 Found 695 hits of ic50 data for polymerid = 1064
Found 695 hits of ic50 data for polymerid = 1064 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM9410

(N-[2-(1-benzylpiperidin-4-yl)ethyl]-N-ethyl-4-(phe...)Show SMILES CCN(CCC1CCN(Cc2ccccc2)CC1)C(=O)c1ccc(cc1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C30H36N2O3S/c1-2-32(22-19-25-17-20-31(21-18-25)23-26-9-5-3-6-10-26)30(33)28-13-15-29(16-14-28)36(34,35)24-27-11-7-4-8-12-27/h3-16,25H,2,17-24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... |
J Med Chem 33: 1880-7 (1990)
Article DOI: 10.1021/jm00169a008
BindingDB Entry DOI: 10.7270/Q20Z71H2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM9410

(N-[2-(1-benzylpiperidin-4-yl)ethyl]-N-ethyl-4-(phe...)Show SMILES CCN(CCC1CCN(Cc2ccccc2)CC1)C(=O)c1ccc(cc1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C30H36N2O3S/c1-2-32(22-19-25-17-20-31(21-18-25)23-26-9-5-3-6-10-26)30(33)28-13-15-29(16-14-28)36(34,35)24-27-11-7-4-8-12-27/h3-16,25H,2,17-24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.301 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition against Acetylcholinesterase (AChE) |
J Med Chem 39: 380-7 (1996)
Article DOI: 10.1021/jm950704x
BindingDB Entry DOI: 10.7270/Q25D8T1Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM9409
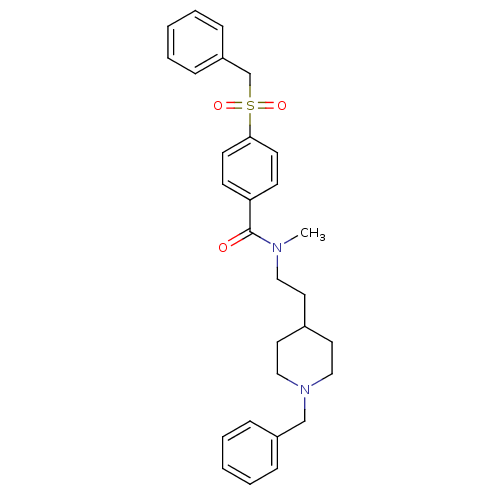
(CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...)Show SMILES CN(CCC1CCN(Cc2ccccc2)CC1)C(=O)c1ccc(cc1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C29H34N2O3S/c1-30(19-16-24-17-20-31(21-18-24)22-25-8-4-2-5-9-25)29(32)27-12-14-28(15-13-27)35(33,34)23-26-10-6-3-7-11-26/h2-15,24H,16-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. |
J Med Chem 35: 4542-8 (1993)
BindingDB Entry DOI: 10.7270/Q25M64P8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM9409

(CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...)Show SMILES CN(CCC1CCN(Cc2ccccc2)CC1)C(=O)c1ccc(cc1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C29H34N2O3S/c1-30(19-16-24-17-20-31(21-18-24)22-25-8-4-2-5-9-25)29(32)27-12-14-28(15-13-27)35(33,34)23-26-10-6-3-7-11-26/h2-15,24H,16-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... |
J Med Chem 33: 1880-7 (1990)
Article DOI: 10.1021/jm00169a008
BindingDB Entry DOI: 10.7270/Q20Z71H2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM9411
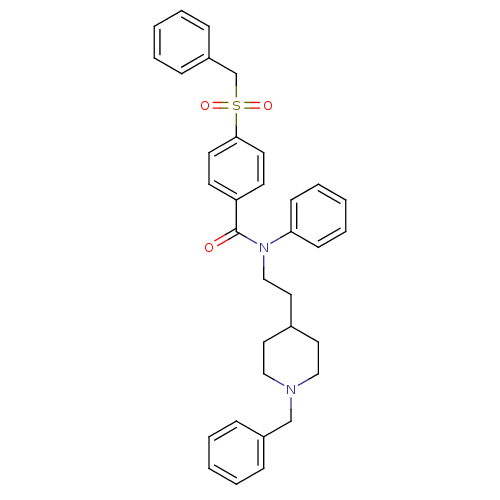
(CHEMBL54058 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...)Show SMILES O=C(N(CCC1CCN(Cc2ccccc2)CC1)c1ccccc1)c1ccc(cc1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C34H36N2O3S/c37-34(31-16-18-33(19-17-31)40(38,39)27-30-12-6-2-7-13-30)36(32-14-8-3-9-15-32)25-22-28-20-23-35(24-21-28)26-29-10-4-1-5-11-29/h1-19,28H,20-27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition against Acetylcholinesterase (AChE) |
J Med Chem 39: 380-7 (1996)
Article DOI: 10.1021/jm950704x
BindingDB Entry DOI: 10.7270/Q25D8T1Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM9409

(CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...)Show SMILES CN(CCC1CCN(Cc2ccccc2)CC1)C(=O)c1ccc(cc1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C29H34N2O3S/c1-30(19-16-24-17-20-31(21-18-24)22-25-8-4-2-5-9-25)29(32)27-12-14-28(15-13-27)35(33,34)23-26-10-6-3-7-11-26/h2-15,24H,16-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase |
J Med Chem 38: 4821-9 (1996)
BindingDB Entry DOI: 10.7270/Q2QC045T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM9409

(CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...)Show SMILES CN(CCC1CCN(Cc2ccccc2)CC1)C(=O)c1ccc(cc1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C29H34N2O3S/c1-30(19-16-24-17-20-31(21-18-24)22-25-8-4-2-5-9-25)29(32)27-12-14-28(15-13-27)35(33,34)23-26-10-6-3-7-11-26/h2-15,24H,16-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Missouri-St. Louis
Curated by ChEMBL
| Assay Description
Inhibition against Acetylcholinesterase (AChE) |
J Med Chem 39: 380-7 (1996)
Article DOI: 10.1021/jm950704x
BindingDB Entry DOI: 10.7270/Q25D8T1Q |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM9411

(CHEMBL54058 | N-[2-(1-benzylpiperidin-4-yl)ethyl]-...)Show SMILES O=C(N(CCC1CCN(Cc2ccccc2)CC1)c1ccccc1)c1ccc(cc1)S(=O)(=O)Cc1ccccc1 Show InChI InChI=1S/C34H36N2O3S/c37-34(31-16-18-33(19-17-31)40(38,39)27-30-12-6-2-7-13-30)36(32-14-8-3-9-15-32)25-22-28-20-23-35(24-21-28)26-29-10-4-1-5-11-29/h1-19,28H,20-27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Laboratories
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. The amount of a yellow substance formed after incubation was d... |
J Med Chem 33: 1880-7 (1990)
Article DOI: 10.1021/jm00169a008
BindingDB Entry DOI: 10.7270/Q20Z71H2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50379273

(CHEMBL1994202 | US9238626, (-)-Huprine Y HCl)Show SMILES CC1=C[C@H]2C[C@@H](C1)c1c(C2)nc2cc(Cl)ccc2c1N |t:1| Show InChI InChI=1S/C17H17ClN2/c1-9-4-10-6-11(5-9)16-15(7-10)20-14-8-12(18)2-3-13(14)17(16)19/h2-4,8,10-11H,5-7H2,1H3,(H2,19,20)/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase |
J Med Chem 38: 4821-9 (1996)
BindingDB Entry DOI: 10.7270/Q2QC045T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. |
J Med Chem 35: 4542-8 (1993)
BindingDB Entry DOI: 10.7270/Q25M64P8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004016
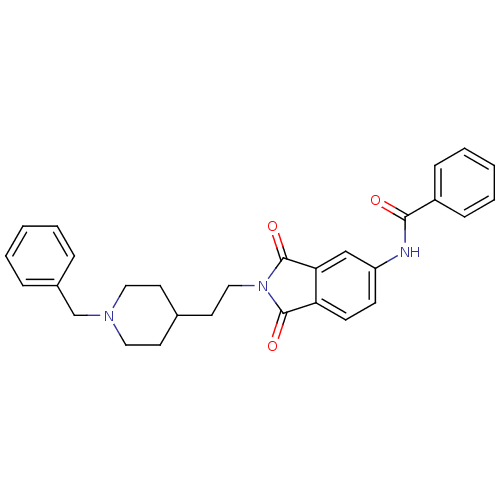
(CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...)Show SMILES O=C(Nc1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1)c1ccccc1 Show InChI InChI=1S/C29H29N3O3/c33-27(23-9-5-2-6-10-23)30-24-11-12-25-26(19-24)29(35)32(28(25)34)18-15-21-13-16-31(17-14-21)20-22-7-3-1-4-8-22/h1-12,19,21H,13-18,20H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase |
J Med Chem 38: 4821-9 (1996)
BindingDB Entry DOI: 10.7270/Q2QC045T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004016

(CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...)Show SMILES O=C(Nc1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1)c1ccccc1 Show InChI InChI=1S/C29H29N3O3/c33-27(23-9-5-2-6-10-23)30-24-11-12-25-26(19-24)29(35)32(28(25)34)18-15-21-13-16-31(17-14-21)20-22-7-3-1-4-8-22/h1-12,19,21H,13-18,20H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. |
J Med Chem 35: 4542-8 (1993)
BindingDB Entry DOI: 10.7270/Q25M64P8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50029933

(2-[3-(1-Benzyl-piperidin-4-yl)-propyl]-5,6-dimetho...)Show SMILES COc1cc2CC(CCCC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C26H33NO3/c1-29-24-16-22-15-21(26(28)23(22)17-25(24)30-2)10-6-9-19-11-13-27(14-12-19)18-20-7-4-3-5-8-20/h3-5,7-8,16-17,19,21H,6,9-15,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase |
J Med Chem 38: 4821-9 (1996)
BindingDB Entry DOI: 10.7270/Q2QC045T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585450

(CHEMBL5076277)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1ccc(cc1)N1CCN(CCCCc2c[nH]c3ccccc23)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585493
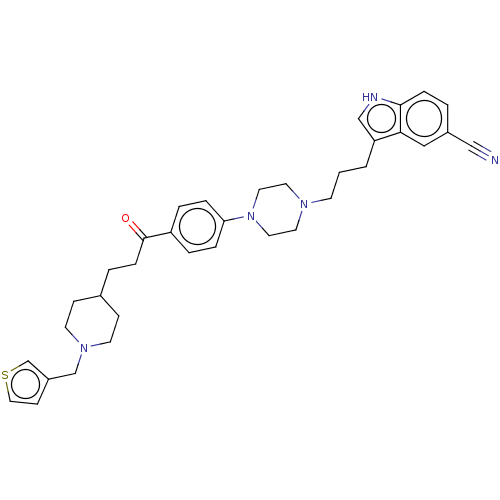
(CHEMBL5086036)Show SMILES O=C(CCC1CCN(Cc2ccsc2)CC1)c1ccc(cc1)N1CCN(CCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM8963

(CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C33H40N4/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33/h4,6,8,10,14,16,18,20H,1-3,5,7,9,11-13,15,17,19,21-23H2,(H,34,36)(H,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul
Curated by ChEMBL
| Assay Description
Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... |
Bioorg Med Chem 26: 5566-5577 (2018)
Article DOI: 10.1016/j.bmc.2018.10.003
BindingDB Entry DOI: 10.7270/Q2B56NFD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50029944

(5,6-Dimethoxy-2-[1-(3-methyl-benzyl)-piperidin-4-y...)Show SMILES COc1cc2CC(CC3CCN(Cc4cccc(C)c4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C25H31NO3/c1-17-5-4-6-19(11-17)16-26-9-7-18(8-10-26)12-21-13-20-14-23(28-2)24(29-3)15-22(20)25(21)27/h4-6,11,14-15,18,21H,7-10,12-13,16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase |
J Med Chem 38: 4821-9 (1996)
BindingDB Entry DOI: 10.7270/Q2QC045T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50467895

(CHEMBL4280262)Show SMILES [H][C@]12O[C@H](CNCCCCCCCCCCNc3c4CCCCc4nc4ccccc34)[C@H](O)[C@@]1([H])OC(C)(C)O2 |r| Show InChI InChI=1S/C31H47N3O4/c1-31(2)37-29-28(35)26(36-30(29)38-31)21-32-19-13-7-5-3-4-6-8-14-20-33-27-22-15-9-11-17-24(22)34-25-18-12-10-16-23(25)27/h9,11,15,17,26,28-30,32,35H,3-8,10,12-14,16,18-21H2,1-2H3,(H,33,34)/t26-,28+,29-,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do Rio Grande do Sul
Curated by ChEMBL
| Assay Description
Inhibition of AChE in Swiss Webster mouse cerebral homogenate using acetylthiocholine iodide as substrate preincubated for 10 mins followed substrate... |
Bioorg Med Chem 26: 5566-5577 (2018)
Article DOI: 10.1016/j.bmc.2018.10.003
BindingDB Entry DOI: 10.7270/Q2B56NFD |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004007

(2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-1,3-dioxo-2,...)Show SMILES O=C(NCc1ccccc1)c1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1 Show InChI InChI=1S/C30H31N3O3/c34-28(31-20-23-7-3-1-4-8-23)25-11-12-26-27(19-25)30(36)33(29(26)35)18-15-22-13-16-32(17-14-22)21-24-9-5-2-6-10-24/h1-12,19,22H,13-18,20-21H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. |
J Med Chem 35: 4542-8 (1993)
BindingDB Entry DOI: 10.7270/Q25M64P8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585484

(CHEMBL5082836)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1ccc(cc1)N1CCN(CCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585455

(CHEMBL5088060)Show SMILES Cc1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc(cc3)C(=O)CCC3CCN(Cc4ccccc4)CC3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585443
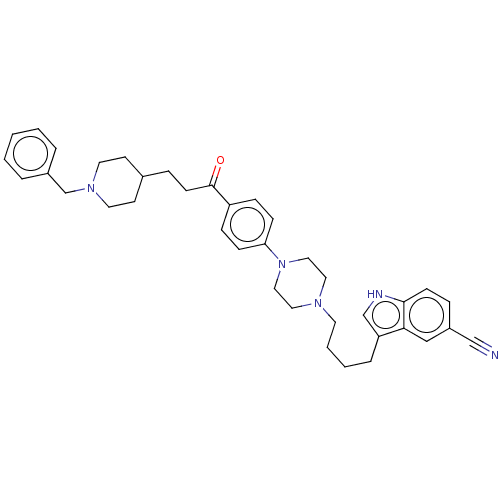
(CHEMBL5082250)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1ccc(cc1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604668
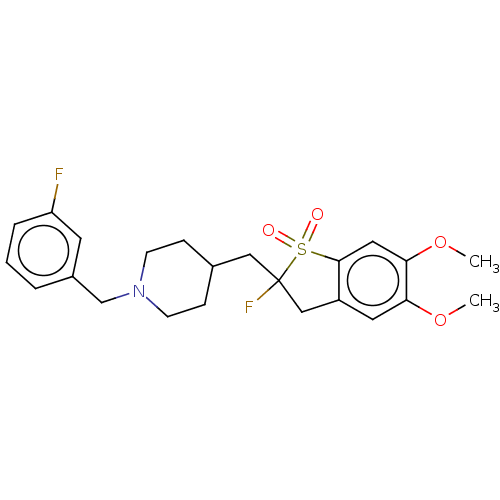
(CHEMBL5180947)Show SMILES COc1cc2CC(F)(CC3CCN(Cc4cccc(F)c4)CC3)S(=O)(=O)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114305
BindingDB Entry DOI: 10.7270/Q26M3BX2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585491
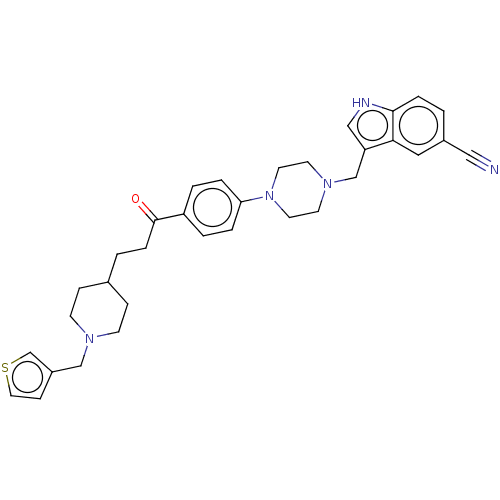
(CHEMBL5086121)Show SMILES O=C(CCC1CCN(Cc2ccsc2)CC1)c1ccc(cc1)N1CCN(Cc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004001
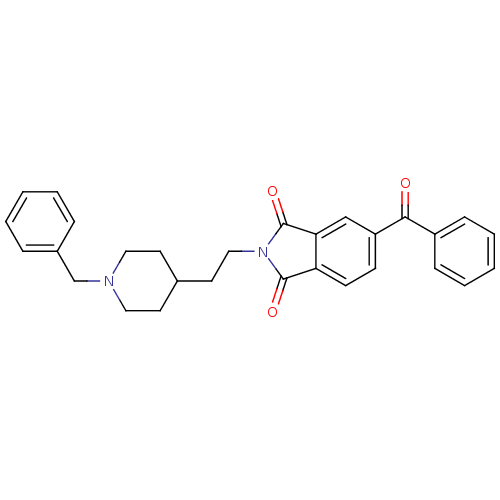
(5-Benzoyl-2-[2-(1-benzyl-piperidin-4-yl)-ethyl]-is...)Show SMILES O=C(c1ccccc1)c1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1 Show InChI InChI=1S/C29H28N2O3/c32-27(23-9-5-2-6-10-23)24-11-12-25-26(19-24)29(34)31(28(25)33)18-15-21-13-16-30(17-14-21)20-22-7-3-1-4-8-22/h1-12,19,21H,13-18,20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. |
J Med Chem 35: 4542-8 (1993)
BindingDB Entry DOI: 10.7270/Q25M64P8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585454
(CHEMBL5082360)Show SMILES COc1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc(cc3)C(=O)CCC3CCN(Cc4ccccc4)CC3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585451

(CHEMBL5087922)Show SMILES Fc1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc(cc3)C(=O)CCC3CCN(Cc4ccccc4)CC3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585492
(CHEMBL5087445)Show SMILES O=C(CCC1CCN(Cc2ccsc2)CC1)c1ccc(cc1)N1CCN(CCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604191
(CHEMBL5207628)Show SMILES [H][C@]12Cc3nc4cc(Cl)ccc4c(NCCCCC(=O)N4CCC(CC4)NC(=O)Nc4ccc(OC(F)(F)F)cc4)c3[C@]([H])(CC(C)=C1)C2 |r,c:50| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50029944

(5,6-Dimethoxy-2-[1-(3-methyl-benzyl)-piperidin-4-y...)Show SMILES COc1cc2CC(CC3CCN(Cc4cccc(C)c4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C25H31NO3/c1-17-5-4-6-19(11-17)16-26-9-7-18(8-10-26)12-21-13-20-14-23(28-2)24(29-3)15-22(20)25(21)27/h4-6,11,14-15,18,21H,7-10,12-13,16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase activity in mouse brain homogenate |
Bioorg Med Chem Lett 2: 871-876 (1992)
Article DOI: 10.1016/S0960-894X(00)80547-8
BindingDB Entry DOI: 10.7270/Q21R6QDV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004035
(CHEMBL433678 | N-(2-(2-(1-benzylpiperidin-4-yl)eth...)Show SMILES CC(=O)Nc1ccc2C(=O)N(CCC3CCN(Cc4ccccc4)CC3)C(=O)c2c1 Show InChI InChI=1S/C24H27N3O3/c1-17(28)25-20-7-8-21-22(15-20)24(30)27(23(21)29)14-11-18-9-12-26(13-10-18)16-19-5-3-2-4-6-19/h2-8,15,18H,9-14,16H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. |
J Med Chem 35: 4542-8 (1993)
BindingDB Entry DOI: 10.7270/Q25M64P8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585477
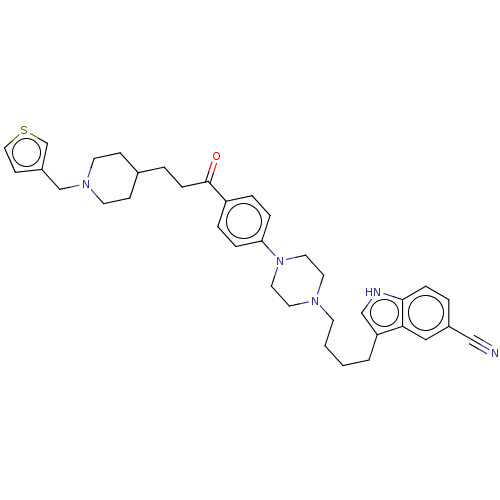
(CHEMBL5088143)Show SMILES O=C(CCC1CCN(Cc2ccsc2)CC1)c1ccc(cc1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585496

(CHEMBL5094829)Show SMILES O=C(CCC1CCN(Cc2cc[nH]c2)CC1)c1ccc(cc1)N1CCN(CCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585456

(CHEMBL5087351)Show SMILES COC(=O)c1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc(cc3)C(=O)CCC3CCN(Cc4ccccc4)CC3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585494

(CHEMBL5088281)Show SMILES O=C(CCC1CCN(Cc2cc[nH]c2)CC1)c1ccc(cc1)N1CCN(Cc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585495

(CHEMBL5075438)Show SMILES O=C(CCC1CCN(Cc2cc[nH]c2)CC1)c1ccc(cc1)N1CCN(CCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585452
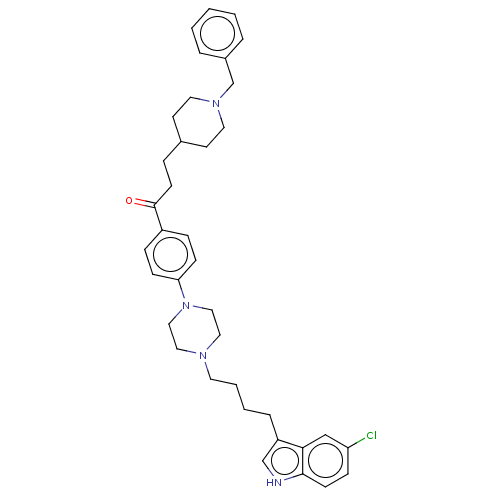
(CHEMBL5090651)Show SMILES Clc1ccc2[nH]cc(CCCCN3CCN(CC3)c3ccc(cc3)C(=O)CCC3CCN(Cc4ccccc4)CC3)c2c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585457

(CHEMBL5094743)Show SMILES O=C(CCC1CCN(Cc2ccccc2)CC1)c1ccc(cc1)N1CCN(CCCCc2c[nH]c3cc(ccc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604667
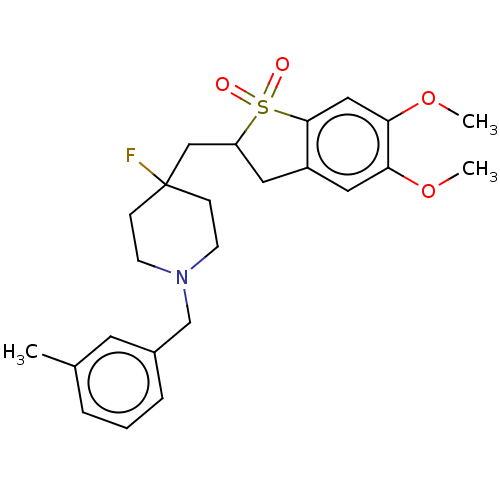
(CHEMBL5198028)Show SMILES COc1cc2CC(CC3(F)CCN(Cc4cccc(C)c4)CC3)S(=O)(=O)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114305
BindingDB Entry DOI: 10.7270/Q26M3BX2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50373109
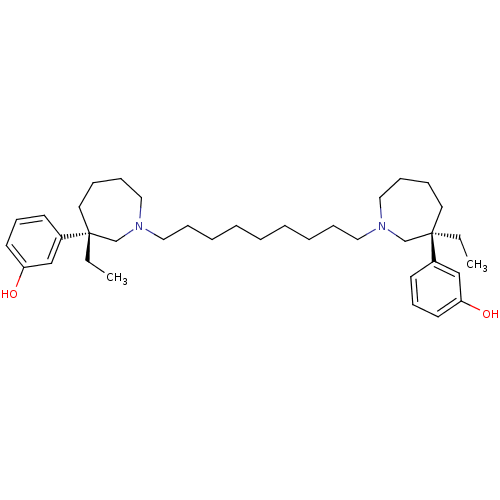
(CHEMBL406645)Show SMILES CC[C@]1(CCCCN(CCCCCCCCCN2CCCC[C@](CC)(C2)c2cccc(O)c2)C1)c1cccc(O)c1 Show InChI InChI=1S/C37H58N2O2/c1-3-36(32-18-16-20-34(40)28-32)22-10-14-26-38(30-36)24-12-8-6-5-7-9-13-25-39-27-15-11-23-37(4-2,31-39)33-19-17-21-35(41)29-33/h16-21,28-29,40-41H,3-15,22-27,30-31H2,1-2H3/t36-,37-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University
Curated by ChEMBL
| Assay Description
Inhibition of mouse brain AChE |
J Med Chem 51: 2027-36 (2008)
Article DOI: 10.1021/jm070154q
BindingDB Entry DOI: 10.7270/Q2C82B4G |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585478

(CHEMBL5080984)Show SMILES O=C(CCC1CCN(Cc2cc[nH]c2)CC1)c1ccc(cc1)N1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50029932
(5,6-Dimethoxy-2-[1-(3-nitro-benzyl)-piperidin-4-yl...)Show SMILES COc1cc2CC(CC3CCN(Cc4cccc(c4)[N+]([O-])=O)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H28N2O5/c1-30-22-13-18-12-19(24(27)21(18)14-23(22)31-2)10-16-6-8-25(9-7-16)15-17-4-3-5-20(11-17)26(28)29/h3-5,11,13-14,16,19H,6-10,12,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase activity in mouse brain homogenate |
Bioorg Med Chem Lett 2: 871-876 (1992)
Article DOI: 10.1016/S0960-894X(00)80547-8
BindingDB Entry DOI: 10.7270/Q21R6QDV |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604189

(CHEMBL5200047)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)CCCNc2c3CCCCc3nc3cc(Cl)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50029932

(5,6-Dimethoxy-2-[1-(3-nitro-benzyl)-piperidin-4-yl...)Show SMILES COc1cc2CC(CC3CCN(Cc4cccc(c4)[N+]([O-])=O)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H28N2O5/c1-30-22-13-18-12-19(24(27)21(18)14-23(22)31-2)10-16-6-8-25(9-7-16)15-17-4-3-5-20(11-17)26(28)29/h3-5,11,13-14,16,19H,6-10,12,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory activity against acetylcholinesterase |
J Med Chem 38: 4821-9 (1996)
BindingDB Entry DOI: 10.7270/Q2QC045T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604657

(CHEMBL5204649)Show SMILES COc1cc2CC(CC3(F)CCN(Cc4ccccc4)CC3)S(=O)(=O)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114305
BindingDB Entry DOI: 10.7270/Q26M3BX2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604190

(CHEMBL5178885)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)CCCCNc2c3CCCCc3nc3cc(Cl)ccc23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02150
BindingDB Entry DOI: 10.7270/Q2F76HMM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50004018

(3-(2-(1-benzylpiperidin-4-yl)ethyl)quinazoline-2,4...)Show SMILES O=c1[nH]c2ccccc2c(=O)n1CCC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C22H25N3O2/c26-21-19-8-4-5-9-20(19)23-22(27)25(21)15-12-17-10-13-24(14-11-17)16-18-6-2-1-3-7-18/h1-9,17H,10-16H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. |
J Med Chem 35: 4542-8 (1993)
BindingDB Entry DOI: 10.7270/Q25M64P8 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50604649

(CHEMBL5202413)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)S(=O)(=O)c2cc1OC | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114305
BindingDB Entry DOI: 10.7270/Q26M3BX2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50585481
(CHEMBL5089999)Show SMILES O=C1C(CC2CCN(Cc3ccccc3)CC2)CC2C=C(C=CC12)N1CCN(CCCc2c[nH]c3ccc(cc23)C#N)CC1 |c:21,23| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of AChE in mouse cortical homogenate using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman's method |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114045
BindingDB Entry DOI: 10.7270/Q29K4G4C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data