 Found 4455 hits of ic50 data for polymerid = 112
Found 4455 hits of ic50 data for polymerid = 112 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM12074

((2R)-2-{[4-(4-bromophenyl)benzene]sulfonamido}-3-m...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(Br)cc1)C(O)=O |r| Show InChI InChI=1S/C17H18BrNO4S/c1-11(2)16(17(20)21)19-24(22,23)15-9-5-13(6-10-15)12-3-7-14(18)8-4-12/h3-11,16,19H,1-2H3,(H,20,21)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 (unknown origin) |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AMPA-activated MMP2 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method |
Bioorg Med Chem 24: 6149-6165 (2016)
Article DOI: 10.1016/j.bmc.2016.09.009
BindingDB Entry DOI: 10.7270/Q2H1341V |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate after 40 mins by spectrofluorimetry |
J Med Chem 57: 8886-902 (2014)
Article DOI: 10.1021/jm500981k
BindingDB Entry DOI: 10.7270/Q28P628P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 using fluorescence peptide Cy3-PLGLK(Cy5Q)AR-NH2 substrate by fluorescence assay |
Bioorg Med Chem 22: 5487-505 (2014)
Article DOI: 10.1016/j.bmc.2014.07.025
BindingDB Entry DOI: 10.7270/Q27S7QBJ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of APMA-activated recombinant human MMP-2 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric m... |
J Med Chem 60: 608-626 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01007
BindingDB Entry DOI: 10.7270/Q2W95CF2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50175548
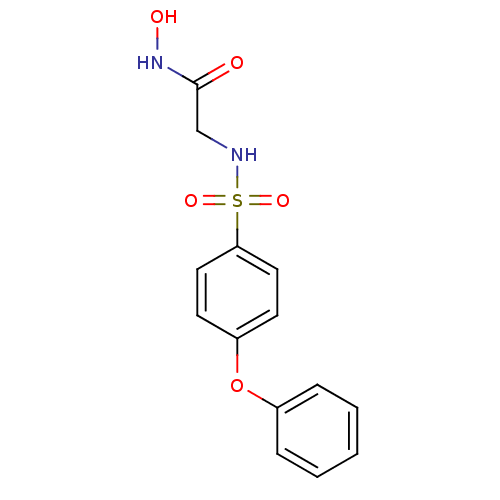
(CHEMBL198778 | N-Hydroxy-2-(4-phenoxy-benzenesulfo...)Show InChI InChI=1S/C14H14N2O5S/c17-14(16-18)10-15-22(19,20)13-8-6-12(7-9-13)21-11-4-2-1-3-5-11/h1-9,15,18H,10H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP2 using Mca-Lys-Pro- Leu-Gly-Leu-Dap(Dnp)-Ala-Arg-NH2 as substrate incubated for 2 hrs prior to substrate addition... |
Eur J Med Chem 62: 379-94 (2013)
Article DOI: 10.1016/j.ejmech.2012.12.058
BindingDB Entry DOI: 10.7270/Q20C4X4W |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM24025
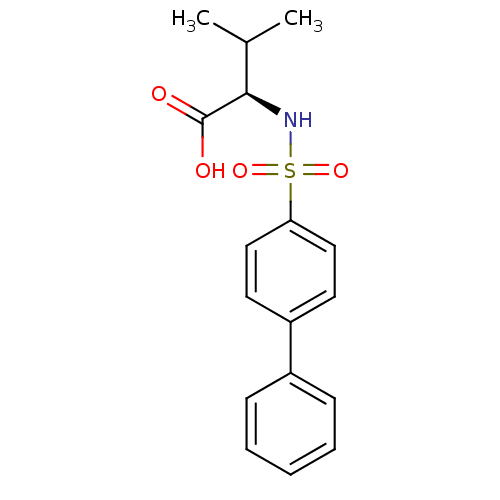
((2R)-3-methyl-2-[(4-phenylbenzene)sulfonamido]buta...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C17H19NO4S/c1-12(2)16(17(19)20)18-23(21,22)15-10-8-14(9-11-15)13-6-4-3-5-7-13/h3-12,16,18H,1-2H3,(H,19,20)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 (unknown origin) |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606383
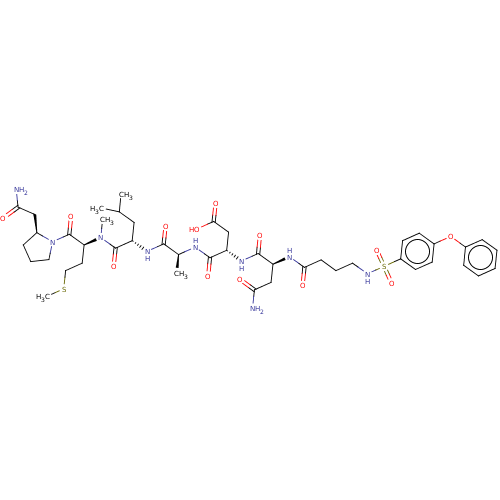
(CHEMBL5209489)Show SMILES CSCC[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | CHEMBL5288452
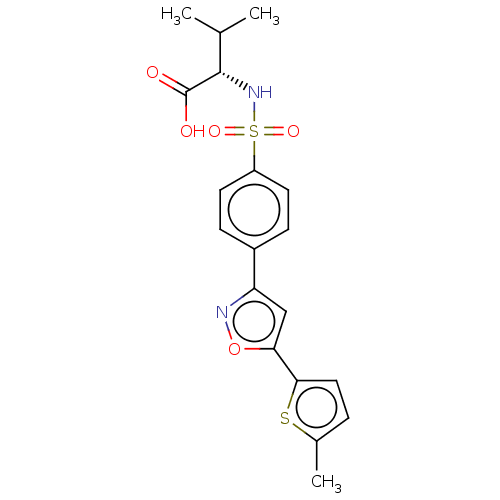
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.0449 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606385
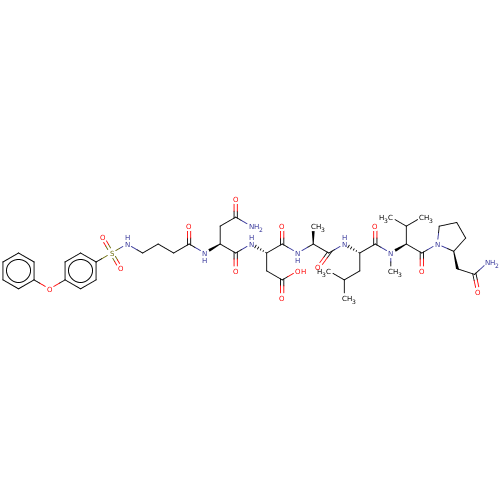
(CHEMBL5203315)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro selective inhibition against matrix metalloprotease-2 (MMP-2) using fluorimetric assay |
J Med Chem 45: 219-32 (2001)
BindingDB Entry DOI: 10.7270/Q2XP747B |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50106026
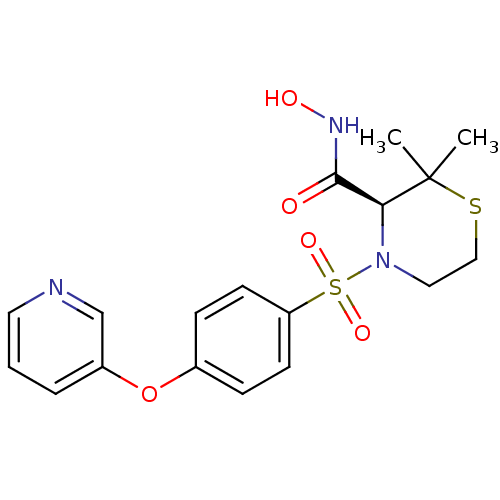
((S)-2,2-Dimethyl-4-[4-(pyridin-3-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2cccnc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(10-11-27-18)28(24,25)15-7-5-13(6-8-15)26-14-4-3-9-19-12-14/h3-9,12,16,23H,10-11H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 2907-10 (2001)
BindingDB Entry DOI: 10.7270/Q2C828MX |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50523895
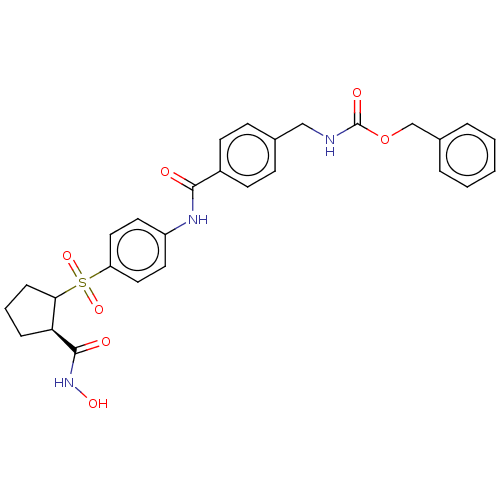
(CHEMBL4522121)Show SMILES ONC(=O)[C@H]1CCCC1S(=O)(=O)c1ccc(NC(=O)c2ccc(CNC(=O)OCc3ccccc3)cc2)cc1 |r| Show InChI InChI=1S/C28H29N3O7S/c32-26(21-11-9-19(10-12-21)17-29-28(34)38-18-20-5-2-1-3-6-20)30-22-13-15-23(16-14-22)39(36,37)25-8-4-7-24(25)27(33)31-35/h1-3,5-6,9-16,24-25,35H,4,7-8,17-18H2,(H,29,34)(H,30,32)(H,31,33)/t24-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 (unknown origin) pre-incubated for 5 mins before Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate addition and measur... |
Bioorg Med Chem 27: 1891-1902 (2019)
Article DOI: 10.1016/j.bmc.2019.03.043
BindingDB Entry DOI: 10.7270/Q2RR22PK |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50592894
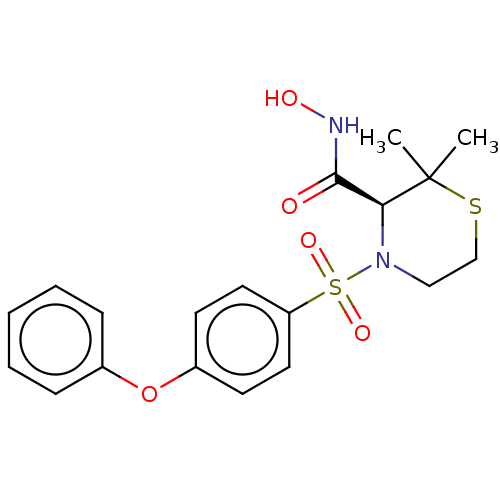
(CHEMBL473539)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606395
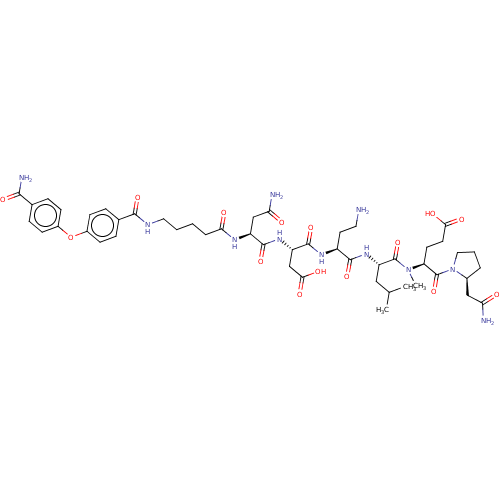
(CHEMBL5207094)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCNC(=O)c1ccc(Oc2ccc(cc2)C(N)=O)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606384
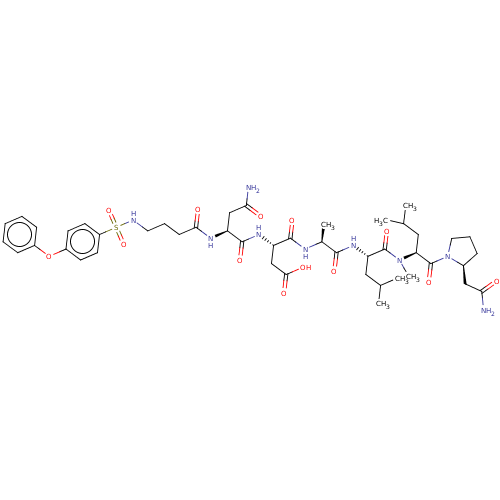
(CHEMBL5183245)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606386
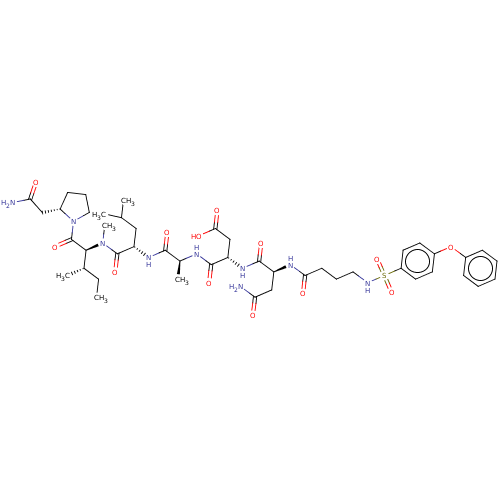
(CHEMBL5182207)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50131941
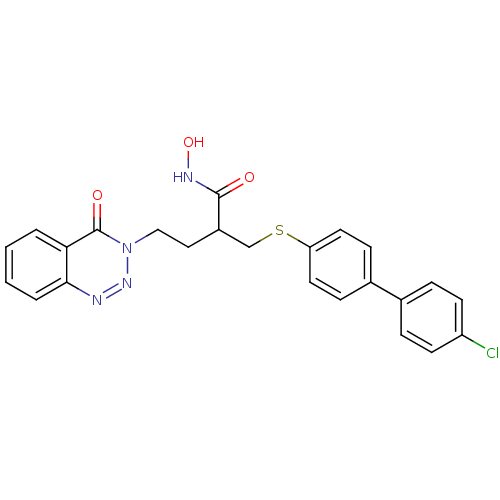
(2-(4'-Chloro-biphenyl-4-ylsulfanylmethyl)-N-hydrox...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)CSc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O3S/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-2 (MMP2) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | CHEMBL5280430

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606380
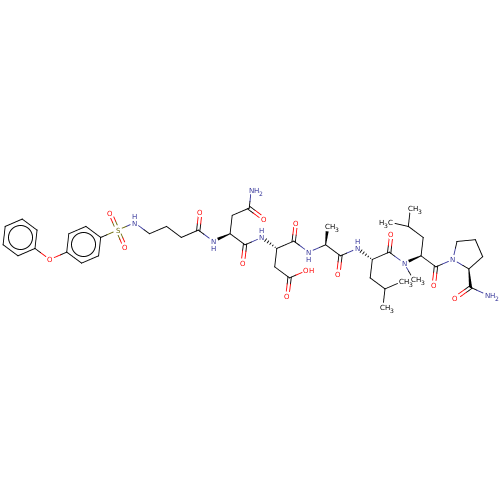
(CHEMBL5191373)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606390
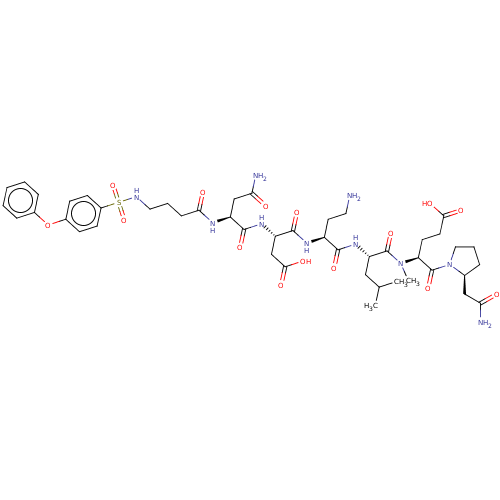
(CHEMBL5185612)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50389101
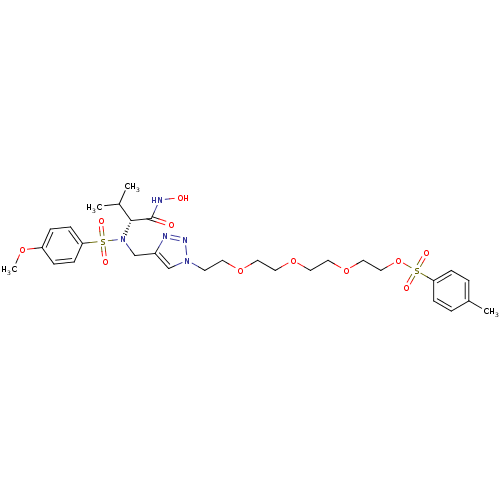
(CHEMBL2064548)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cn(CCOCCOCCOCCOS(=O)(=O)c2ccc(C)cc2)nn1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C30H43N5O11S2/c1-23(2)29(30(36)32-37)35(47(38,39)27-11-7-26(42-4)8-12-27)22-25-21-34(33-31-25)13-14-43-15-16-44-17-18-45-19-20-46-48(40,41)28-9-5-24(3)6-10-28/h5-12,21,23,29,37H,13-20,22H2,1-4H3,(H,32,36)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital M£nster
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diaminopropionyl)Ala-Arg-NH2 as substrate incuba... |
J Med Chem 55: 4714-27 (2012)
Article DOI: 10.1021/jm300199g
BindingDB Entry DOI: 10.7270/Q2GQ6ZTT |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50137279
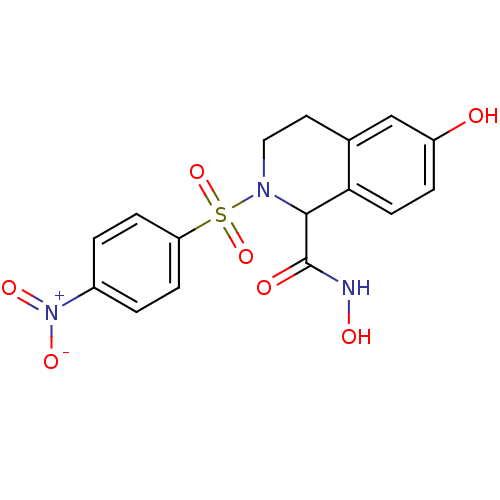
(6-Hydroxy-2-(4-nitro-benzenesulfonyl)-1,2,3,4-tetr...)Show SMILES ONC(=O)C1N(CCc2cc(O)ccc12)S(=O)(=O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C16H15N3O7S/c20-12-3-6-14-10(9-12)7-8-18(15(14)16(21)17-22)27(25,26)13-4-1-11(2-5-13)19(23)24/h1-6,9,15,20,22H,7-8H2,(H,17,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 (unknown origin) |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606381
(CHEMBL5198135)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N(C)[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606377
(CHEMBL5186594)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606378
(CHEMBL5170274)Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCCS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50161330
((R)-2-[(Biphenyl-4-sulfonyl)-isopropoxy-amino]-N-h...)Show SMILES CC(C)ON([C@H](C(C)C)C(=O)NO)S(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C20H26N2O5S/c1-14(2)19(20(23)21-24)22(27-15(3)4)28(25,26)18-12-10-17(11-13-18)16-8-6-5-7-9-16/h5-15,19,24H,1-4H3,(H,21,23)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-2 |
Bioorg Med Chem Lett 15: 1321-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.024
BindingDB Entry DOI: 10.7270/Q2X34Z6T |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-2 |
Bioorg Med Chem Lett 15: 1321-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.024
BindingDB Entry DOI: 10.7270/Q2X34Z6T |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50606388
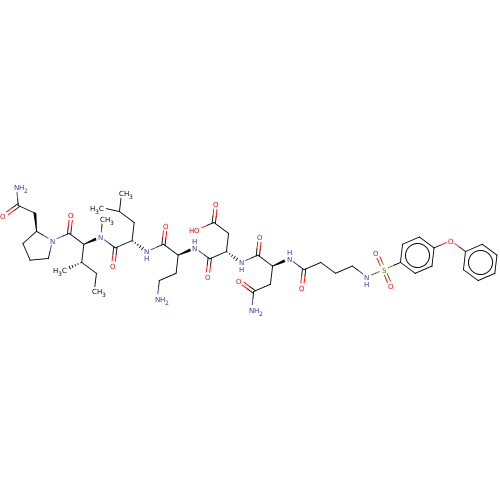
(CHEMBL5203492)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](CCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1CC(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
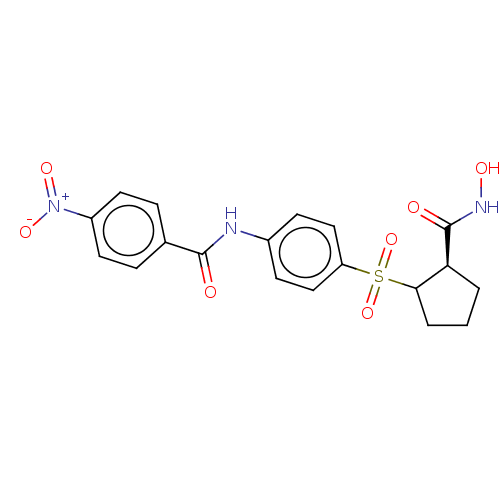
(Homo sapiens (Human)) | BDBM50523894
(CHEMBL4535400)Show SMILES ONC(=O)[C@H]1CCCC1S(=O)(=O)c1ccc(NC(=O)c2ccc(cc2)[N+]([O-])=O)cc1 |r| Show InChI InChI=1S/C19H19N3O7S/c23-18(12-4-8-14(9-5-12)22(26)27)20-13-6-10-15(11-7-13)30(28,29)17-3-1-2-16(17)19(24)21-25/h4-11,16-17,25H,1-3H2,(H,20,23)(H,21,24)/t16-,17?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 (unknown origin) pre-incubated for 5 mins before Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate addition and measur... |
Bioorg Med Chem 27: 1891-1902 (2019)
Article DOI: 10.1016/j.bmc.2019.03.043
BindingDB Entry DOI: 10.7270/Q2RR22PK |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
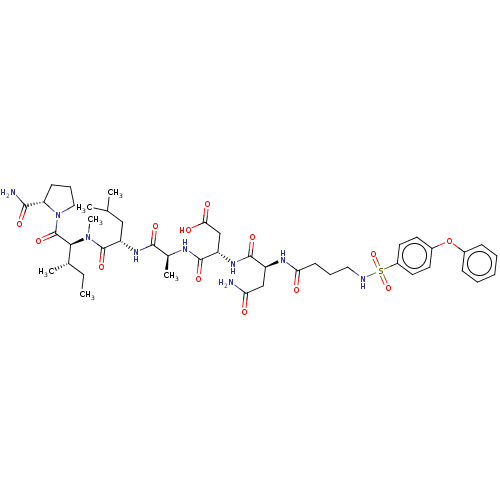
(Homo sapiens (Human)) | BDBM50606382
(CHEMBL5191820)Show SMILES CC[C@H](C)[C@H](N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)CCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00613
BindingDB Entry DOI: 10.7270/Q2KD231G |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
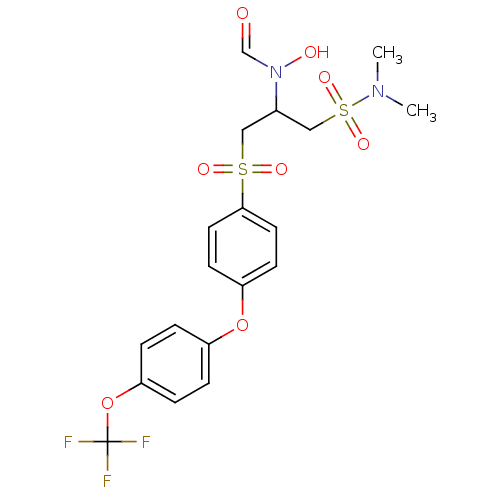
(Homo sapiens (Human)) | BDBM50108135
(2-(Formyl-hydroxy-amino)-3-[4-(4-trifluoromethoxy-...)Show SMILES CN(C)S(=O)(=O)CC(CS(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1)N(O)C=O Show InChI InChI=1S/C19H21F3N2O8S2/c1-23(2)34(29,30)12-14(24(26)13-25)11-33(27,28)18-9-7-16(8-10-18)31-15-3-5-17(6-4-15)32-19(20,21)22/h3-10,13-14,26H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro selective inhibition against matrix metalloprotease-2 (MMP-2) using fluorimetric assay |
J Med Chem 45: 219-32 (2001)
BindingDB Entry DOI: 10.7270/Q2XP747B |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50389096
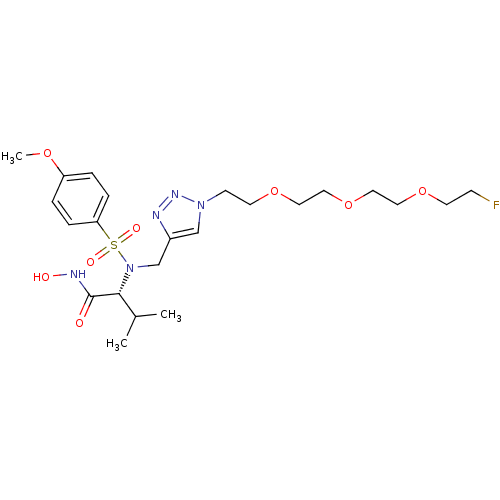
(CHEMBL2064549)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cn(CCOCCOCCOCCF)nn1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C23H36FN5O8S/c1-18(2)22(23(30)26-31)29(38(32,33)21-6-4-20(34-3)5-7-21)17-19-16-28(27-25-19)9-11-36-13-15-37-14-12-35-10-8-24/h4-7,16,18,22,31H,8-15,17H2,1-3H3,(H,26,30)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University Hospital M£nster
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 using (7-methoxycoumarin-4-yl)acetyl-Pro-Leu-Gly-Leu-(3-(2,4-dinitrophenyl)-L-2,3-diaminopropionyl)Ala-Arg-NH2 as substrate incuba... |
J Med Chem 55: 4714-27 (2012)
Article DOI: 10.1021/jm300199g
BindingDB Entry DOI: 10.7270/Q2GQ6ZTT |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348687
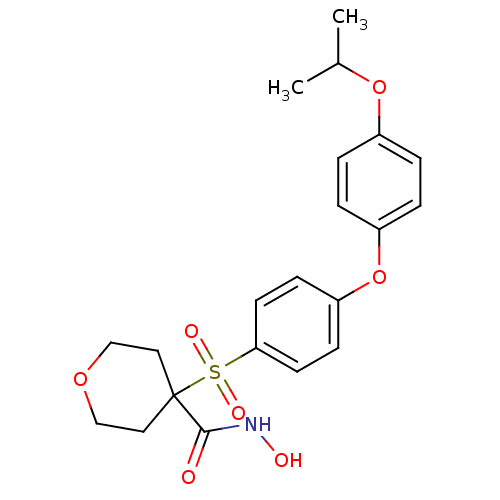
(CHEMBL1801049)Show SMILES CC(C)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCOCC2)C(=O)NO)cc1 Show InChI InChI=1S/C21H25NO7S/c1-15(2)28-16-3-5-17(6-4-16)29-18-7-9-19(10-8-18)30(25,26)21(20(23)22-24)11-13-27-14-12-21/h3-10,15,24H,11-14H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348658
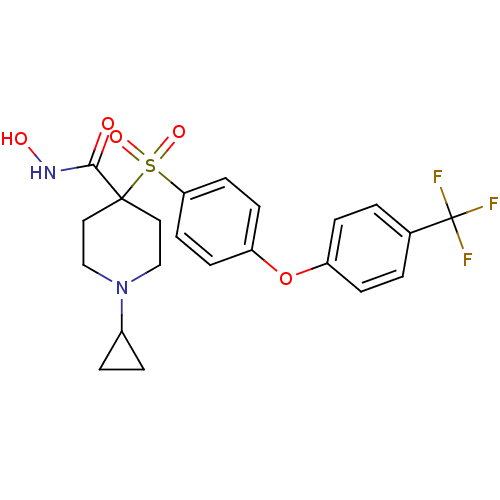
(CHEMBL1801424)Show SMILES ONC(=O)C1(CCN(CC1)C1CC1)S(=O)(=O)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C22H23F3N2O5S/c23-22(24,25)15-1-5-17(6-2-15)32-18-7-9-19(10-8-18)33(30,31)21(20(28)26-29)11-13-27(14-12-21)16-3-4-16/h1-2,5-10,16,29H,3-4,11-14H2,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348664
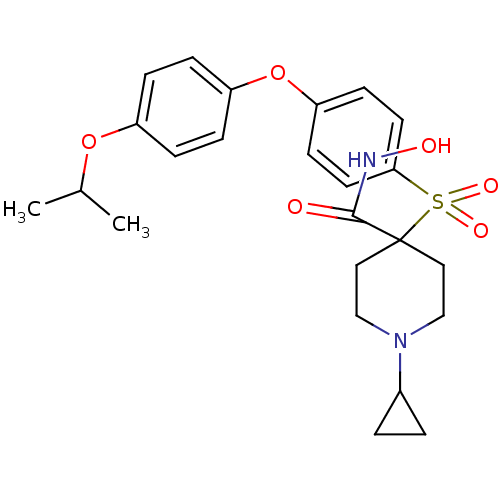
(CHEMBL1801417)Show SMILES CC(C)Oc1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCN(CC2)C2CC2)C(=O)NO)cc1 Show InChI InChI=1S/C24H30N2O6S/c1-17(2)31-19-5-7-20(8-6-19)32-21-9-11-22(12-10-21)33(29,30)24(23(27)25-28)13-15-26(16-14-24)18-3-4-18/h5-12,17-18,28H,3-4,13-16H2,1-2H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348680

(CHEMBL1801414)Show SMILES COCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(cc2)C(C)C)cc1 Show InChI InChI=1S/C24H32N2O6S/c1-18(2)19-4-6-20(7-5-19)32-21-8-10-22(11-9-21)33(29,30)24(23(27)25-28)12-14-26(15-13-24)16-17-31-3/h4-11,18,28H,12-17H2,1-3H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
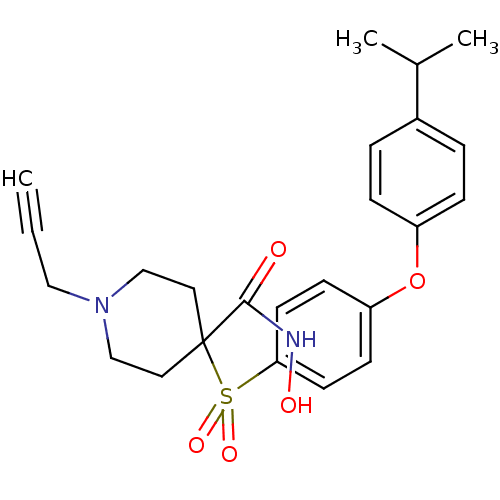
(Homo sapiens (Human)) | BDBM50348666
(CHEMBL1801413)Show SMILES CC(C)c1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCN(CC#C)CC2)C(=O)NO)cc1 Show InChI InChI=1S/C24H28N2O5S/c1-4-15-26-16-13-24(14-17-26,23(27)25-28)32(29,30)22-11-9-21(10-12-22)31-20-7-5-19(6-8-20)18(2)3/h1,5-12,18,28H,13-17H2,2-3H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
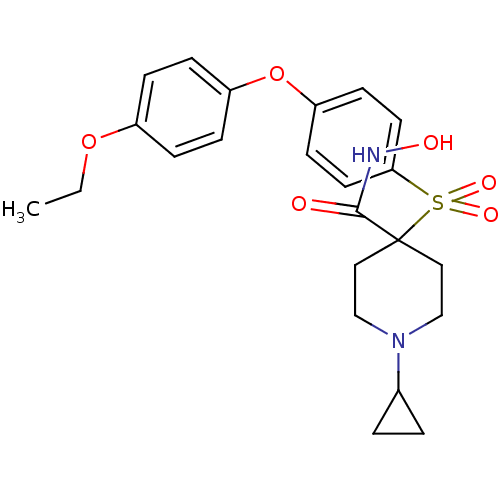
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348681
(CHEMBL1801412)Show SMILES CCOc1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCN(CC2)C2CC2)C(=O)NO)cc1 Show InChI InChI=1S/C23H28N2O6S/c1-2-30-18-5-7-19(8-6-18)31-20-9-11-21(12-10-20)32(28,29)23(22(26)24-27)13-15-25(16-14-23)17-3-4-17/h5-12,17,27H,2-4,13-16H2,1H3,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
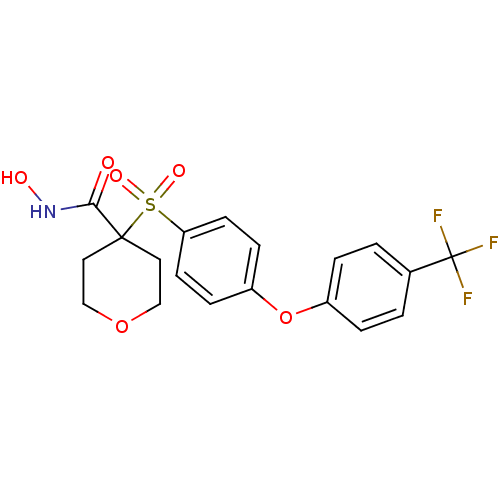
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348671
(CHEMBL1229868)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C19H18F3NO6S/c20-19(21,22)13-1-3-14(4-2-13)29-15-5-7-16(8-6-15)30(26,27)18(17(24)23-25)9-11-28-12-10-18/h1-8,25H,9-12H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
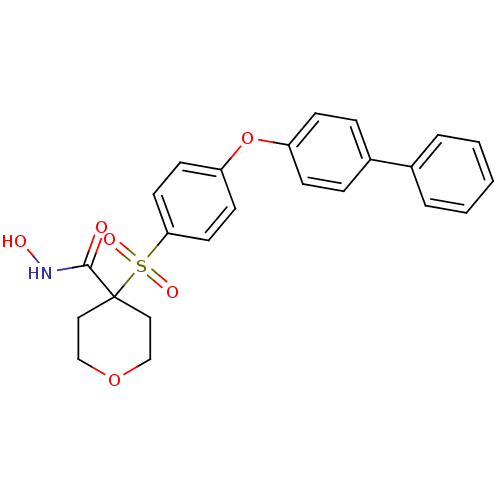
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348673
(CHEMBL1801050)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccc(cc2)-c2ccccc2)cc1 Show InChI InChI=1S/C24H23NO6S/c26-23(25-27)24(14-16-30-17-15-24)32(28,29)22-12-10-21(11-13-22)31-20-8-6-19(7-9-20)18-4-2-1-3-5-18/h1-13,27H,14-17H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348676
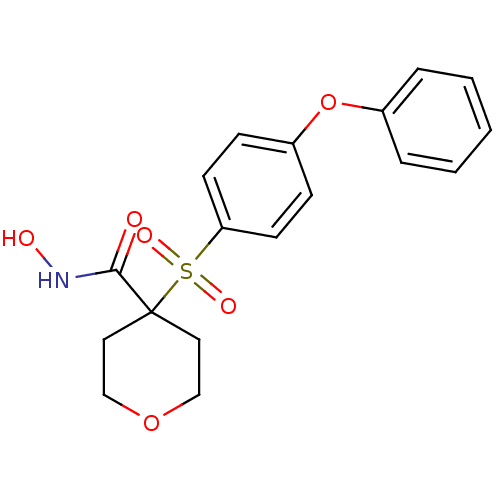
(CHEMBL1801044)Show SMILES ONC(=O)C1(CCOCC1)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H19NO6S/c20-17(19-21)18(10-12-24-13-11-18)26(22,23)16-8-6-15(7-9-16)25-14-4-2-1-3-5-14/h1-9,21H,10-13H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50105450
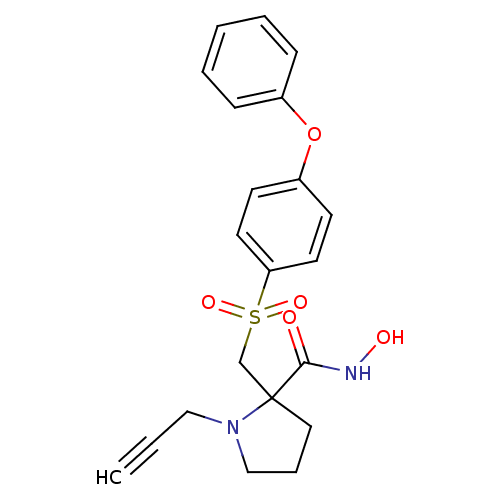
(2-(4-Phenoxy-benzenesulfonylmethyl)-1-prop-2-ynyl-...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccccc3)cc2)CCCN1CC#C Show InChI InChI=1S/C21H22N2O5S/c1-2-14-23-15-6-13-21(23,20(24)22-25)16-29(26,27)19-11-9-18(10-12-19)28-17-7-4-3-5-8-17/h1,3-5,7-12,25H,6,13-16H2,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Matrix metalloprotease-2 |
Bioorg Med Chem Lett 11: 2723-5 (2001)
BindingDB Entry DOI: 10.7270/Q29G5NCX |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50026872
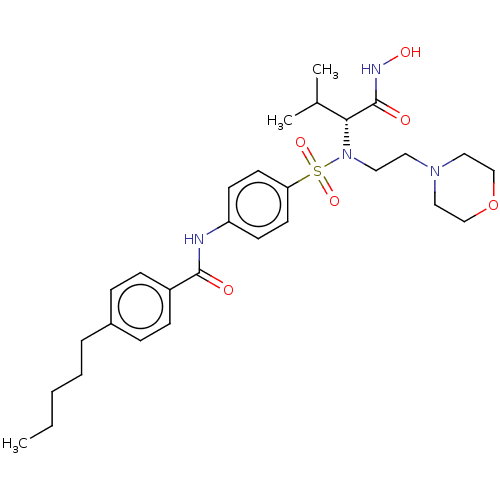
(CHEMBL1233506 | SC-74020)Show SMILES CCCCCc1ccc(cc1)C(=O)Nc1ccc(cc1)S(=O)(=O)N(CCN1CCOCC1)[C@H](C(C)C)C(=O)NO |r| Show InChI InChI=1S/C29H42N4O6S/c1-4-5-6-7-23-8-10-24(11-9-23)28(34)30-25-12-14-26(15-13-25)40(37,38)33(27(22(2)3)29(35)31-36)17-16-32-18-20-39-21-19-32/h8-15,22,27,36H,4-7,16-21H2,1-3H3,(H,30,34)(H,31,35)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Colosseum Combinatorial Chemistry Centre for Technology (C4T SCarl)
Curated by ChEMBL
| Assay Description
Inhibition of full length MMP2 using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate preincubated for 1 hr prior substrate addition measured after 3... |
Bioorg Med Chem 20: 2323-37 (2012)
Article DOI: 10.1016/j.bmc.2012.02.010
BindingDB Entry DOI: 10.7270/Q2N29XD5 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348647
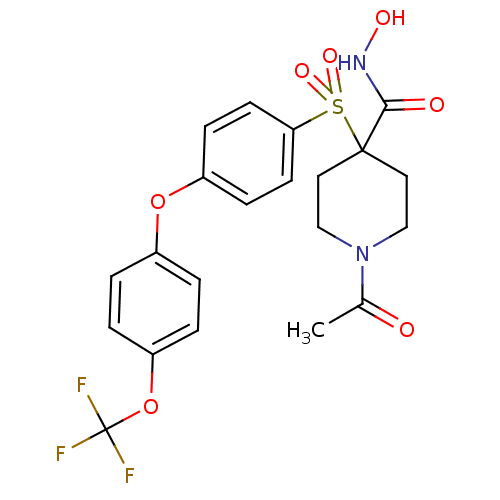
(CHEMBL1801396)Show SMILES CC(=O)N1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C21H21F3N2O7S/c1-14(27)26-12-10-20(11-13-26,19(28)25-29)34(30,31)18-8-6-16(7-9-18)32-15-2-4-17(5-3-15)33-21(22,23)24/h2-9,29H,10-13H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50071267
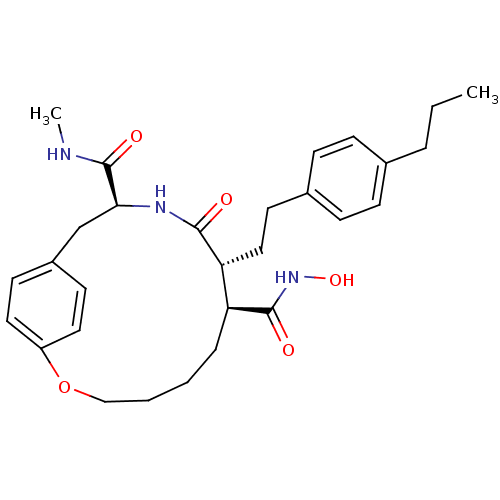
((7S,8R,11S)-9-Oxo-8-[2-(4-propyl-phenyl)-ethyl]-2-...)Show SMILES CCCc1ccc(CC[C@@H]2[C@H](CCCCOc3ccc(C[C@H](NC2=O)C(=O)NC)cc3)C(=O)NO)cc1 Show InChI InChI=1S/C29H39N3O5/c1-3-6-20-8-10-21(11-9-20)14-17-25-24(28(34)32-36)7-4-5-18-37-23-15-12-22(13-16-23)19-26(29(35)30-2)31-27(25)33/h8-13,15-16,24-26,36H,3-7,14,17-19H2,1-2H3,(H,30,35)(H,31,33)(H,32,34)/t24-,25+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast gelatinase A (MMP-2) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343077
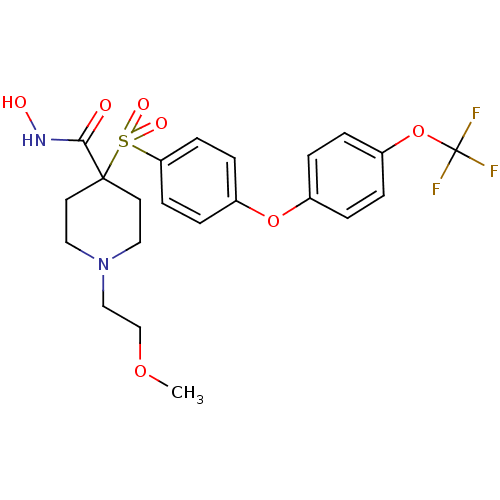
(CHEMBL1771212 | N-hydroxy-1-(2-methoxyethyl)-4-(4-...)Show SMILES COCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C22H25F3N2O7S/c1-32-15-14-27-12-10-21(11-13-27,20(28)26-29)35(30,31)19-8-6-17(7-9-19)33-16-2-4-18(5-3-16)34-22(23,24)25/h2-9,29H,10-15H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-2 |
Bioorg Med Chem Lett 21: 2820-2 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.099
BindingDB Entry DOI: 10.7270/Q2416XCK |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50096646
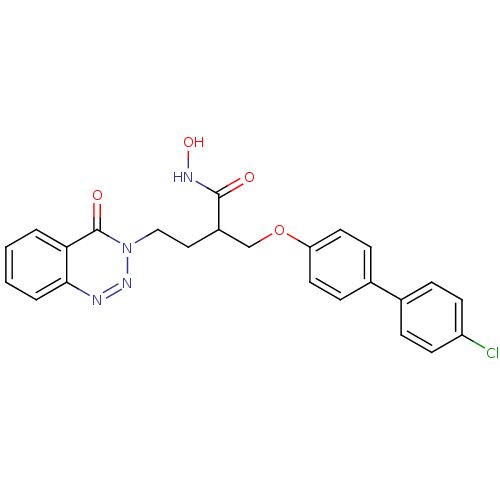
(2-(4'-Chloro-biphenyl-4-yloxymethyl)-N-hydroxy-4-(...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)COc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O4/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50343077

(CHEMBL1771212 | N-hydroxy-1-(2-methoxyethyl)-4-(4-...)Show SMILES COCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C22H25F3N2O7S/c1-32-15-14-27-12-10-21(11-13-27,20(28)26-29)35(30,31)19-8-6-17(7-9-19)33-16-2-4-18(5-3-16)34-22(23,24)25/h2-9,29H,10-15H2,1H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50348652
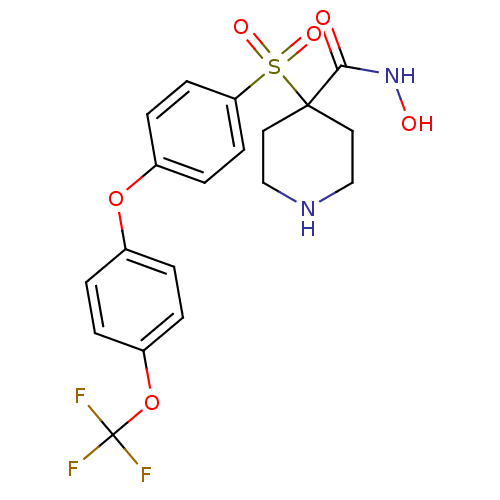
(CHEMBL1801429)Show SMILES ONC(=O)C1(CCNCC1)S(=O)(=O)c1ccc(Oc2ccc(OC(F)(F)F)cc2)cc1 Show InChI InChI=1S/C19H19F3N2O6S/c20-19(21,22)30-15-3-1-13(2-4-15)29-14-5-7-16(8-6-14)31(27,28)18(17(25)24-26)9-11-23-12-10-18/h1-8,23,26H,9-12H2,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP2 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data