63,96.101,35.35,77.82,wD:4.4,12.21,63.66,85.90,(2.68,-6.73,;1.36,-7.52,;.01,-6.75,;-1.31,-7.53,;-2.65,-6.78,;-3.99,-7.57,;-3.97,-9.11,;-2.62,-9.86,;-5.3,-9.89,;-5.28,-11.43,;-6.61,-12.22,;-7.94,-11.46,;-6.59,-13.75,;-5.25,-14.51,;-3.92,-13.72,;-3.94,-12.19,;-2.61,-11.4,;-1.26,-12.16,;.06,-11.37,;-1.25,-13.71,;-2.58,-14.49,;-7.92,-14.54,;-7.92,-16.08,;-9.23,-16.86,;-6.56,-16.83,;-6.64,-9.14,;-6.65,-7.59,;-7.95,-9.92,;-2.67,-5.24,;-4.01,-4.49,;-1.35,-4.46,;-1.37,-2.92,;-.03,-2.13,;1.32,-2.89,;-.05,-.59,;1.27,.2,;1.26,1.73,;-.08,2.49,;-.25,4.02,;-1.77,4.33,;-2.52,2.99,;-1.48,1.85,;2.62,-.57,;2.64,-2.11,;3.94,.22,;5.29,-.54,;5.31,-2.09,;6.64,-2.84,;6.66,-4.39,;8,-5.15,;9.32,-4.36,;9.3,-2.82,;7.96,-2.06,;6.65,.17,;7.9,-.73,;6.72,1.71,;5.7,2.87,;6.48,4.19,;7.98,3.86,;8.13,2.32,;9.45,1.54,;9.44,-0,;10.8,2.29,;12.11,1.49,;12.1,-.05,;13.38,-.9,;14.82,-.37,;15.78,-1.58,;14.93,-2.86,;15.31,-4.35,;14.21,-5.43,;12.73,-5.02,;12.34,-3.52,;13.44,-2.45,;13.46,2.25,;13.47,3.79,;14.78,1.46,;16.13,2.22,;16.14,3.76,;17.48,4.52,;18.82,3.75,;17.5,6.06,;17.45,1.44,;17.43,-.1,;18.79,2.2,;20.11,1.4,;20.09,-.14,;21.42,-.92,;21.41,-2.46,;22.74,-3.24,;22.71,-4.78,;21.38,-5.53,;24.05,-5.55,;21.45,2.16,;21.46,3.7,;22.79,1.39,;24.13,2.15,;24.14,3.68,;25.48,4.44,;26.81,3.66,;28.16,4.41,;28.17,5.95,;26.84,6.74,;25.51,5.98,;25.46,1.36,;25.44,-.18,;26.8,2.12,;28.12,1.33,;29.46,2.09,;30.79,1.3,;29.47,3.63,)| Show InChI InChI=1S/C76H99N19O16/c1-5-6-22-53(89-74(110)65(43(2)3)94-72(108)56(86-44(4)96)33-47-26-28-50(97)29-27-47)66(102)84-41-63(99)87-58(36-49-39-80-42-85-49)70(106)93-60(34-46-19-11-8-12-20-46)75(111)95-31-16-25-61(95)73(109)92-57(35-48-38-82-52-23-14-13-21-51(48)52)69(105)91-59(37-64(100)101)71(107)88-54(24-15-30-81-76(78)79)68(104)90-55(67(103)83-40-62(77)98)32-45-17-9-7-10-18-45/h7-14,17-21,23,26-29,38-39,42-43,53-61,65,82,97H,5-6,15-16,22,24-25,30-37,40-41H2,1-4H3,(H2,77,98)(H,80,85)(H,83,103)(H,84,102)(H,86,96)(H,87,99)(H,88,107)(H,89,110)(H,90,104)(H,91,105)(H,92,109)(H,93,106)(H,94,108)(H,100,101)(H4,78,79,81)/t53-,54-,55-,56-,57-,58-,59-,60+,61-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590039
(CHEMBL5185775)Show SMILES [H][C@]12CSC\C(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)=N\OCC(=O)NCCOCCOCCOCCN=[N+]=[N-] |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.105 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590040
(CHEMBL5185945)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCOCCOCCN)C2CCc31 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.107 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Effective concentration required for the biological activity against human Melanocortin 3 receptor |
J Med Chem 46: 4215-31 (2003)
Article DOI: 10.1021/jm0303103
BindingDB Entry DOI: 10.7270/Q2JW8FM1 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM82411
(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.132 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Evaluated for agonist activity at cloned Melanocortin 3 receptor |
J Med Chem 38: 3454-61 (1995)
BindingDB Entry DOI: 10.7270/Q2Z32094 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50581319

(BIM-22493 | RM-493 | SETMELANOTIDE | Setmelanotide)Show SMILES C[C@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.151 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human melanocortin receptor 3 expressed in human T-REx-293 cells assessed as stimulation of intracellular cAMP accumulation incub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50581319

(BIM-22493 | RM-493 | SETMELANOTIDE | Setmelanotide)Show SMILES C[C@H]1NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.151 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590038
(CHEMBL5172738)Show SMILES [H][C@]12CSC3=C(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)C(=O)N(CCCCC#C)C3=O |r,c:4| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.158 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590037
(CHEMBL5191309)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)nn3 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.182 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50033133

((3R,6S,9R,12S,15S,23R)-15-((S)-2-Acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc(F)cc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O Show InChI InChI=1S/C50H68FN15O9/c1-3-4-11-36(60-28(2)67)44(70)66-41-24-42(68)56-19-8-7-13-35(43(52)69)61-47(73)39(22-30-25-58-34-12-6-5-10-33(30)34)64-45(71)37(14-9-20-57-50(53)54)62-46(72)38(21-29-15-17-31(51)18-16-29)63-48(74)40(65-49(41)75)23-32-26-55-27-59-32/h5-6,10,12,15-18,25-27,35-41,58H,3-4,7-9,11,13-14,19-24H2,1-2H3,(H2,52,69)(H,55,59)(H,56,68)(H,60,67)(H,61,73)(H,62,72)(H,63,74)(H,64,71)(H,65,75)(H,66,70)(H4,53,54,57)/t35-,36+,37+,38-,39-,40+,41+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.191 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Evaluated for agonist activity at cloned Melanocortin 3 receptor |
J Med Chem 38: 3454-61 (1995)
BindingDB Entry DOI: 10.7270/Q2Z32094 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.204 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in low doxycyclin-treated HEK293 cell membranes assessed as increase in cAMP production after 45 mins by HTR... |
J Med Chem 61: 3674-3684 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00170
BindingDB Entry DOI: 10.7270/Q2736TCZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590028
(CHEMBL5195641)Show SMILES [H][C@]12CSSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.229 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Effective concentration required for intracellular cAMP accumulation against Melanocortin 3 receptor |
J Med Chem 43: 4998-5002 (2001)
BindingDB Entry DOI: 10.7270/Q2P55P7V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Effective concentration that was able to generate 50% maximal intracellular cAMP in L-cells transfected with Melanocortin 3 receptor |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Effective concentration required for the biological activity against human Melanocortin 3 receptor |
J Med Chem 46: 4215-31 (2003)
Article DOI: 10.1021/jm0303103
BindingDB Entry DOI: 10.7270/Q2JW8FM1 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590032
(CHEMBL5178164)Show SMILES [H][C@]12CSc3c(F)c(F)c(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c(F)c3F |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.275 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Departments of Medicinal Chemistry and Pharmacodynamics , University of Florida , Gainesville , Florida 32610 , United States.
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as increase in cAMP production incubated for 2 hrs by AlphaScreen assay |
J Med Chem 61: 3738-3744 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00251
BindingDB Entry DOI: 10.7270/Q2CV4M69 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590033
(CHEMBL5170533)Show SMILES [H][C@]12CSCCCSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.302 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590030
(CHEMBL5175487)Show SMILES [H][C@]12CSCc3cccc(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c3 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.309 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50163143
(CHEMBL405282 | H-Tyr-Val-Met-Gly-His-Phe-Arg-D-Trp...)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(O)=O Show InChI InChI=1S/C74H99N21O16S/c1-41(2)62(95-63(102)49(75)30-44-22-24-47(96)25-23-44)72(111)90-53(26-29-112-3)64(103)84-38-59(97)87-57(34-46-37-80-40-86-46)70(109)92-55(32-43-16-8-5-9-17-43)68(107)88-52(21-13-28-82-74(78)79)67(106)93-56(33-45-36-83-50-19-11-10-18-48(45)50)69(108)94-58(35-60(98)99)71(110)89-51(20-12-27-81-73(76)77)66(105)91-54(65(104)85-39-61(100)101)31-42-14-6-4-7-15-42/h4-11,14-19,22-25,36-37,40-41,49,51-58,62,83,96H,12-13,20-21,26-35,38-39,75H2,1-3H3,(H,80,86)(H,84,103)(H,85,104)(H,87,97)(H,88,107)(H,89,110)(H,90,111)(H,91,105)(H,92,109)(H,93,106)(H,94,108)(H,95,102)(H,98,99)(H,100,101)(H4,76,77,81)(H4,78,79,82)/t49-,51-,52-,53-,54-,55-,56+,57-,58-,62-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Effective concentration for intracellular cAMP accumulation in human melanocortin 3 receptor expressing HEK 293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50026884
(CHEMBL405282)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(O)=O Show InChI InChI=1S/C74H99N21O16S/c1-41(2)62(95-63(102)49(75)30-44-22-24-47(96)25-23-44)72(111)90-53(26-29-112-3)64(103)84-38-59(97)87-57(34-46-37-80-40-86-46)70(109)92-55(32-43-16-8-5-9-17-43)68(107)88-52(21-13-28-82-74(78)79)67(106)93-56(33-45-36-83-50-19-11-10-18-48(45)50)69(108)94-58(35-60(98)99)71(110)89-51(20-12-27-81-73(76)77)66(105)91-54(65(104)85-39-61(100)101)31-42-14-6-4-7-15-42/h4-11,14-19,22-25,36-37,40-41,49,51-58,62,83,96H,12-13,20-21,26-35,38-39,75H2,1-3H3,(H,80,86)(H,84,103)(H,85,104)(H,87,97)(H,88,107)(H,89,110)(H,90,111)(H,91,105)(H,92,109)(H,93,106)(H,94,108)(H,95,102)(H,98,99)(H,100,101)(H4,76,77,81)(H4,78,79,82)/t49-,51-,52-,53-,54-,55-,56+,57-,58-,62-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Effective concentration required for intracellular cAMP accumulation against Melanocortin 3 receptor |
J Med Chem 43: 4998-5002 (2001)
BindingDB Entry DOI: 10.7270/Q2P55P7V |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590031
(CHEMBL5203986)Show SMILES [H][C@]12CSCc3ccc(CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)cc3 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.331 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590029
(CHEMBL5192329)Show SMILES [H][C@]12CSCc3ccccc3CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.347 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM82411

(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
In vitro agonist potency was evaluated in HEK293 cells transfected with human melanocortin receptor (hMC3R) |
Bioorg Med Chem Lett 13: 2647-50 (2003)
BindingDB Entry DOI: 10.7270/Q2474BD9 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50152271
(Ac-Ser-Tyr-Ser-Nle-Glu-His6-D-Phe7-Arg8-Trp9-Gly-L...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C78H111N21O19/c1-6-43(4)65(98-74(115)60(40-101)96-71(112)56(33-46-23-25-49(103)26-24-46)93-73(114)59(39-100)88-44(5)102)76(117)91-53(27-28-63(105)106)69(110)95-58(35-48-37-83-41-87-48)72(113)92-55(32-45-16-8-7-9-17-45)70(111)90-52(21-14-30-84-78(81)82)68(109)94-57(34-47-36-85-51-19-11-10-18-50(47)51)67(108)86-38-62(104)89-54(20-12-13-29-79)77(118)99-31-15-22-61(99)75(116)97-64(42(2)3)66(80)107/h7-11,16-19,23-26,36-37,41-43,52-61,64-65,85,100-101,103H,6,12-15,20-22,27-35,38-40,79H2,1-5H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,111)(H,91,117)(H,92,113)(H,93,114)(H,94,109)(H,95,110)(H,96,112)(H,97,116)(H,98,115)(H,105,106)(H4,81,82,84)/t43-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
University of Cincinnati
Curated by ChEMBL
| Assay Description
Agonistic activity against against human Melanocortin 3 receptor |
Bioorg Med Chem Lett 14: 4839-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.046
BindingDB Entry DOI: 10.7270/Q2445KX8 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590035
(CHEMBL5185405)Show SMILES [H][C@]12CSC3=C(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)C(=O)NC3=O |r,c:4| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50189013
((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCN=C(N)N)C1=O)C(=O)[C@@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(C)=O |wU:20.22,4.17,32.43,43.54,(1.84,-9.34,;3.18,-8.56,;4.51,-9.34,;4.51,-10.88,;5.85,-8.58,;7.18,-9.35,;7.18,-10.89,;5.84,-11.66,;5.83,-13.19,;7.17,-13.96,;7.17,-15.49,;8.51,-16.27,;9.84,-15.49,;9.83,-13.95,;8.51,-13.19,;8.51,-11.65,;5.85,-7.03,;4.52,-6.26,;4.52,-4.73,;5.86,-3.95,;7.19,-4.72,;8.53,-3.94,;9.85,-4.71,;11.19,-3.94,;12.52,-4.71,;13.86,-3.94,;15.2,-4.7,;13.86,-2.4,;7.19,-6.27,;8.52,-7.04,;5.85,-2.41,;7.19,-1.65,;4.52,-1.65,;3.18,-2.41,;1.84,-1.66,;.51,-2.41,;-.83,-1.66,;-.82,-.11,;.5,.67,;1.84,-.11,;4.52,-.11,;5.85,.66,;7.2,-.11,;5.85,2.21,;7.2,2.98,;8.54,2.21,;8.7,.67,;10.21,.35,;10.99,1.69,;9.95,2.84,;4.52,2.98,;4.51,4.52,;3.18,5.29,;5.85,5.29,)| Show InChI InChI=1S/C39H48N10O5/c1-25(50)46-31(22-30-23-43-24-45-30)35(51)47-32(20-26-9-4-3-5-10-26)37(53)48-17-18-49(38(54)33(48)13-8-16-44-39(40)41)34(36(52)42-2)21-27-14-15-28-11-6-7-12-29(28)19-27/h3-7,9-12,14-15,19,23-24,31-34H,8,13,16-18,20-22H2,1-2H3,(H,42,52)(H,43,45)(H,46,50)(H,47,51)(H4,40,41,44)/t31-,32+,33-,34-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50189025

((S)-2-acetamido-N1-((R)-3-(4-fluorophenyl)-1-((S)-...)Show SMILES CNC(=O)[C@H](Cc1ccc2ccccc2c1)N1CCN([C@@H](CCCNC(N)=N)C1=O)C(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@H](CCC(N)=O)NC(C)=O Show InChI InChI=1S/C38H48FN9O6/c1-23(49)45-29(15-16-33(40)50)34(51)46-30(21-24-10-13-28(39)14-11-24)36(53)47-18-19-48(37(54)31(47)8-5-17-44-38(41)42)32(35(52)43-2)22-25-9-12-26-6-3-4-7-27(26)20-25/h3-4,6-7,9-14,20,29-32H,5,8,15-19,21-22H2,1-2H3,(H2,40,50)(H,43,52)(H,45,49)(H,46,51)(H4,41,42,44)/t29-,30+,31-,32-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R |
Bioorg Med Chem Lett 16: 4668-73 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.087
BindingDB Entry DOI: 10.7270/Q2W66KDF |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590041
(CHEMBL5207936)Show SMILES [H][C@]12CSc3nnc(SC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc4c[nH]c5ccccc45)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc4ccccc4)NC(=O)[C@H](Cc4cnc[nH]4)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c4ccccc14)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2)c1CCC2C(COC(=O)NCCOCCOCCOCCOCCC(O)=O)C2CCc31 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50029747
((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.669 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Evaluated for agonist activity at cloned Melanocortin 3 receptor |
J Med Chem 38: 3454-61 (1995)
BindingDB Entry DOI: 10.7270/Q2Z32094 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50029747

((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Effective concentration required for the biological activity against human Melanocortin 3 receptor |
J Med Chem 46: 4215-31 (2003)
Article DOI: 10.1021/jm0303103
BindingDB Entry DOI: 10.7270/Q2JW8FM1 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50389769
(BREMELANOTIDE)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(O)=O |r,wU:61.64,50.52,39.41,25.25,4.4,wD:12.11,21.75,(-11.27,-.98,;-11.98,-2.34,;-11.15,-3.64,;-9.61,-3.57,;-8.78,-4.87,;-9.49,-6.24,;-11.03,-6.31,;-11.73,-7.67,;-11.86,-5.01,;-7.24,-4.8,;-6.41,-6.1,;-6.53,-3.43,;-4.99,-3.36,;-4.15,-4.65,;-4.16,-6.3,;-5.38,-7.46,;9.13,-7.95,;9.83,-6.57,;8.99,-5.29,;9.68,-3.91,;8.85,-2.63,;9.54,-1.25,;8.7,.04,;7.17,-.04,;6.47,-1.42,;6.33,1.25,;7.03,2.62,;8.56,2.7,;9.53,1.55,;10.96,2.1,;10.89,3.65,;11.98,4.73,;11.57,6.22,;10.08,6.61,;9,5.52,;9.4,4.04,;4.79,1.16,;3.95,2.46,;4.65,3.83,;2.41,2.38,;1.57,3.67,;2.27,5.04,;3.81,5.12,;4.51,6.49,;6.04,6.57,;6.88,5.28,;6.74,7.95,;1.71,1,;.18,.92,;-.66,2.21,;-.52,-.45,;.32,-1.74,;1.86,-1.66,;2.69,-2.95,;4.24,-2.86,;4.93,-1.49,;4.09,-.2,;2.55,-.28,;-2.06,-.53,;-2.9,.76,;-2.2,2.13,;-4.44,.67,;-5.28,1.97,;-4.58,3.34,;-5.29,4.71,;-4.2,5.81,;-2.82,5.1,;-3.06,3.57,;-5.14,-.69,;-4.3,-1.99,;-2.76,-1.9,;11.08,-1.17,;11.92,-2.46,;11.78,.2,)| Show InChI InChI=1S/C50H68N14O10/c1-3-4-16-35(58-29(2)65)43(67)64-41-25-42(66)54-20-11-10-18-37(49(73)74)60-46(70)39(23-31-26-56-34-17-9-8-15-33(31)34)62-44(68)36(19-12-21-55-50(51)52)59-45(69)38(22-30-13-6-5-7-14-30)61-47(71)40(63-48(41)72)24-32-27-53-28-57-32/h5-9,13-15,17,26-28,35-41,56H,3-4,10-12,16,18-25H2,1-2H3,(H,53,57)(H,54,66)(H,58,65)(H,59,69)(H,60,70)(H,61,71)(H,62,68)(H,63,72)(H,64,67)(H,73,74)(H4,51,52,55)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.676 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human melanocortin receptor 3 expressed in human T-REx-293 cells assessed as stimulation of intracellular cAMP accumulation incub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00095
BindingDB Entry DOI: 10.7270/Q2N301TQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50389769

(BREMELANOTIDE)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(O)=O |r,wU:61.64,50.52,39.41,25.25,4.4,wD:12.11,21.75,(-11.27,-.98,;-11.98,-2.34,;-11.15,-3.64,;-9.61,-3.57,;-8.78,-4.87,;-9.49,-6.24,;-11.03,-6.31,;-11.73,-7.67,;-11.86,-5.01,;-7.24,-4.8,;-6.41,-6.1,;-6.53,-3.43,;-4.99,-3.36,;-4.15,-4.65,;-4.16,-6.3,;-5.38,-7.46,;9.13,-7.95,;9.83,-6.57,;8.99,-5.29,;9.68,-3.91,;8.85,-2.63,;9.54,-1.25,;8.7,.04,;7.17,-.04,;6.47,-1.42,;6.33,1.25,;7.03,2.62,;8.56,2.7,;9.53,1.55,;10.96,2.1,;10.89,3.65,;11.98,4.73,;11.57,6.22,;10.08,6.61,;9,5.52,;9.4,4.04,;4.79,1.16,;3.95,2.46,;4.65,3.83,;2.41,2.38,;1.57,3.67,;2.27,5.04,;3.81,5.12,;4.51,6.49,;6.04,6.57,;6.88,5.28,;6.74,7.95,;1.71,1,;.18,.92,;-.66,2.21,;-.52,-.45,;.32,-1.74,;1.86,-1.66,;2.69,-2.95,;4.24,-2.86,;4.93,-1.49,;4.09,-.2,;2.55,-.28,;-2.06,-.53,;-2.9,.76,;-2.2,2.13,;-4.44,.67,;-5.28,1.97,;-4.58,3.34,;-5.29,4.71,;-4.2,5.81,;-2.82,5.1,;-3.06,3.57,;-5.14,-.69,;-4.3,-1.99,;-2.76,-1.9,;11.08,-1.17,;11.92,-2.46,;11.78,.2,)| Show InChI InChI=1S/C50H68N14O10/c1-3-4-16-35(58-29(2)65)43(67)64-41-25-42(66)54-20-11-10-18-37(49(73)74)60-46(70)39(23-31-26-56-34-17-9-8-15-33(31)34)62-44(68)36(19-12-21-55-50(51)52)59-45(69)38(22-30-13-6-5-7-14-30)61-47(71)40(63-48(41)72)24-32-27-53-28-57-32/h5-9,13-15,17,26-28,35-41,56H,3-4,10-12,16,18-25H2,1-2H3,(H,53,57)(H,54,66)(H,58,65)(H,59,69)(H,60,70)(H,61,71)(H,62,68)(H,63,72)(H,64,67)(H,73,74)(H4,51,52,55)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.676 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50165941

(Ac-R[CEHdFRWC]-NH2 | CHEMBL408257)Show SMILES CC(=O)N[C@H](CCCNC(N)=N)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@H](CCC(O)=O)NC1=O)C(N)=O Show InChI InChI=1S/C51H70N18O11S2/c1-27(70)62-33(13-7-17-58-50(53)54)43(74)69-40-25-82-81-24-39(42(52)73)68-47(78)37(20-29-22-60-32-12-6-5-11-31(29)32)66-44(75)34(14-8-18-59-51(55)56)63-46(77)36(19-28-9-3-2-4-10-28)65-48(79)38(21-30-23-57-26-61-30)67-45(76)35(64-49(40)80)15-16-41(71)72/h2-6,9-12,22-23,26,33-40,60H,7-8,13-21,24-25H2,1H3,(H2,52,73)(H,57,61)(H,62,70)(H,63,77)(H,64,80)(H,65,79)(H,66,75)(H,67,76)(H,68,78)(H,69,74)(H,71,72)(H4,53,54,58)(H4,55,56,59)/t33-,34-,35+,36-,37+,38-,39+,40+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human Melanocortin-3 receptor as peptide required for 50% maximal cAMP release |
J Med Chem 48: 3095-8 (2005)
Article DOI: 10.1021/jm0501432
BindingDB Entry DOI: 10.7270/Q2251HQZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50590034
(CHEMBL5190042)Show SMILES [H][C@]12CSCC(=O)CSC[C@]([H])(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc3ccccc3)NC(=O)[C@H](Cc3cnc[nH]3)NC(=O)CNC(=O)[C@H](CCCC)NC1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c3ccccc13)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N2 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.692 | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00793
BindingDB Entry DOI: 10.7270/Q20C50R5 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50263819
(CHEMBL4060381)Show SMILES C[C@@H](NC(=O)[C@H](CS)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CS)C(N)=O |r| Show InChI InChI=1S/C49H70N18O9S2/c1-26(60-47(76)39(24-78)67-42(71)33(61-27(2)68)14-8-16-56-48(51)52)41(70)63-37(20-30-22-55-25-59-30)46(75)64-35(18-28-10-4-3-5-11-28)44(73)62-34(15-9-17-57-49(53)54)43(72)65-36(45(74)66-38(23-77)40(50)69)19-29-21-58-32-13-7-6-12-31(29)32/h3-7,10-13,21-22,25-26,33-39,58,77-78H,8-9,14-20,23-24H2,1-2H3,(H2,50,69)(H,55,59)(H,60,76)(H,61,68)(H,62,73)(H,63,70)(H,64,75)(H,65,72)(H,66,74)(H,67,71)(H4,51,52,56)(H4,53,54,57)/t26-,33+,34+,35-,36+,37+,38+,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.692 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in low doxycyclin-treated HEK293 cell membranes assessed as increase in cAMP production after 45 mins by HTR... |
J Med Chem 61: 3674-3684 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00170
BindingDB Entry DOI: 10.7270/Q2736TCZ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50250586
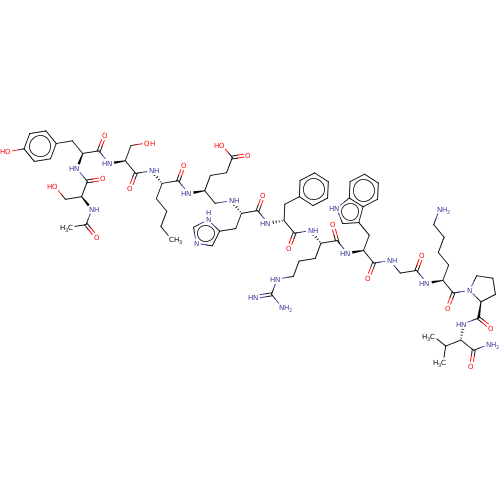
(CHEMBL4093140)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)CN[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H113N21O18/c1-5-6-19-54(93-75(115)62(42-101)97-73(113)59(34-47-24-27-51(103)28-25-47)95-74(114)61(41-100)89-45(4)102)69(109)90-49(26-29-65(105)106)39-86-57(36-50-38-83-43-88-50)71(111)94-58(33-46-16-8-7-9-17-46)72(112)92-55(22-14-31-84-78(81)82)70(110)96-60(35-48-37-85-53-20-11-10-18-52(48)53)68(108)87-40-64(104)91-56(21-12-13-30-79)77(117)99-32-15-23-63(99)76(116)98-66(44(2)3)67(80)107/h7-11,16-18,20,24-25,27-28,37-38,43-44,49,54-63,66,85-86,100-101,103H,5-6,12-15,19,21-23,26,29-36,39-42,79H2,1-4H3,(H2,80,107)(H,83,88)(H,87,108)(H,89,102)(H,90,109)(H,91,104)(H,92,112)(H,93,115)(H,94,111)(H,95,114)(H,96,110)(H,97,113)(H,98,116)(H,105,106)(H4,81,82,84)/t49-,54-,55-,56-,57-,58+,59-,60-,61-,62-,63-,66-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC3R expressed in HEK293 cells assessed as induction of intracellular cAMP accumulation after 3 mins |
J Med Chem 60: 9320-9329 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01295
BindingDB Entry DOI: 10.7270/Q2XG9TJB |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50017181

(CHEMBL441738)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Effective concentration for intracellular cAMP accumulation in human melanocortin 3 receptor expressing HEK 293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data