 Found 1891 hits of ic50 data for polymerid = 114
Found 1891 hits of ic50 data for polymerid = 114 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM12074

((2R)-2-{[4-(4-bromophenyl)benzene]sulfonamido}-3-m...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(Br)cc1)C(O)=O |r| Show InChI InChI=1S/C17H18BrNO4S/c1-11(2)16(17(20)21)19-24(22,23)15-9-5-13(6-10-15)12-3-7-14(18)8-4-12/h3-11,16,19H,1-2H3,(H,20,21)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 (unknown origin) |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM24025
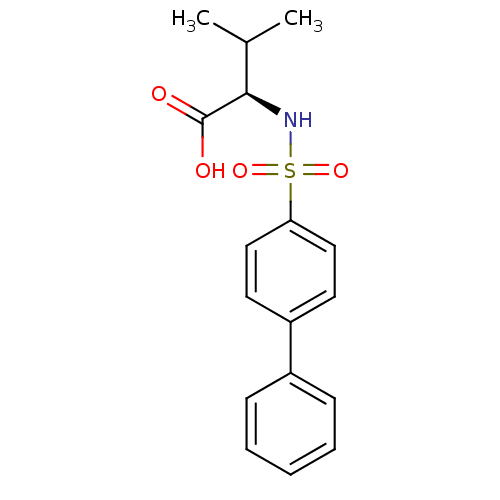
((2R)-3-methyl-2-[(4-phenylbenzene)sulfonamido]buta...)Show SMILES CC(C)[C@@H](NS(=O)(=O)c1ccc(cc1)-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C17H19NO4S/c1-12(2)16(17(19)20)18-23(21,22)15-10-8-14(9-11-15)13-6-4-3-5-7-13/h3-12,16,18H,1-2H3,(H,19,20)/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a |
Indian Institute of Technology (BHU)
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 (unknown origin) |
Eur J Med Chem 60: 89-100 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.016
BindingDB Entry DOI: 10.7270/Q2MC91CR |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50062351

((R)-N*4*-Hydroxy-N*1*-[(S)-2-(1H-indol-3-yl)-1-met...)Show SMILES CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CC(C)C)CC(=O)NO |r| Show InChI InChI=1S/C20H28N4O4/c1-12(2)8-13(10-18(25)24-28)19(26)23-17(20(27)21-3)9-14-11-22-16-7-5-4-6-15(14)16/h4-7,11-13,17,22,28H,8-10H2,1-3H3,(H,21,27)(H,23,26)(H,24,25)/t13-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.191 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR/CNRS 6013
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2843-6 (2003)
BindingDB Entry DOI: 10.7270/Q2PN952R |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50348668

(CHEMBL1801056)Show SMILES COc1ccc(Oc2ccc(cc2)S(=O)(=O)C2(CCN(CC#C)CC2)C(=O)NO)cc1 Show InChI InChI=1S/C22H24N2O6S/c1-3-14-24-15-12-22(13-16-24,21(25)23-26)31(27,28)20-10-8-19(9-11-20)30-18-6-4-17(29-2)5-7-18/h1,4-11,26H,12-16H2,2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP3 assessed as cleavage of fluorogenic peptide MCAPro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 by fluorometric assay |
J Med Chem 53: 6653-80 (2010)
Article DOI: 10.1021/jm100669j
BindingDB Entry DOI: 10.7270/Q2KH0NQB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Pisa
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-3 |
Bioorg Med Chem Lett 15: 1321-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.024
BindingDB Entry DOI: 10.7270/Q2X34Z6T |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227724

(CHEMBL400083 | N-hydroxy-2-methyl-2-{4-[2-methyl-3...)Show SMILES CNCCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C25H35N3O5S/c1-18-16-20(8-9-23(18)21-6-5-7-22(17-21)33-15-12-26-4)19-10-13-28(14-11-19)34(31,32)25(2,3)24(29)27-30/h5-9,16-17,19,26,30H,10-15H2,1-4H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of APMA-activated recombinant human MMP-3 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric m... |
J Med Chem 60: 608-626 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01007
BindingDB Entry DOI: 10.7270/Q2W95CF2 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP3 using fluorescence peptide Cy3-PLGLK(Cy5Q)AR-NH2 substrate by fluorescence assay |
Bioorg Med Chem 22: 5487-505 (2014)
Article DOI: 10.1016/j.bmc.2014.07.025
BindingDB Entry DOI: 10.7270/Q27S7QBJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50592894
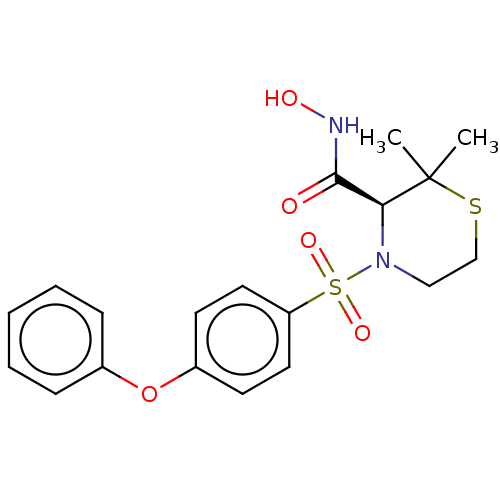
(CHEMBL473539)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01855
BindingDB Entry DOI: 10.7270/Q2GB281X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11863

(4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccc(Cl)cc3)cc2)CCOCC1 Show InChI InChI=1S/C19H20ClNO6S/c20-14-1-3-15(4-2-14)27-16-5-7-17(8-6-16)28(24,25)13-19(18(22)21-23)9-11-26-12-10-19/h1-8,23H,9-13H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP3 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate after 40 mins by spectrofluorimetry |
J Med Chem 57: 8886-902 (2014)
Article DOI: 10.1021/jm500981k
BindingDB Entry DOI: 10.7270/Q28P628P |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227723
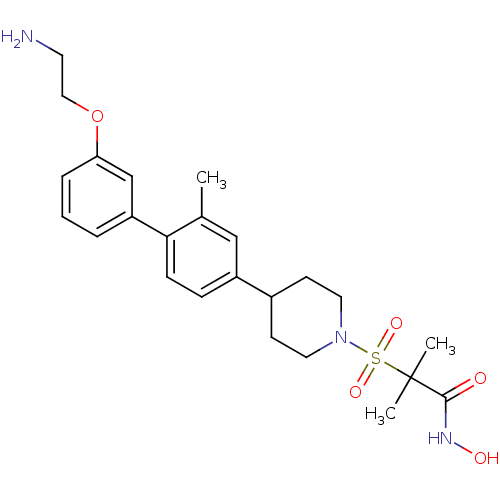
(2-{4-[3'-(2-amino-ethoxy)-2-methyl-biphenyl-4-yl]-...)Show SMILES Cc1cc(ccc1-c1cccc(OCCN)c1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H33N3O5S/c1-17-15-19(7-8-22(17)20-5-4-6-21(16-20)32-14-11-25)18-9-12-27(13-10-18)33(30,31)24(2,3)23(28)26-29/h4-8,15-16,18,29H,9-14,25H2,1-3H3,(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50097273
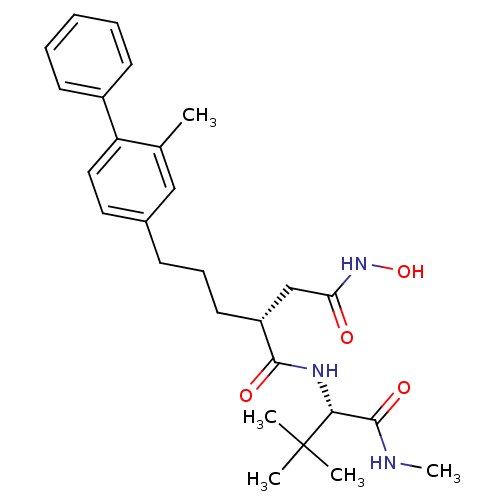
((R)-N*1*-((S)-2,2-dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C27H37N3O4/c1-18-16-19(14-15-22(18)20-11-7-6-8-12-20)10-9-13-21(17-23(31)30-34)25(32)29-24(26(33)28-5)27(2,3)4/h6-8,11-12,14-16,21,24,34H,9-10,13,17H2,1-5H3,(H,28,33)(H,29,32)(H,30,31)/t21-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Concentration required to inhibit the catalytic domain Matrix metalloprotease-3 using Nagase fluorogenic as a substrate. |
Bioorg Med Chem Lett 11: 571-4 (2001)
BindingDB Entry DOI: 10.7270/Q22B8X9X |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50097273

((R)-N*1*-((S)-2,2-dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C27H37N3O4/c1-18-16-19(14-15-22(18)20-11-7-6-8-12-20)10-9-13-21(17-23(31)30-34)25(32)29-24(26(33)28-5)27(2,3)4/h6-8,11-12,14-16,21,24,34H,9-10,13,17H2,1-5H3,(H,28,33)(H,29,32)(H,30,31)/t21-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 2223-68 (2007)
Article DOI: 10.1016/j.bmc.2007.01.011
BindingDB Entry DOI: 10.7270/Q2571DBD |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-3 (MMP-3) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063915

((2R,3S)-N*4*-Hydroxy-2-isobutyl-3-methyl-N*1*-[(S)...)Show SMILES CNC(=O)[C@H](Cc1cccc2CCCCc12)NC(=O)[C@H](CC(C)C)[C@H](C)C(=O)NO Show InChI InChI=1S/C23H35N3O4/c1-14(2)12-19(15(3)21(27)26-30)22(28)25-20(23(29)24-4)13-17-10-7-9-16-8-5-6-11-18(16)17/h7,9-10,14-15,19-20,30H,5-6,8,11-13H2,1-4H3,(H,24,29)(H,25,28)(H,26,27)/t15-,19+,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-3 (MMP-3). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Kanebo Ltd.
Curated by ChEMBL
| Assay Description
Activity against Matrix metalloproteinase-3 (MMP-3). |
J Med Chem 41: 1209-17 (1998)
Article DOI: 10.1021/jm970404a
BindingDB Entry DOI: 10.7270/Q2SQ8ZHB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50104001
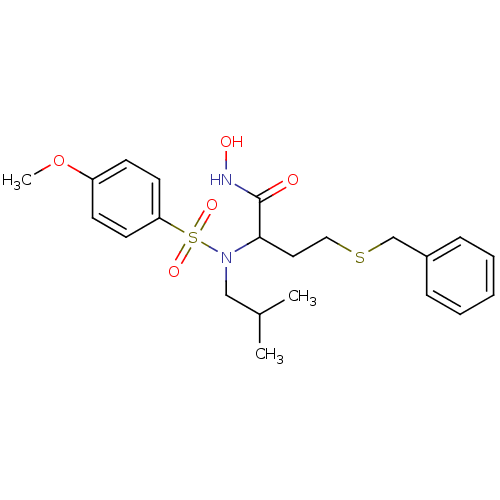
(4-(benzylthio)-N-hydroxy-2-(N-isobutyl-4-methoxyph...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C(CCSCc1ccccc1)C(=O)NO Show InChI InChI=1S/C22H30N2O5S2/c1-17(2)15-24(31(27,28)20-11-9-19(29-3)10-12-20)21(22(25)23-26)13-14-30-16-18-7-5-4-6-8-18/h4-12,17,21,26H,13-16H2,1-3H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma Deutschland GmbH
Curated by ChEMBL
| Assay Description
Binding affinity to MMP3 |
J Med Chem 49: 51-69 (2006)
Article DOI: 10.1021/jm050363f
BindingDB Entry DOI: 10.7270/Q20K285S |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50104001

(4-(benzylthio)-N-hydroxy-2-(N-isobutyl-4-methoxyph...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)C(CCSCc1ccccc1)C(=O)NO Show InChI InChI=1S/C22H30N2O5S2/c1-17(2)15-24(31(27,28)20-11-9-19(29-3)10-12-20)21(22(25)23-26)13-14-30-16-18-7-5-4-6-8-18/h4-12,17,21,26H,13-16H2,1-3H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3 |
J Med Chem 44: 3066-73 (2001)
BindingDB Entry DOI: 10.7270/Q27S7N24 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50078566
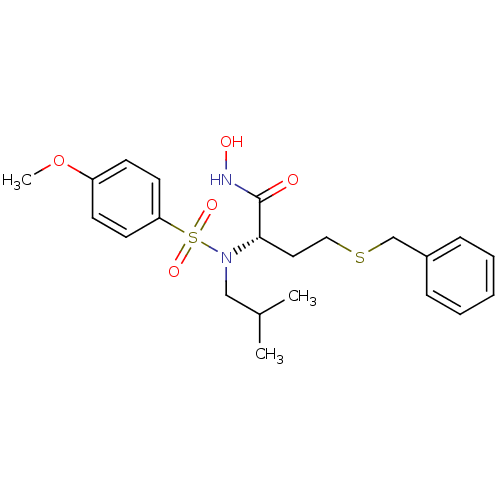
((S)-4-Benzylsulfanyl-N-hydroxy-2-[isobutyl-(4-meth...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@@H](CCSCc1ccccc1)C(=O)NO Show InChI InChI=1S/C22H30N2O5S2/c1-17(2)15-24(31(27,28)20-11-9-19(29-3)10-12-20)21(22(25)23-26)13-14-30-16-18-7-5-4-6-8-18/h4-12,17,21,26H,13-16H2,1-3H3,(H,23,25)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 |
Bioorg Med Chem Lett 9: 1691-6 (1999)
BindingDB Entry DOI: 10.7270/Q20002K2 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082537
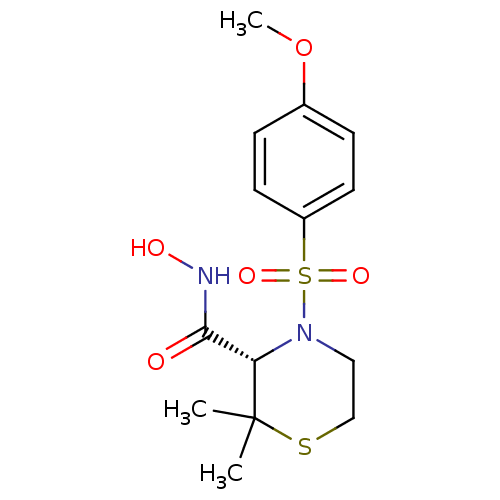
((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-thi...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCSC(C)(C)[C@@H]1C(=O)NO Show InChI InChI=1S/C14H20N2O5S2/c1-14(2)12(13(17)15-18)16(8-9-22-14)23(19,20)11-6-4-10(21-3)5-7-11/h4-7,12,18H,8-9H2,1-3H3,(H,15,17)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3. |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082545
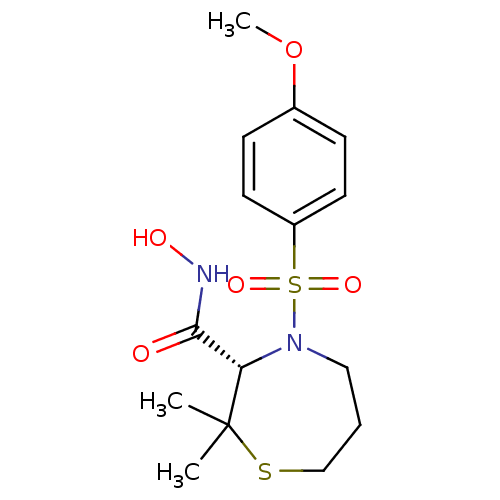
((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-[1,...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCCSC(C)(C)[C@@H]1C(=O)NO Show InChI InChI=1S/C15H22N2O5S2/c1-15(2)13(14(18)16-19)17(9-4-10-23-15)24(20,21)12-7-5-11(22-3)6-8-12/h5-8,13,19H,4,9-10H2,1-3H3,(H,16,18)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3. |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082545

((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-[1,...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCCSC(C)(C)[C@@H]1C(=O)NO Show InChI InChI=1S/C15H22N2O5S2/c1-15(2)13(14(18)16-19)17(9-4-10-23-15)24(20,21)12-7-5-11(22-3)6-8-12/h5-8,13,19H,4,9-10H2,1-3H3,(H,16,18)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrin |
J Med Chem 61: 2166-2210 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00315
BindingDB Entry DOI: 10.7270/Q2B56N68 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50254802
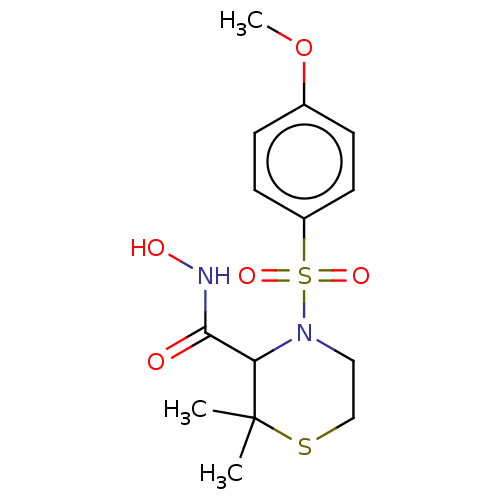
(CHEMBL4088630)Show InChI InChI=1S/C14H20N2O5S2/c1-14(2)12(13(17)15-18)16(8-9-22-14)23(19,20)11-6-4-10(21-3)5-7-11/h4-7,12,18H,8-9H2,1-3H3,(H,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences , St. John's University , Queens , New York 11439 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of truncated human recombinant MMP3 catalytic domain expressed in Escherichia coli BL21(DE3) after 3 hrs in presence of [H]-transferrin |
J Med Chem 61: 2166-2210 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00315
BindingDB Entry DOI: 10.7270/Q2B56N68 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50104006
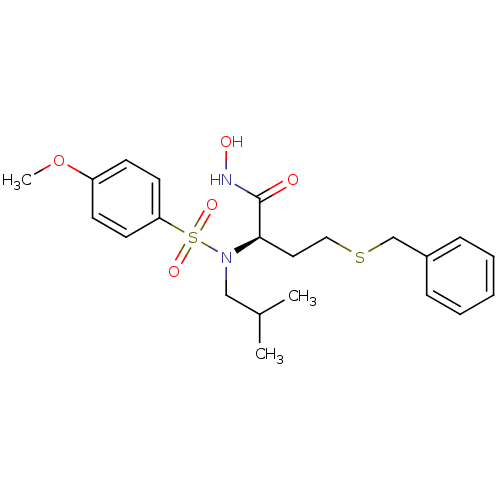
(4-Benzylsulfanyl-N-hydroxy-2-[isobutyl-(4-methoxy-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(C)C)[C@H](CCSCc1ccccc1)C(=O)NO Show InChI InChI=1S/C22H30N2O5S2/c1-17(2)15-24(31(27,28)20-11-9-19(29-3)10-12-20)21(22(25)23-26)13-14-30-16-18-7-5-4-6-8-18/h4-12,17,21,26H,13-16H2,1-3H3,(H,23,25)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 |
J Med Chem 44: 3074-82 (2001)
BindingDB Entry DOI: 10.7270/Q2416WBQ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082542
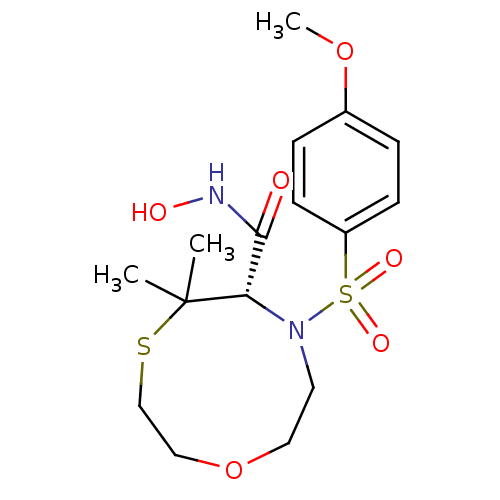
((S)-7-(4-Methoxy-benzenesulfonyl)-5,5-dimethyl-[1,...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCOCCSC(C)(C)[C@@H]1C(=O)NO Show InChI InChI=1S/C16H24N2O6S2/c1-16(2)14(15(19)17-20)18(8-9-24-10-11-25-16)26(21,22)13-6-4-12(23-3)5-7-13/h4-7,14,20H,8-11H2,1-3H3,(H,17,19)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3. |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082545

((S)-4-(4-Methoxy-benzenesulfonyl)-2,2-dimethyl-[1,...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CCCSC(C)(C)[C@@H]1C(=O)NO Show InChI InChI=1S/C15H22N2O5S2/c1-15(2)13(14(18)16-19)17(9-4-10-23-15)24(20,21)12-7-5-11(22-3)6-8-12/h5-8,13,19H,4,9-10H2,1-3H3,(H,16,18)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a |
Pomona College
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 15: 2223-68 (2007)
Article DOI: 10.1016/j.bmc.2007.01.011
BindingDB Entry DOI: 10.7270/Q2571DBD |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Ability to inhibit the matrix metalloprotease-3 by method of Knight et al using the fluorogenic peptide substrate. |
Bioorg Med Chem Lett 11: 567-70 (2001)
BindingDB Entry DOI: 10.7270/Q2610ZK1 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50287379

(4-[(S)-2-((R)-2-Hydroxycarbamoylmethyl-4-methyl-pe...)Show SMILES COC(=O)c1ccc(NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)CC(=O)NO)cc1 Show InChI InChI=1S/C22H33N3O6/c1-13(2)10-16(12-19(26)25-30)20(27)24-18(11-14(3)4)21(28)23-17-8-6-15(7-9-17)22(29)31-5/h6-9,13-14,16,18,30H,10-12H2,1-5H3,(H,23,28)(H,24,27)(H,25,26)/t16-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human fibroblast stromelysin, matrix metalloproteinase-3 |
Bioorg Med Chem Lett 6: 1601-1606 (1996)
Article DOI: 10.1016/S0960-894X(96)00283-1
BindingDB Entry DOI: 10.7270/Q2WD40JH |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50103993
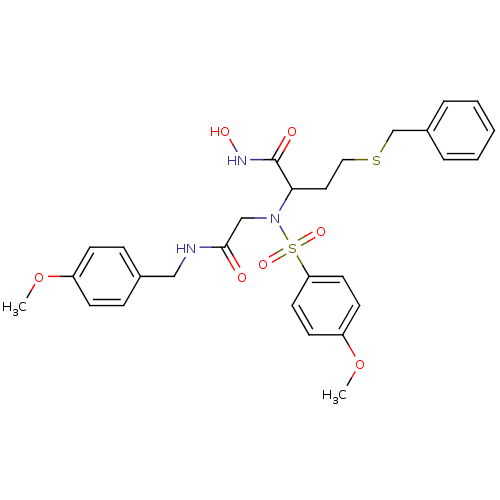
(4-Benzylsulfanyl-N-hydroxy-2-{(4-methoxy-benzenesu...)Show SMILES COc1ccc(CNC(=O)CN(C(CCSCc2ccccc2)C(=O)NO)S(=O)(=O)c2ccc(OC)cc2)cc1 Show InChI InChI=1S/C28H33N3O7S2/c1-37-23-10-8-21(9-11-23)18-29-27(32)19-31(40(35,36)25-14-12-24(38-2)13-15-25)26(28(33)30-34)16-17-39-20-22-6-4-3-5-7-22/h3-15,26,34H,16-20H2,1-2H3,(H,29,32)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3 |
J Med Chem 44: 3066-73 (2001)
BindingDB Entry DOI: 10.7270/Q27S7N24 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227722

(CHEMBL398641 | N-hydroxy-2-{4-[3'-(2-hydroxy-ethox...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)c1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C24H32N2O6S/c1-17-15-19(7-8-22(17)20-5-4-6-21(16-20)32-14-13-27)18-9-11-26(12-10-18)33(30,31)24(2,3)23(28)25-29/h4-8,15-16,18,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227709

(CHEMBL251917 | N-hydroxy-2-(4-(4-(6-(2-hydroxyetho...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)n1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-15-18(7-8-19(16)20-5-4-6-21(24-20)32-14-13-27)17-9-11-26(12-10-17)33(30,31)23(2,3)22(28)25-29/h4-8,15,17,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Athens
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem 16: 8781-94 (2008)
Article DOI: 10.1016/j.bmc.2008.08.058
BindingDB Entry DOI: 10.7270/Q2JD4WM2 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50084206
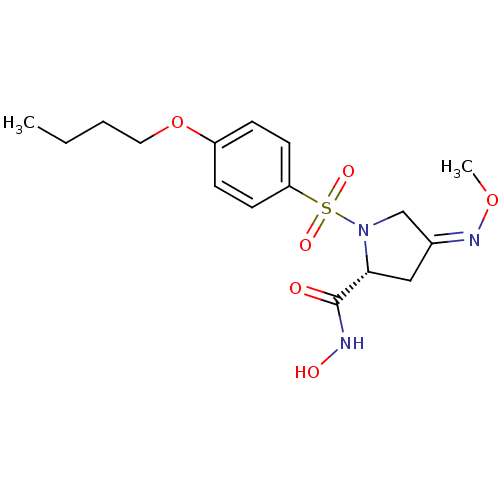
((R)-1-(4-Butoxy-benzenesulfonyl)-4-[(Z)-methoxyimi...)Show SMILES CCCCOc1ccc(cc1)S(=O)(=O)N1C\C(C[C@@H]1C(=O)NO)=N/OC Show InChI InChI=1S/C16H23N3O6S/c1-3-4-9-25-13-5-7-14(8-6-13)26(22,23)19-11-12(18-24-2)10-15(19)16(20)17-21/h5-8,15,21H,3-4,9-11H2,1-2H3,(H,17,20)/b18-12-/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
In vitro inhibition activity of human recombinant stromelysin (Matrix metalloproteinase-3) |
J Med Chem 42: 5426-36 (2000)
BindingDB Entry DOI: 10.7270/Q2Q23ZGT |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227725
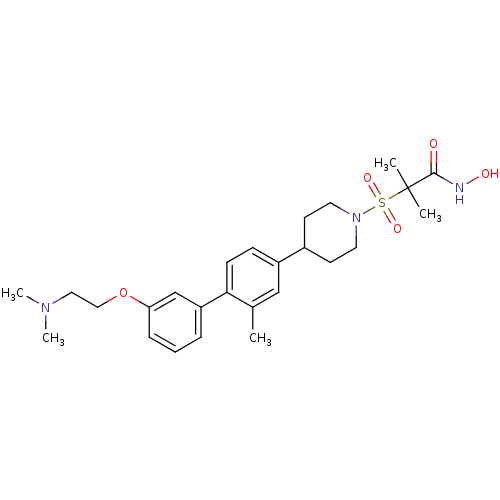
(2-{4-[3'-(2-dimethylamino-ethoxy)-2-methyl-bipheny...)Show SMILES CN(C)CCOc1cccc(c1)-c1ccc(cc1C)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C26H37N3O5S/c1-19-17-21(9-10-24(19)22-7-6-8-23(18-22)34-16-15-28(4)5)20-11-13-29(14-12-20)35(32,33)26(2,3)25(30)27-31/h6-10,17-18,20,31H,11-16H2,1-5H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082550
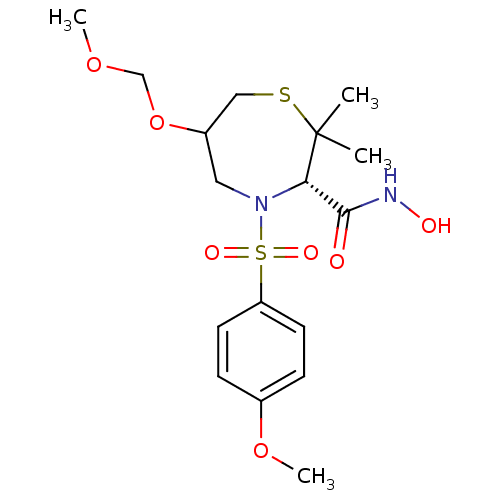
((S)-4-(4-Methoxy-benzenesulfonyl)-6-methoxymethoxy...)Show SMILES COCOC1CSC(C)(C)[C@@H](N(C1)S(=O)(=O)c1ccc(OC)cc1)C(=O)NO Show InChI InChI=1S/C17H26N2O7S2/c1-17(2)15(16(20)18-21)19(9-13(10-27-17)26-11-24-3)28(22,23)14-7-5-12(25-4)6-8-14/h5-8,13,15,21H,9-11H2,1-4H3,(H,18,20)/t13?,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3. |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50227709

(CHEMBL251917 | N-hydroxy-2-(4-(4-(6-(2-hydroxyetho...)Show SMILES Cc1cc(ccc1-c1cccc(OCCO)n1)C1CCN(CC1)S(=O)(=O)C(C)(C)C(=O)NO Show InChI InChI=1S/C23H31N3O6S/c1-16-15-18(7-8-19(16)20-5-4-6-21(24-20)32-14-13-27)17-9-11-26(12-10-17)33(30,31)23(2,3)22(28)25-29/h4-8,15,17,27,29H,9-14H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 17: 6750-3 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.042
BindingDB Entry DOI: 10.7270/Q20K289K |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-3 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50103988
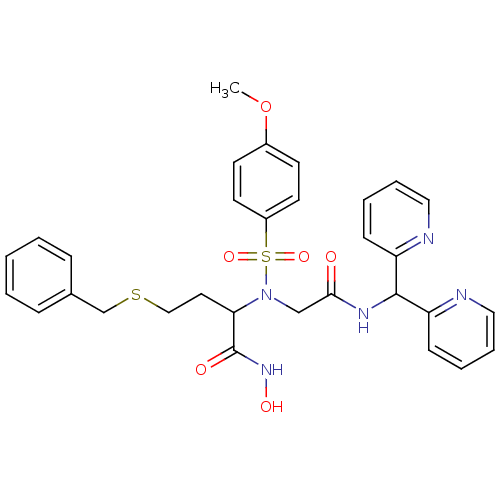
(4-Benzylsulfanyl-2-[{[(di-pyridin-2-yl-methyl)-car...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(=O)NC(c1ccccn1)c1ccccn1)C(CCSCc1ccccc1)C(=O)NO Show InChI InChI=1S/C31H33N5O6S2/c1-42-24-13-15-25(16-14-24)44(40,41)36(28(31(38)35-39)17-20-43-22-23-9-3-2-4-10-23)21-29(37)34-30(26-11-5-7-18-32-26)27-12-6-8-19-33-27/h2-16,18-19,28,30,39H,17,20-22H2,1H3,(H,34,37)(H,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3 |
J Med Chem 44: 3066-73 (2001)
BindingDB Entry DOI: 10.7270/Q27S7N24 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50096651
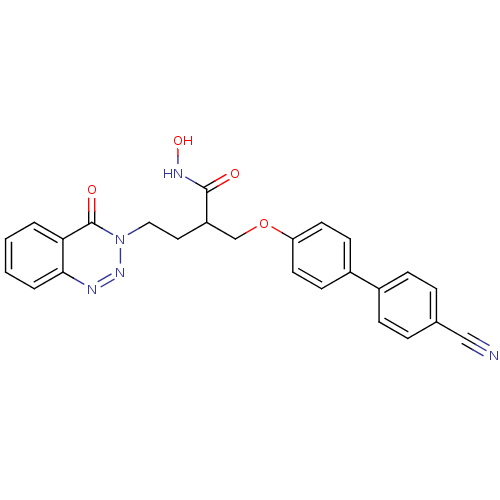
(2-(4'-Cyano-biphenyl-4-yloxymethyl)-N-hydroxy-4-(4...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)COc1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C25H21N5O4/c26-15-17-5-7-18(8-6-17)19-9-11-21(12-10-19)34-16-20(24(31)28-33)13-14-30-25(32)22-3-1-2-4-23(22)27-29-30/h1-12,20,33H,13-14,16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-3 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082534
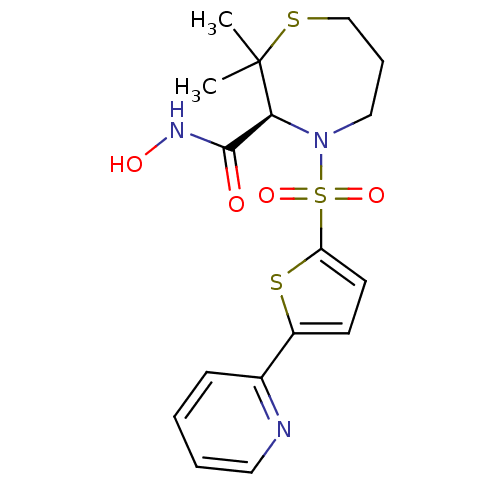
((S)-2,2-Dimethyl-4-(5-pyridin-2-yl-thiophene-2-sul...)Show SMILES CC1(C)SCCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(s1)-c1ccccn1 Show InChI InChI=1S/C17H21N3O4S3/c1-17(2)15(16(21)19-22)20(10-5-11-25-17)27(23,24)14-8-7-13(26-14)12-6-3-4-9-18-12/h3-4,6-9,15,22H,5,10-11H2,1-2H3,(H,19,21)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3. |
J Med Chem 42: 4547-62 (1999)
BindingDB Entry DOI: 10.7270/Q2D79C32 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP3) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131944
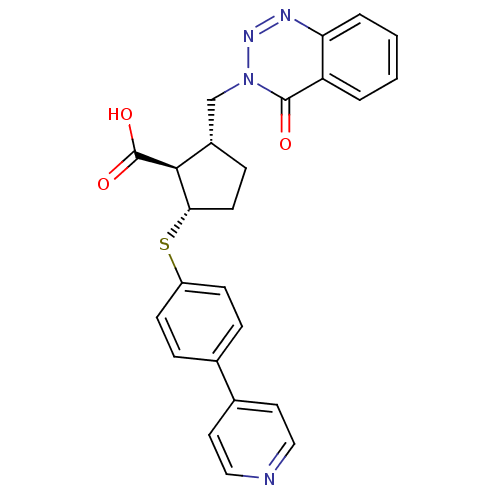
(2-(4-Oxo-4H-benzo[d][1,2,3]triazin-3-ylmethyl)-5-(...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C25H22N4O3S/c30-24-20-3-1-2-4-21(20)27-28-29(24)15-18-7-10-22(23(18)25(31)32)33-19-8-5-16(6-9-19)17-11-13-26-14-12-17/h1-6,8-9,11-14,18,22-23H,7,10,15H2,(H,31,32)/t18-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP3) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50072563
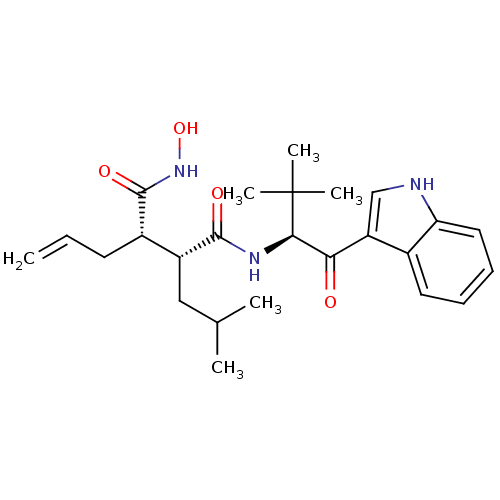
((2R,3S)-2-Allyl-N*1*-hydroxy-N*4*-[(S)-1-(1H-indol...)Show SMILES CC(C)C[C@H]([C@H](CC=C)C(=O)NO)C(=O)N[C@H](C(=O)c1c[nH]c2ccccc12)C(C)(C)C Show InChI InChI=1S/C25H35N3O4/c1-7-10-17(24(31)28-32)18(13-15(2)3)23(30)27-22(25(4,5)6)21(29)19-14-26-20-12-9-8-11-16(19)20/h7-9,11-12,14-15,17-18,22,26,32H,1,10,13H2,2-6H3,(H,27,30)(H,28,31)/t17-,18+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50072583
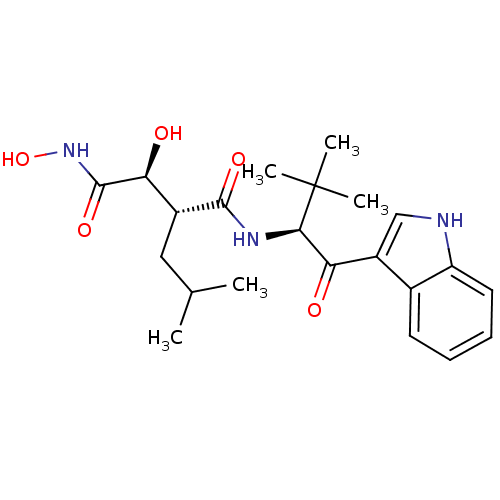
((2R,3S)-2,N*1*-Dihydroxy-N*4*-[(S)-1-(1H-indole-3-...)Show SMILES CC(C)C[C@H]([C@H](O)C(=O)NO)C(=O)N[C@H](C(=O)c1c[nH]c2ccccc12)C(C)(C)C Show InChI InChI=1S/C22H31N3O5/c1-12(2)10-14(18(27)21(29)25-30)20(28)24-19(22(3,4)5)17(26)15-11-23-16-9-7-6-8-13(15)16/h6-9,11-12,14,18-19,23,27,30H,10H2,1-5H3,(H,24,28)(H,25,29)/t14-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant matrix metalloproteinase 3 |
Bioorg Med Chem Lett 8: 3251-6 (1999)
BindingDB Entry DOI: 10.7270/Q289152J |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50098915
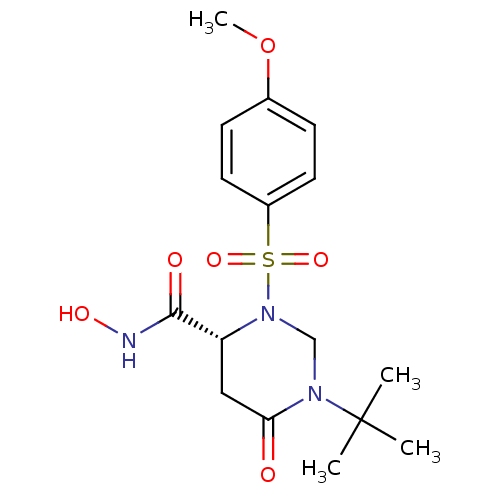
((R)-1-tert-Butyl-3-(4-methoxy-benzenesulfonyl)-6-o...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CN(C(=O)C[C@@H]1C(=O)NO)C(C)(C)C Show InChI InChI=1S/C16H23N3O6S/c1-16(2,3)18-10-19(13(9-14(18)20)15(21)17-22)26(23,24)12-7-5-11(25-4)6-8-12/h5-8,13,22H,9-10H2,1-4H3,(H,17,21)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-3 |
Bioorg Med Chem Lett 11: 1009-13 (2001)
BindingDB Entry DOI: 10.7270/Q2X34Z04 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50203953
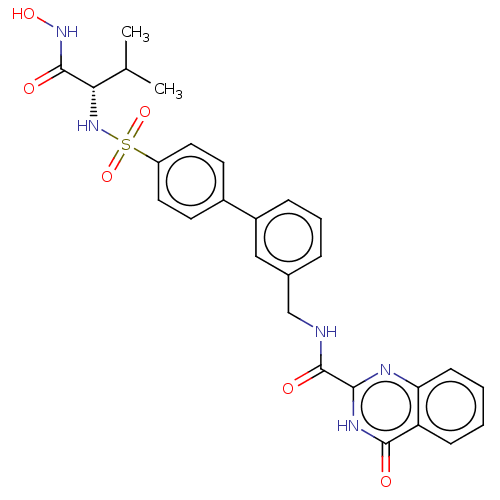
(CHEMBL3932562)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1cccc(CNC(=O)c2nc3ccccc3c(=O)[nH]2)c1)C(=O)NO |r| Show InChI InChI=1S/C27H27N5O6S/c1-16(2)23(26(34)31-36)32-39(37,38)20-12-10-18(11-13-20)19-7-5-6-17(14-19)15-28-27(35)24-29-22-9-4-3-8-21(22)25(33)30-24/h3-14,16,23,32,36H,15H2,1-2H3,(H,28,35)(H,31,34)(H,29,30,33)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human AMPA-activated MMP3 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method |
Bioorg Med Chem 24: 6149-6165 (2016)
Article DOI: 10.1016/j.bmc.2016.09.009
BindingDB Entry DOI: 10.7270/Q2H1341V |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50071267
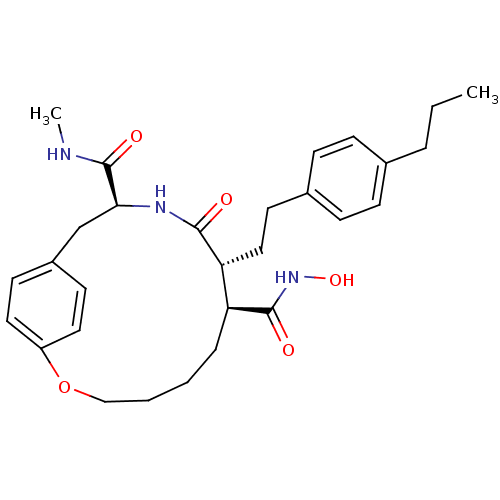
((7S,8R,11S)-9-Oxo-8-[2-(4-propyl-phenyl)-ethyl]-2-...)Show SMILES CCCc1ccc(CC[C@@H]2[C@H](CCCCOc3ccc(C[C@H](NC2=O)C(=O)NC)cc3)C(=O)NO)cc1 Show InChI InChI=1S/C29H39N3O5/c1-3-6-20-8-10-21(11-9-20)14-17-25-24(28(34)32-36)7-4-5-18-37-23-15-12-22(13-16-23)19-26(29(35)30-2)31-27(25)33/h8-13,15-16,24-26,36H,3-7,14,17-19H2,1-2H3,(H,30,35)(H,31,33)(H,32,34)/t24-,25+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of fibroblast stromelysin (MMP-3) |
Bioorg Med Chem Lett 8: 2087-92 (1999)
BindingDB Entry DOI: 10.7270/Q2MW2G89 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM11868

(CHEMBL256157 | N-Hydroxy-4-{[(4-phenoxyphenyl)sulf...)Show SMILES ONC(=O)C1(CS(=O)(=O)c2ccc(Oc3ccccc3)cc2)CCN(CC#C)CC1 Show InChI InChI=1S/C22H24N2O5S/c1-2-14-24-15-12-22(13-16-24,21(25)23-26)17-30(27,28)20-10-8-19(9-11-20)29-18-6-4-3-5-7-18/h1,3-11,26H,12-17H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Incyte Corporation
Curated by ChEMBL
| Assay Description
Inhibition of MMP3 |
Bioorg Med Chem Lett 18: 560-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.086
BindingDB Entry DOI: 10.7270/Q2CN73MJ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50104002
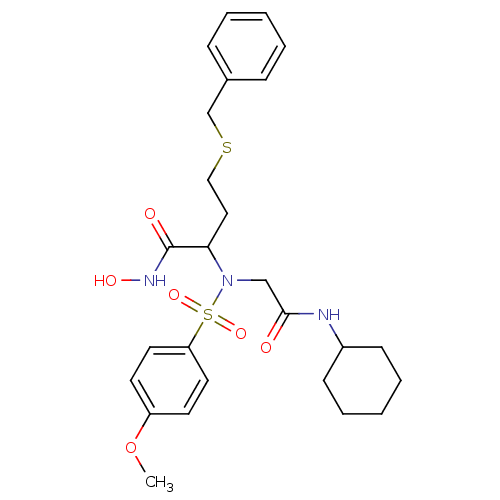
(4-Benzylsulfanyl-2-[cyclohexylcarbamoylmethyl-(4-m...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(=O)NC1CCCCC1)C(CCSCc1ccccc1)C(=O)NO Show InChI InChI=1S/C26H35N3O6S2/c1-35-22-12-14-23(15-13-22)37(33,34)29(18-25(30)27-21-10-6-3-7-11-21)24(26(31)28-32)16-17-36-19-20-8-4-2-5-9-20/h2,4-5,8-9,12-15,21,24,32H,3,6-7,10-11,16-19H2,1H3,(H,27,30)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3 |
J Med Chem 44: 3066-73 (2001)
BindingDB Entry DOI: 10.7270/Q27S7N24 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50103992
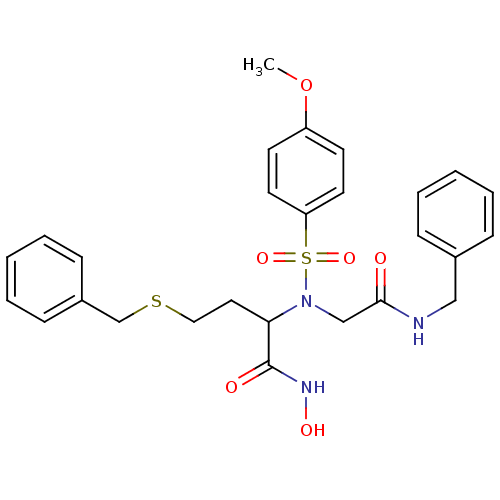
(2-[(Benzylcarbamoyl-methyl)-(4-methoxy-benzenesulf...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(CC(=O)NCc1ccccc1)C(CCSCc1ccccc1)C(=O)NO Show InChI InChI=1S/C27H31N3O6S2/c1-36-23-12-14-24(15-13-23)38(34,35)30(19-26(31)28-18-21-8-4-2-5-9-21)25(27(32)29-33)16-17-37-20-22-10-6-3-7-11-22/h2-15,25,33H,16-20H2,1H3,(H,28,31)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against matrix metalloprotease-3 |
J Med Chem 44: 3066-73 (2001)
BindingDB Entry DOI: 10.7270/Q27S7N24 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data