 Found 327 hits of ic50 data for polymerid = 1233
Found 327 hits of ic50 data for polymerid = 1233 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10881

(CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...)Show InChI InChI=1S/C5H8N4O3S2/c1-3(10)7-4-9(2)8-5(13-4)14(6,11)12/h1-2H3,(H2,6,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 62: 597-604 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.030
BindingDB Entry DOI: 10.7270/Q2X92CNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50430551
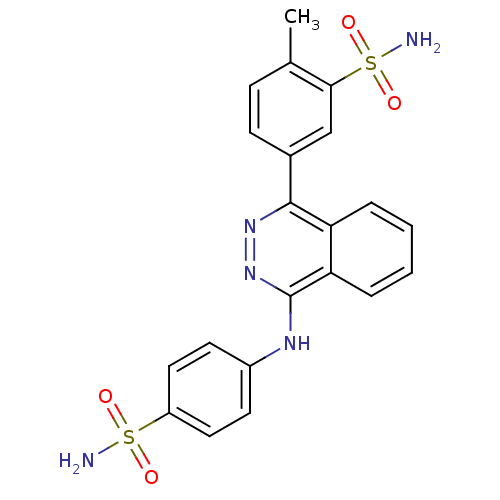
(CHEMBL2336905)Show SMILES Cc1ccc(cc1S(N)(=O)=O)-c1nnc(Nc2ccc(cc2)S(N)(=O)=O)c2ccccc12 Show InChI InChI=1S/C21H19N5O4S2/c1-13-6-7-14(12-19(13)32(23,29)30)20-17-4-2-3-5-18(17)21(26-25-20)24-15-8-10-16(11-9-15)31(22,27)28/h2-12H,1H3,(H,24,26)(H2,22,27,28)(H2,23,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 62: 597-604 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.030
BindingDB Entry DOI: 10.7270/Q2X92CNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10882

(6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...)Show InChI InChI=1S/C9H10N2O3S2/c1-2-14-6-3-4-7-8(5-6)15-9(11-7)16(10,12)13/h3-5H,2H2,1H3,(H2,10,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50005003

(CHEMBL2392423)Show InChI InChI=1S/C14H13N3O3S2/c15-22(19,20)12-8-6-11(7-9-12)16-14(21)17-13(18)10-4-2-1-3-5-10/h1-9H,(H2,15,19,20)(H2,16,17,18,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50005002

(CHEMBL2392424)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1cccc1\C=C1\C(=O)NN(C1=O)c1ccc(F)cc1 Show InChI InChI=1S/C20H15FN4O4S/c21-13-3-5-15(6-4-13)25-20(27)18(19(26)23-25)12-16-2-1-11-24(16)14-7-9-17(10-8-14)30(22,28)29/h1-12H,(H,23,26)(H2,22,28,29)/b18-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50430550
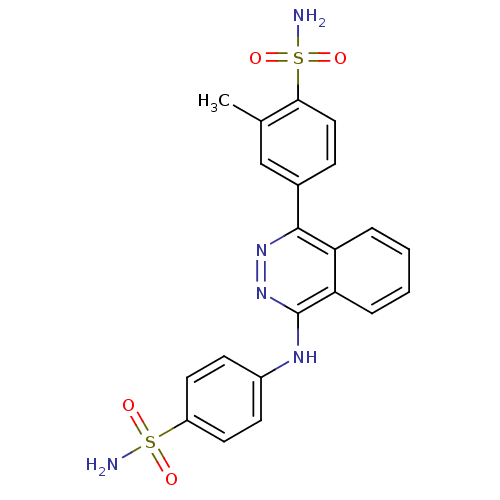
(CHEMBL2336906)Show SMILES Cc1cc(ccc1S(N)(=O)=O)-c1nnc(Nc2ccc(cc2)S(N)(=O)=O)c2ccccc12 Show InChI InChI=1S/C21H19N5O4S2/c1-13-12-14(6-11-19(13)32(23,29)30)20-17-4-2-3-5-18(17)21(26-25-20)24-15-7-9-16(10-8-15)31(22,27)28/h2-12H,1H3,(H,24,26)(H2,22,27,28)(H2,23,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 62: 597-604 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.030
BindingDB Entry DOI: 10.7270/Q2X92CNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50546171
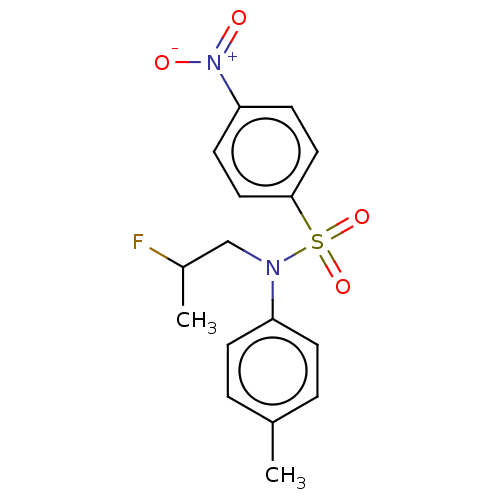
(CHEMBL4790443)Show SMILES CC(F)CN(c1ccc(C)cc1)S(=O)(=O)c1ccc(cc1)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA9 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2019.111731
BindingDB Entry DOI: 10.7270/Q24B34WR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50005000
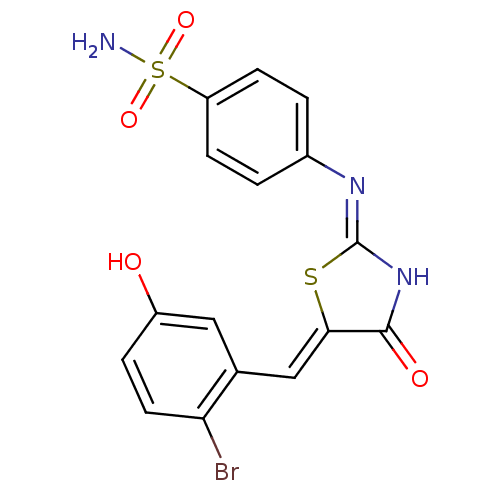
(CHEMBL2392425)Show SMILES NS(=O)(=O)c1ccc(cc1)\N=C1\NC(=O)\C(S1)=C\c1cc(O)ccc1Br Show InChI InChI=1S/C16H12BrN3O4S2/c17-13-6-3-11(21)7-9(13)8-14-15(22)20-16(25-14)19-10-1-4-12(5-2-10)26(18,23)24/h1-8,21H,(H2,18,23,24)(H,19,20,22)/b14-8- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50589087
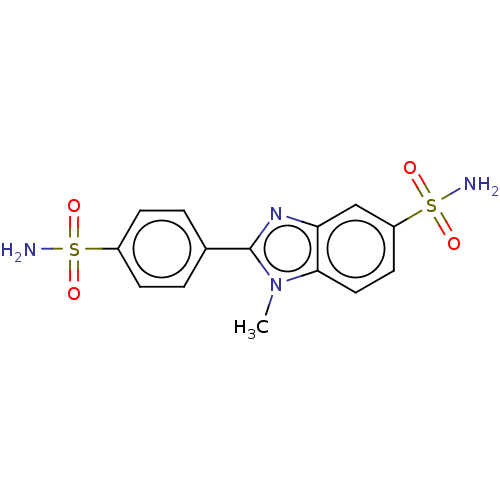
(CHEMBL5199602)Show SMILES Cn1c(nc2cc(ccc12)S(N)(=O)=O)-c1ccc(cc1)S(N)(=O)=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50589086

(CHEMBL5195955) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248709
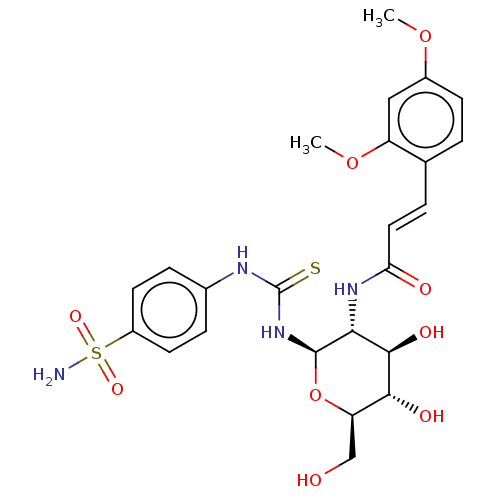
(CHEMBL4103517)Show SMILES COc1ccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c(OC)c1 |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-15-7-3-13(17(11-15)36-2)4-10-19(30)27-20-22(32)21(31)18(12-29)37-23(20)28-24(38)26-14-5-8-16(9-6-14)39(25,33)34/h3-11,18,20-23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b10-4+/t18-,20-,21-,22-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 62: 597-604 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.030
BindingDB Entry DOI: 10.7270/Q2X92CNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50004999
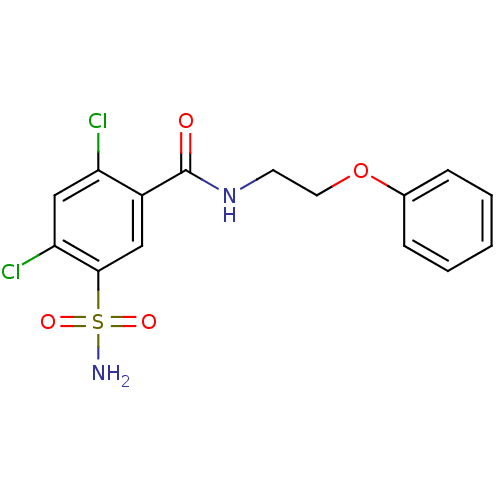
(CHEMBL1387347)Show SMILES NS(=O)(=O)c1cc(C(=O)NCCOc2ccccc2)c(Cl)cc1Cl Show InChI InChI=1S/C15H14Cl2N2O4S/c16-12-9-13(17)14(24(18,21)22)8-11(12)15(20)19-6-7-23-10-4-2-1-3-5-10/h1-5,8-9H,6-7H2,(H,19,20)(H2,18,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50004998
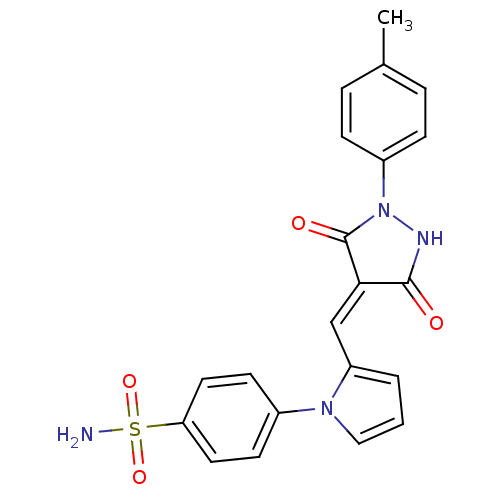
(CHEMBL2392426)Show SMILES Cc1ccc(cc1)N1NC(=O)\C(=C\c2cccn2-c2ccc(cc2)S(N)(=O)=O)C1=O Show InChI InChI=1S/C21H18N4O4S/c1-14-4-6-16(7-5-14)25-21(27)19(20(26)23-25)13-17-3-2-12-24(17)15-8-10-18(11-9-15)30(22,28)29/h2-13H,1H3,(H,23,26)(H2,22,28,29)/b19-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against carbonic anhydrase IX |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 20 |
University of Marburg
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 47: 550-7 (2004)
Article DOI: 10.1021/jm030912m
BindingDB Entry DOI: 10.7270/Q2W957DZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50004961

(CHEMBL2392428)Show SMILES CC1=NN(C(=O)\C1=C/C=C/c1ccccc1)c1ccc(cc1)S(N)(=O)=O |t:1| Show InChI InChI=1S/C19H17N3O3S/c1-14-18(9-5-8-15-6-3-2-4-7-15)19(23)22(21-14)16-10-12-17(13-11-16)26(20,24)25/h2-13H,1H3,(H2,20,24,25)/b8-5+,18-9- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50430552

(CHEMBL2336904)Show SMILES NS(=O)(=O)c1ccc(Nc2nnc(-c3cccc(c3)S(N)(=O)=O)c3ccccc23)cc1 Show InChI InChI=1S/C20H17N5O4S2/c21-30(26,27)15-10-8-14(9-11-15)23-20-18-7-2-1-6-17(18)19(24-25-20)13-4-3-5-16(12-13)31(22,28)29/h1-12H,(H,23,25)(H2,21,26,27)(H2,22,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 62: 597-604 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.030
BindingDB Entry DOI: 10.7270/Q2X92CNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50004997

(CHEMBL2392427)Show InChI InChI=1S/C19H20N2O2S/c20-24(22,23)18-10-8-15(9-11-18)12-13-21-14-17-6-3-5-16-4-1-2-7-19(16)17/h1-11,21H,12-14H2,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 |
Bioorg Med Chem Lett 18: 1898-903 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.008
BindingDB Entry DOI: 10.7270/Q2RN37M3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50004959
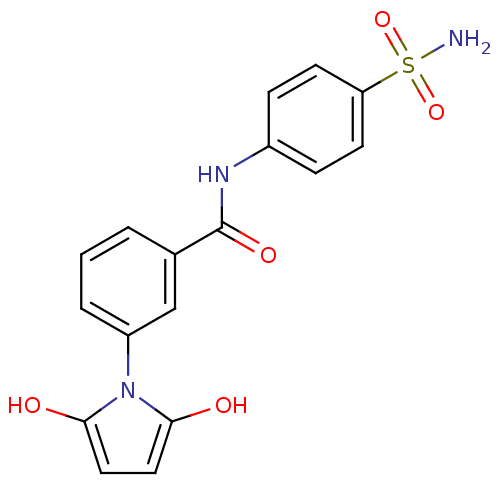
(CHEMBL2392429)Show SMILES NS(=O)(=O)c1ccc(NC(=O)c2cccc(c2)N2C(=O)CCC2=O)cc1 Show InChI InChI=1S/C17H15N3O5S/c18-26(24,25)14-6-4-12(5-7-14)19-17(23)11-2-1-3-13(10-11)20-15(21)8-9-16(20)22/h1-7,10H,8-9H2,(H,19,23)(H2,18,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM103909
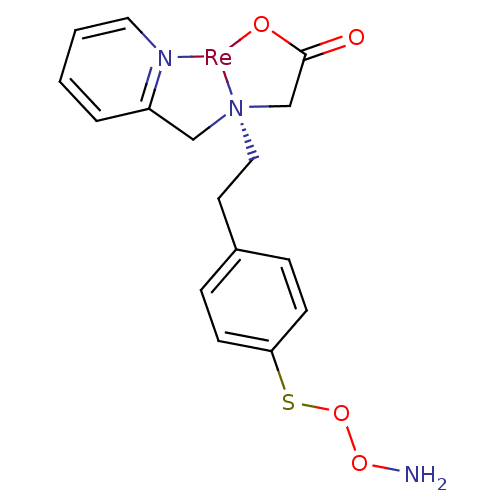
(JNK3 inhibitor 6 | US8562945, 242)Show SMILES NOOSc1ccc(CC[N@@]23CC(=O)O[Re]2[N]2=C(C3)C=CC=C2)cc1 |r,c:17,21,23| Show InChI InChI=1S/C16H19N3O4S.Re/c17-22-23-24-15-6-4-13(5-7-15)8-10-19(12-16(20)21)11-14-3-1-2-9-18-14;/h1-7,9H,8,10-12,17H2,(H,20,21);/q;+1/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50542352
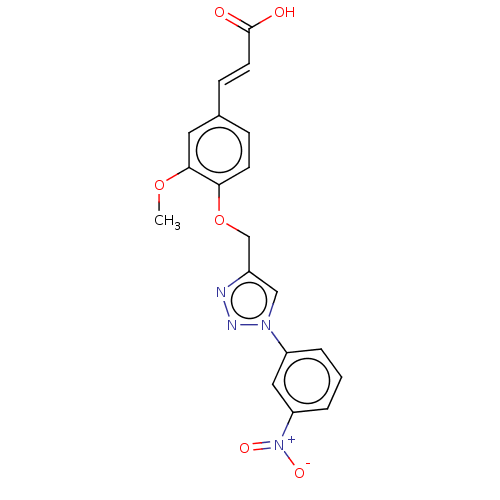
(CHEMBL4640224)Show SMILES COc1cc(\C=C\C(O)=O)ccc1OCc1cn(nn1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C19H16N4O6/c1-28-18-9-13(6-8-19(24)25)5-7-17(18)29-12-14-11-22(21-20-14)15-3-2-4-16(10-15)23(26)27/h2-11H,12H2,1H3,(H,24,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 using p-nitrophenyl acetate as substrate by UV-VIS spectrophotometric analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115424
BindingDB Entry DOI: 10.7270/Q22Z192W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492590
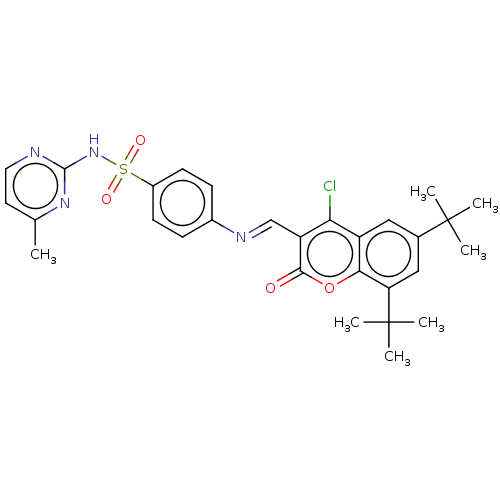
(CHEMBL2408085)Show SMILES Cc1ccnc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(cc(c3oc2=O)C(C)(C)C)C(C)(C)C)n1 Show InChI InChI=1S/C29H31ClN4O4S/c1-17-12-13-31-27(33-17)34-39(36,37)20-10-8-19(9-11-20)32-16-22-24(30)21-14-18(28(2,3)4)15-23(29(5,6)7)25(21)38-26(22)35/h8-16H,1-7H3,(H,31,33,34)/b32-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM13063

(4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...)Show InChI InChI=1S/C16H14N2O3S/c1-11-15(12-7-9-14(10-8-12)22(17,19)20)16(18-21-11)13-5-3-2-4-6-13/h2-10H,1H3,(H2,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | 20 |
University of Marburg
| Assay Description
An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... |
J Med Chem 47: 550-7 (2004)
Article DOI: 10.1021/jm030912m
BindingDB Entry DOI: 10.7270/Q2W957DZ |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 expressed in Escherichia coli BL21 D3 strain using p-nitrophenyl acetate as substrate by UV/visible spectrop... |
Eur J Med Chem 155: 13-23 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.034
BindingDB Entry DOI: 10.7270/Q2GM89V4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50004958
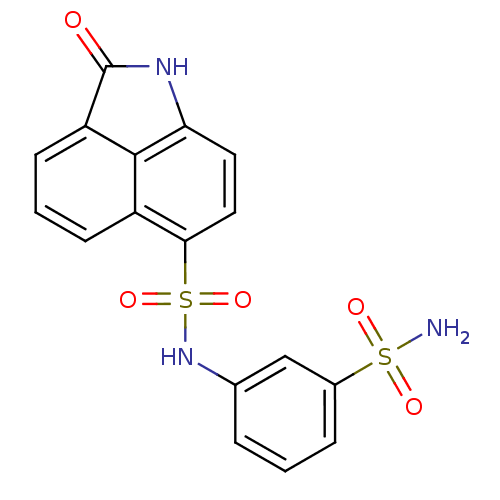
(CHEMBL2392430)Show SMILES NS(=O)(=O)c1cccc(NS(=O)(=O)c2ccc3NC(=O)c4cccc2c34)c1 Show InChI InChI=1S/C17H13N3O5S2/c18-26(22,23)11-4-1-3-10(9-11)20-27(24,25)15-8-7-14-16-12(15)5-2-6-13(16)17(21)19-14/h1-9,20H,(H,19,21)(H2,18,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant carbonic anhydrase-9 catalytic domain (unknown origin) |
Bioorg Med Chem Lett 23: 3496-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.048
BindingDB Entry DOI: 10.7270/Q2GH9KFD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 preincubated with enzyme for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112994
BindingDB Entry DOI: 10.7270/Q2CC14FP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50430540
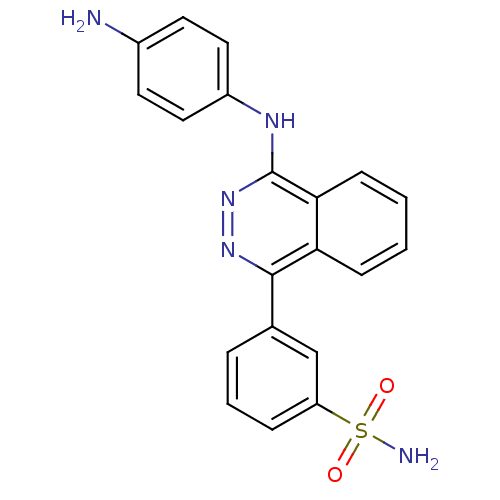
(CHEMBL2336902)Show SMILES Nc1ccc(Nc2nnc(-c3cccc(c3)S(N)(=O)=O)c3ccccc23)cc1 Show InChI InChI=1S/C20H17N5O2S/c21-14-8-10-15(11-9-14)23-20-18-7-2-1-6-17(18)19(24-25-20)13-4-3-5-16(12-13)28(22,26)27/h1-12H,21H2,(H,23,25)(H2,22,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 62: 597-604 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.030
BindingDB Entry DOI: 10.7270/Q2X92CNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50430539

(CHEMBL2336903)Show SMILES CC(C)(C)c1ccc(Nc2nnc(-c3cccc(c3)S(N)(=O)=O)c3ccccc23)cc1 Show InChI InChI=1S/C24H24N4O2S/c1-24(2,3)17-11-13-18(14-12-17)26-23-21-10-5-4-9-20(21)22(27-28-23)16-7-6-8-19(15-16)31(25,29)30/h4-15H,1-3H3,(H,26,28)(H2,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 after 15 mins by stopped flow CO2 hydration assay |
Eur J Med Chem 62: 597-604 (2013)
Article DOI: 10.1016/j.ejmech.2013.01.030
BindingDB Entry DOI: 10.7270/Q2X92CNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112923
BindingDB Entry DOI: 10.7270/Q21N852G |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human CA9 using 4-nitrophenylacetate as substrate by esterase assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128420
BindingDB Entry DOI: 10.7270/Q2VX0MC2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128291
BindingDB Entry DOI: 10.7270/Q2FT8QXR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Jamia Millia Islamia
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 using p-nitrophenyl acetate as substrate by UV-VIS spectrophotometric analysis |
Bioorg Med Chem 28: (2020)
Article DOI: 10.1016/j.bmc.2020.115424
BindingDB Entry DOI: 10.7270/Q22Z192W |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain |
Bioorg Med Chem Lett 18: 1898-903 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.008
BindingDB Entry DOI: 10.7270/Q2RN37M3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248718

(CHEMBL4075252)Show SMILES COc1cccc(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)c1OC |r| Show InChI InChI=1S/C24H30N4O9S2/c1-35-16-5-3-4-13(22(16)36-2)6-11-18(30)27-19-21(32)20(31)17(12-29)37-23(19)28-24(38)26-14-7-9-15(10-8-14)39(25,33)34/h3-11,17,19-21,23,29,31-32H,12H2,1-2H3,(H,27,30)(H2,25,33,34)(H2,26,28,38)/b11-6+/t17-,19-,20-,21-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492593
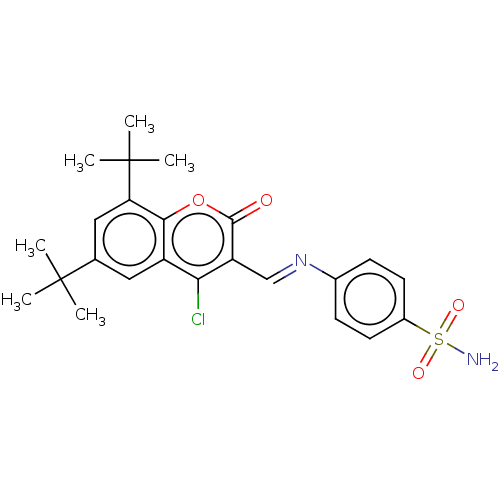
(CHEMBL2408080)Show SMILES CC(C)(C)c1cc(c2oc(=O)c(\C=N\c3ccc(cc3)S(N)(=O)=O)c(Cl)c2c1)C(C)(C)C Show InChI InChI=1S/C24H27ClN2O4S/c1-23(2,3)14-11-17-20(25)18(22(28)31-21(17)19(12-14)24(4,5)6)13-27-15-7-9-16(10-8-15)32(26,29)30/h7-13H,1-6H3,(H2,26,29,30)/b27-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 in presence of 0.01 mM tris(carboxyethyl)phosphine |
Bioorg Med Chem Lett 18: 1898-903 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.008
BindingDB Entry DOI: 10.7270/Q2RN37M3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 in presence of 0.01 mM threitol |
Bioorg Med Chem Lett 18: 1898-903 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.008
BindingDB Entry DOI: 10.7270/Q2RN37M3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50248710
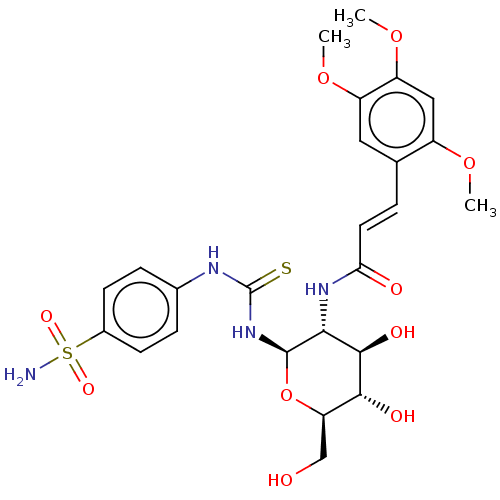
(CHEMBL4070358)Show SMILES COc1cc(OC)c(\C=C\C(=O)N[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]2NC(=S)Nc2ccc(cc2)S(N)(=O)=O)cc1OC |r| Show InChI InChI=1S/C25H32N4O10S2/c1-36-16-11-18(38-3)17(37-2)10-13(16)4-9-20(31)28-21-23(33)22(32)19(12-30)39-24(21)29-25(40)27-14-5-7-15(8-6-14)41(26,34)35/h4-11,19,21-24,30,32-33H,12H2,1-3H3,(H,28,31)(H2,26,34,35)(H2,27,29,40)/b9-4+/t19-,21-,22-,23-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 using 4-nitrophenylacetate as substrate pretreated for 15 mins prior to test by spectrophotometr... |
Eur J Med Chem 132: 1-10 (2017)
Article DOI: 10.1016/j.ejmech.2017.03.023
BindingDB Entry DOI: 10.7270/Q2WS8WNX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50568586

(CHEMBL4859439)Show SMILES Cc1ncc(n1CCOc1cccc(\C=N/c2ccc(cc2)S(=O)(=O)Nc2nccs2)c1)[N+]([O-])=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human carbonic anhydrase 9 preincubated with enzyme for 15 mins by phenol red dye based stopped flow CO2 hydration assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112994
BindingDB Entry DOI: 10.7270/Q2CC14FP |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM103908
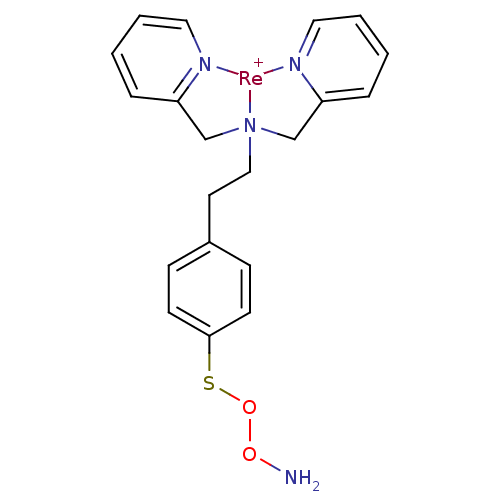
(JNK3 inhibitor 5 | US8562945, 241)Show SMILES NOOSc1ccc(CC[N]23CC4=CC=CC=[N]4[Re+]2[N]2=C(C3)C=CC=C2)cc1 |c:14,16,21,25,27,t:12| Show InChI InChI=1S/C20H22N4O2S.Re/c21-25-26-27-20-9-7-17(8-10-20)11-14-24(15-18-5-1-3-12-22-18)16-19-6-2-4-13-23-19;/h1-10,12-13H,11,14-16,21H2;/q;+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
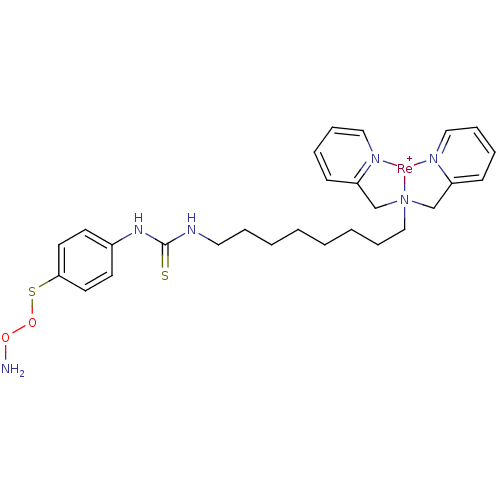
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM103911
(JNK3 inhibitor 8 | US8562945, 246)Show SMILES NOOSc1ccc(NC(=S)NCCCCCCCC[N]23CC4=CC=CC=[N]4[Re+]2[N]2=C(C3)C=CC=C2)cc1 |c:24,26,31,35,37,t:22| Show InChI InChI=1S/C27H36N6O2S2.Re/c28-34-35-37-26-15-13-23(14-16-26)32-27(36)31-19-7-3-1-2-4-10-20-33(21-24-11-5-8-17-29-24)22-25-12-6-9-18-30-25;/h5-6,8-9,11-18H,1-4,7,10,19-22,28H2,(H2,31,32,36);/q;+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc.
US Patent
| Assay Description
Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. |
US Patent US8562945 (2013)
BindingDB Entry DOI: 10.7270/Q2XP73KD |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 in presence of 0.01 mM beta-mercaptoethanol |
Bioorg Med Chem Lett 18: 1898-903 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.008
BindingDB Entry DOI: 10.7270/Q2RN37M3 |
More data for this
Ligand-Target Pair | |
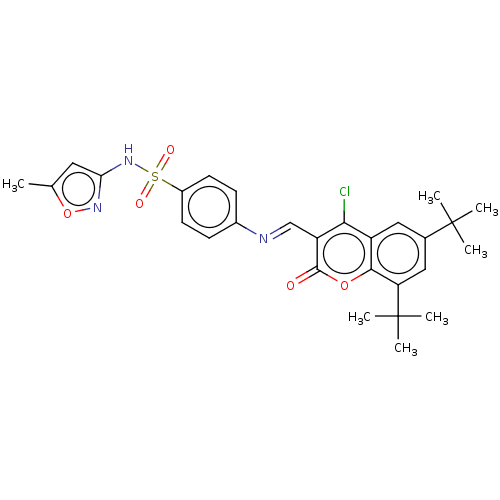
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50492588
(CHEMBL2408084)Show SMILES Cc1cc(NS(=O)(=O)c2ccc(cc2)\N=C\c2c(Cl)c3cc(cc(c3oc2=O)C(C)(C)C)C(C)(C)C)no1 Show InChI InChI=1S/C28H30ClN3O5S/c1-16-12-23(31-37-16)32-38(34,35)19-10-8-18(9-11-19)30-15-21-24(29)20-13-17(27(2,3)4)14-22(28(5,6)7)25(20)36-26(21)33/h8-15H,1-7H3,(H,31,32)/b30-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CA9 using 4-nitrophenylacetate as substrate preincubated for 15 mins by stopped-flow CO2 hydration assay |
Eur J Med Chem 66: 1-11 (2013)
Article DOI: 10.1016/j.ejmech.2013.04.035
BindingDB Entry DOI: 10.7270/Q2M61P55 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 9 catalytic domain in presence of 0.01 mM beta-mercaptoethanol |
Bioorg Med Chem Lett 18: 1898-903 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.008
BindingDB Entry DOI: 10.7270/Q2RN37M3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data