 Found 412 hits of ic50 data for polymerid = 1612
Found 412 hits of ic50 data for polymerid = 1612 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606310
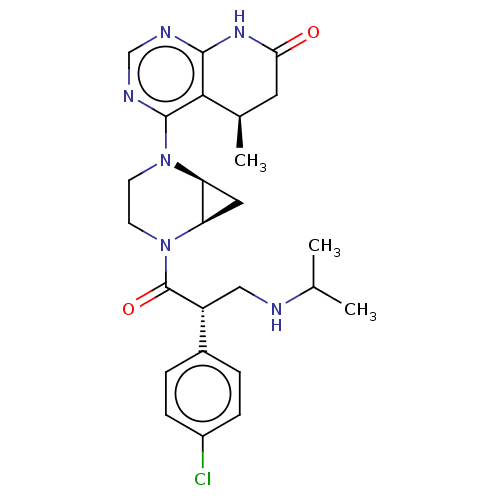
(CHEMBL5169427 | US20230286979, Isomer 2 of Example...)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1)c1ncnc2NC(=O)C[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2W95F93 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606310

(CHEMBL5169427 | US20230286979, Isomer 2 of Example...)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1)c1ncnc2NC(=O)C[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606310

(CHEMBL5169427 | US20230286979, Isomer 2 of Example...)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1)c1ncnc2NC(=O)C[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
CELGENE QUANTICEL RESEARCH, INC.
US Patent
| |
US Patent US10815234 (2020)
BindingDB Entry DOI: 10.7270/Q2RV0STB |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50528415
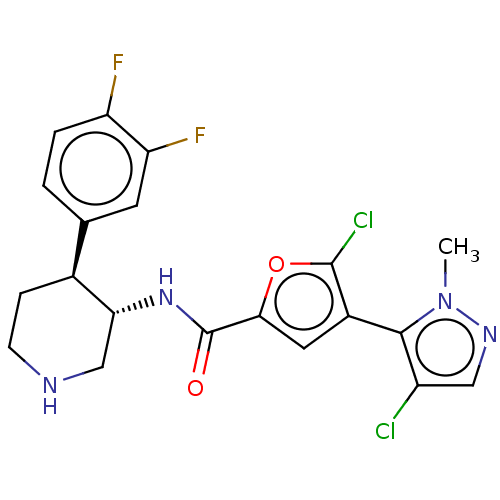
(CHEMBL4457064)Show SMILES O[C@H]([C@@H](O)C(O)=O)C(O)=O.Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@@H]1CNCC[C@H]1c1ccc(F)c(F)c1 |r,wU:26.26,wD:1.0,2.2,31.33,(23.87,-52,;23.87,-53.52,;25.19,-54.29,;25.19,-55.81,;26.51,-53.52,;27.84,-54.29,;26.51,-52,;22.55,-54.29,;21.23,-53.52,;22.55,-55.81,;7.85,-50.48,;7.53,-51.98,;6.12,-52.61,;6.28,-54.14,;7.78,-54.46,;8.41,-55.87,;8.56,-53.13,;10.09,-52.98,;11.12,-54.12,;12.52,-53.5,;12.37,-51.97,;10.87,-51.64,;10.24,-50.24,;13.85,-54.28,;13.85,-55.82,;15.19,-53.51,;16.52,-54.28,;16.52,-55.82,;17.85,-56.59,;19.19,-55.83,;19.19,-54.28,;17.86,-53.51,;17.87,-51.98,;16.53,-51.21,;16.53,-49.66,;17.86,-48.89,;17.86,-47.35,;19.2,-49.66,;20.53,-48.88,;19.2,-51.21,)| Show InChI InChI=1S/C20H18Cl2F2N4O2.C4H6O6/c1-28-18(13(21)8-26-28)12-7-17(30-19(12)22)20(29)27-16-9-25-5-4-11(16)10-2-3-14(23)15(24)6-10;5-1(3(7)8)2(6)4(9)10/h2-3,6-8,11,16,25H,4-5,9H2,1H3,(H,27,29);1-2,5-6H,(H,7,8)(H,9,10)/t11-,16+;1-,2-/m01/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Akt3 (unknown origin) by mobile shift assay |
J Med Chem 62: 7264-7288 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00891
BindingDB Entry DOI: 10.7270/Q2XW4P7W |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606310

(CHEMBL5169427 | US20230286979, Isomer 2 of Example...)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1)c1ncnc2NC(=O)C[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606346
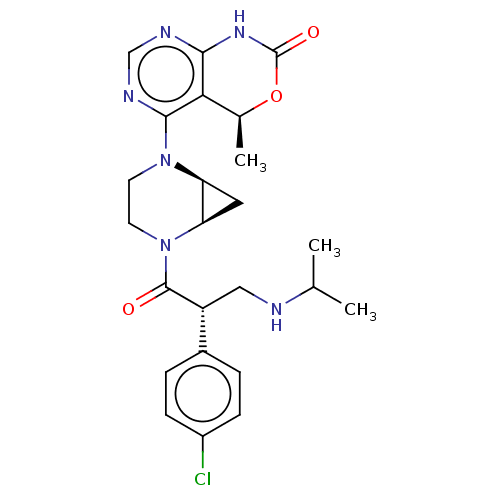
(CHEMBL5197007 | US20230321108, Isomer 4 of Example...)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1)c1ncnc2NC(=O)O[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606311
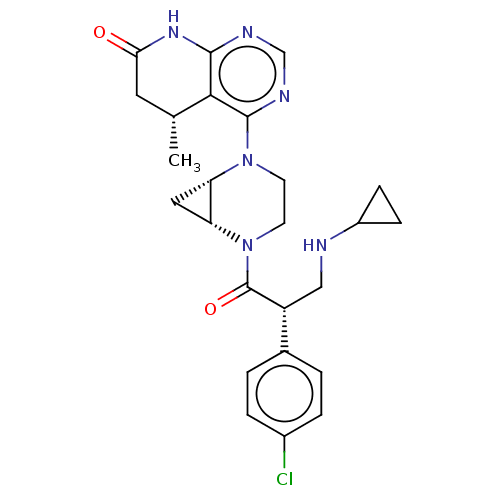
(CHEMBL5182446 | US20230321108, Isomer 2 of Example...)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC1CC1)c1ccc(Cl)cc1)c1ncnc2NC(=O)C[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606311

(CHEMBL5182446 | US20230321108, Isomer 2 of Example...)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC1CC1)c1ccc(Cl)cc1)c1ncnc2NC(=O)C[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50528409

(CHEMBL4441825)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@@H]1CN[C@H](CCO)C[C@H]1c1ccc(F)c(F)c1 |r,wU:16.17,19.21,wD:24.27,(5.73,-5.85,;5.41,-7.35,;4,-7.98,;4.16,-9.51,;5.66,-9.83,;6.29,-11.24,;6.44,-8.5,;7.97,-8.34,;9,-9.49,;10.41,-8.86,;10.25,-7.33,;8.75,-7.01,;8.12,-5.6,;11.75,-9.64,;11.74,-11.18,;13.08,-8.87,;14.41,-9.65,;14.41,-11.18,;15.74,-11.96,;17.08,-11.19,;18.41,-11.96,;19.74,-11.2,;21.07,-11.97,;17.08,-9.64,;15.75,-8.88,;15.75,-7.35,;14.42,-6.58,;14.42,-5.03,;15.75,-4.26,;15.74,-2.72,;17.08,-5.03,;18.41,-4.25,;17.09,-6.58,)| Show InChI InChI=1S/C22H22Cl2F2N4O3/c1-30-20(15(23)9-28-30)14-8-19(33-21(14)24)22(32)29-18-10-27-12(4-5-31)7-13(18)11-2-3-16(25)17(26)6-11/h2-3,6,8-9,12-13,18,27,31H,4-5,7,10H2,1H3,(H,29,32)/t12-,13+,18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Akt3 (unknown origin) by mobile shift assay |
J Med Chem 62: 7264-7288 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00891
BindingDB Entry DOI: 10.7270/Q2XW4P7W |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606346

(CHEMBL5197007 | US20230321108, Isomer 4 of Example...)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1)c1ncnc2NC(=O)O[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606329
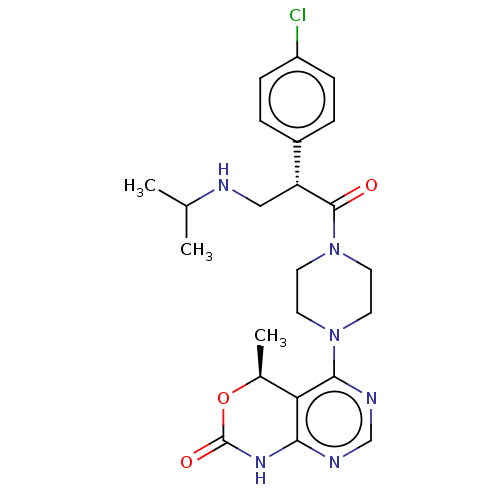
(CHEMBL5175332)Show SMILES CC(C)NC[C@@H](C(=O)N1CCN(CC1)c1ncnc2NC(=O)O[C@@H](C)c12)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606341
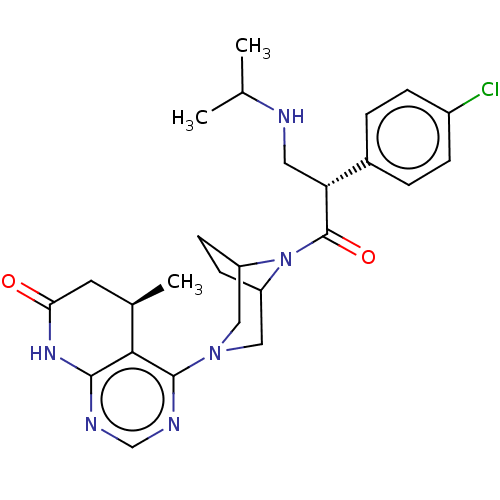
(CHEMBL5195745)Show SMILES CC(C)NC[C@@H](C(=O)N1C2CCC1CN(C2)c1ncnc2NC(=O)C[C@@H](C)c12)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606347
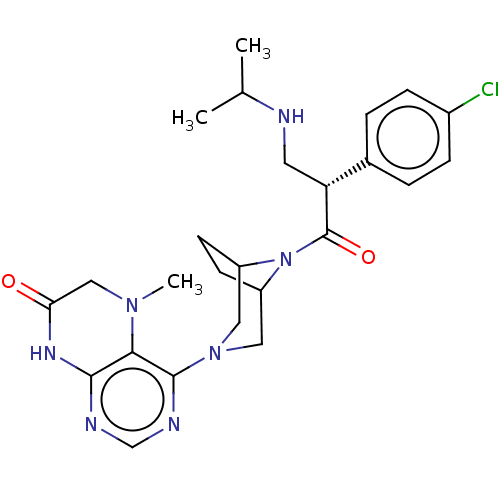
(CHEMBL5190230)Show SMILES OC=O.CC(C)NC[C@@H](C(=O)N1C2CCC1CN(C2)c1ncnc2NC(=O)CN(C)c12)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606345

(CHEMBL5180219)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1)N2c1ncnc2NC(=O)O[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606309
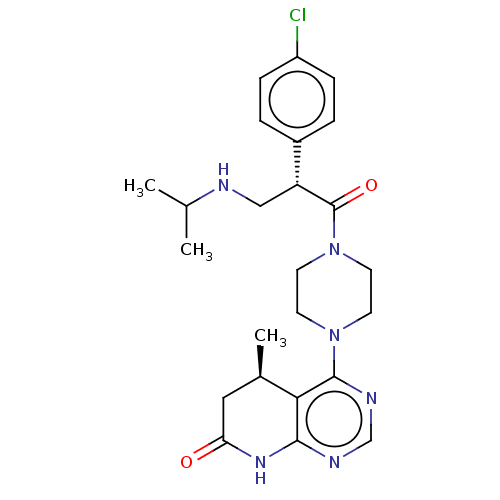
(CHEMBL5206905)Show SMILES CC(C)NC[C@@H](C(=O)N1CCN(CC1)c1ncnc2NC(=O)C[C@@H](C)c12)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606342
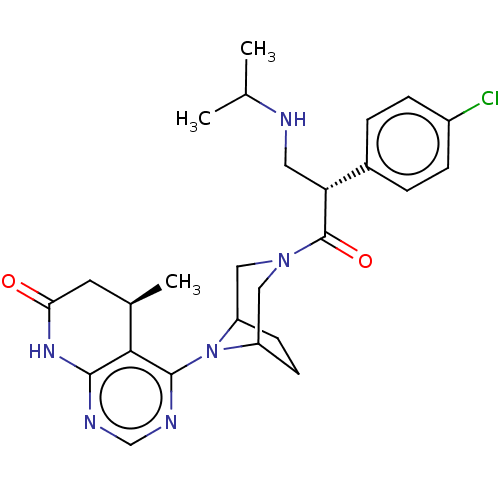
(CHEMBL5177491)Show SMILES CC(C)NC[C@@H](C(=O)N1CC2CCC(C1)N2c1ncnc2NC(=O)C[C@@H](C)c12)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606309

(CHEMBL5206905)Show SMILES CC(C)NC[C@@H](C(=O)N1CCN(CC1)c1ncnc2NC(=O)C[C@@H](C)c12)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606319
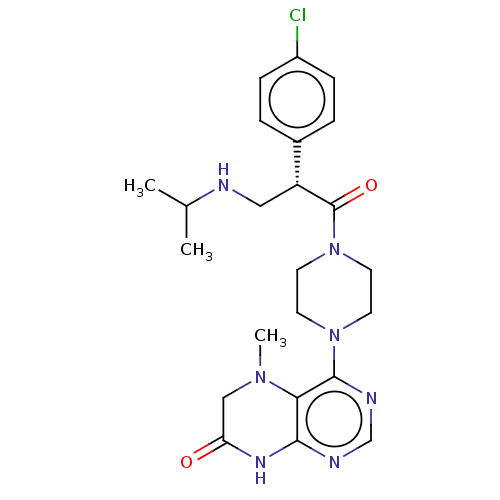
(CHEMBL5195664)Show SMILES Cl.CC(C)NC[C@@H](C(=O)N1CCN(CC1)c1ncnc2NC(=O)CN(C)c12)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606344
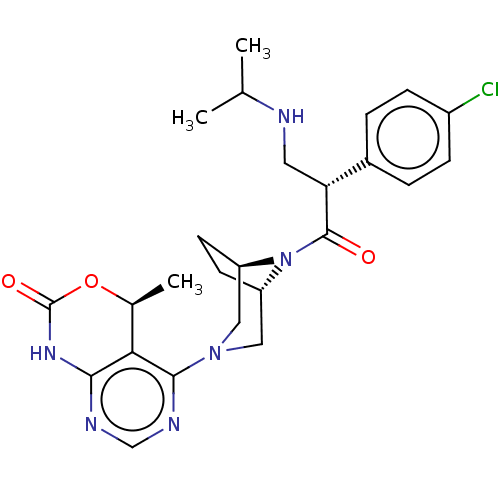
(CHEMBL5189707)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)c1ncnc3NC(=O)O[C@@H](C)c13)N2C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606349
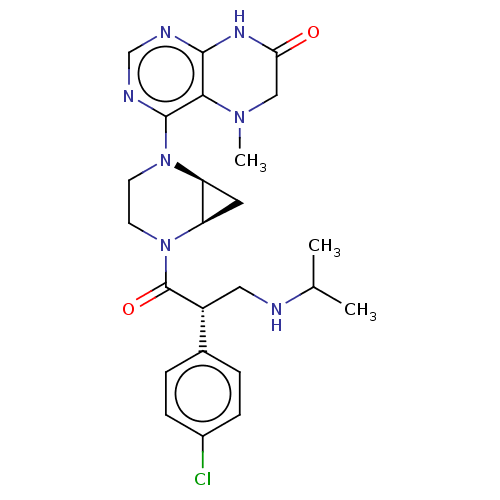
(CHEMBL5171561)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1)c1ncnc2NC(=O)CN(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606313
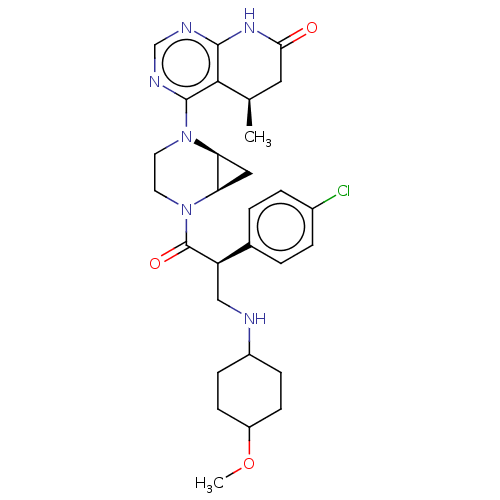
(CHEMBL5193325)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC1CCC(CC1)OC)c1ccc(Cl)cc1)c1ncnc2NC(=O)C[C@@H](C)c12 |r,wU:11.13,1.0,3.4,wD:38.42,(3.08,-1.58,;2,-2.67,;3.33,-3.45,;2,-4.22,;3.48,-4.62,;.66,-5,;-.68,-4.22,;-.68,-2.67,;.66,-1.91,;.66,-.37,;1.99,.4,;-.67,.4,;-.67,1.94,;.66,2.71,;.66,4.25,;-.67,5.02,;-.67,6.56,;.66,7.33,;1.99,6.56,;1.99,5.02,;.66,8.87,;-.67,9.64,;-2.01,-.37,;-2,-1.91,;-3.34,-2.68,;-4.67,-1.92,;-6.01,-2.69,;-4.68,-.37,;-3.34,.4,;.66,-6.54,;-.68,-7.32,;-.68,-8.87,;.66,-9.64,;2,-8.87,;3.33,-9.64,;4.67,-8.87,;6.01,-9.64,;4.67,-7.32,;3.33,-6.54,;3.33,-5,;2,-7.32,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606348
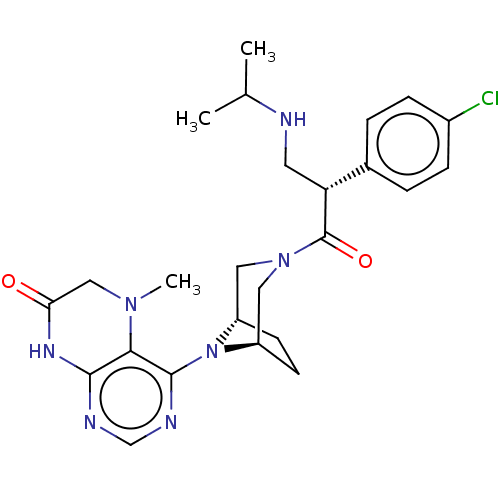
(CHEMBL5187172)Show SMILES [H][C@]12CC[C@]([H])(CN(C1)C(=O)[C@H](CNC(C)C)c1ccc(Cl)cc1)N2c1ncnc2NC(=O)CN(C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606314
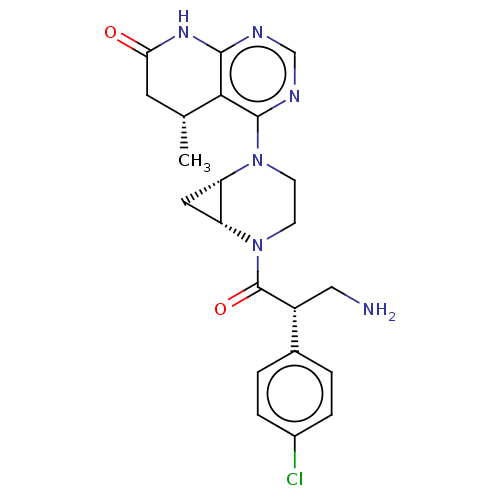
(CHEMBL5187508)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CN)c1ccc(Cl)cc1)c1ncnc2NC(=O)C[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606323
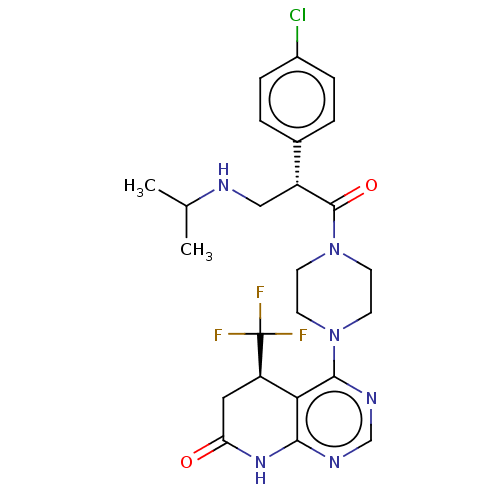
(CHEMBL5189610)Show SMILES CC(C)NC[C@@H](C(=O)N1CCN(CC1)c1ncnc2NC(=O)C[C@H](c12)C(F)(F)F)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306165
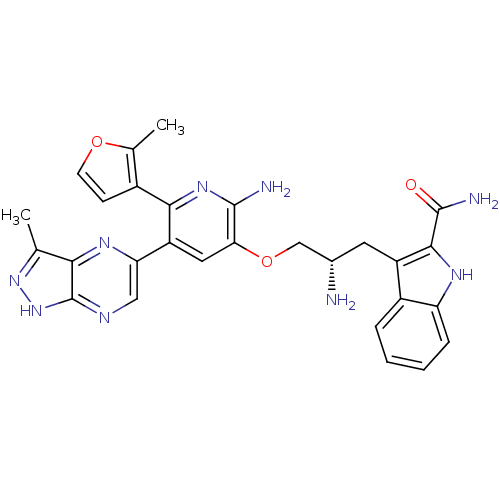
(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C(N)=O)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N9O3/c1-13-23-28(37-36-13)32-11-21(34-23)19-10-22(26(30)35-24(19)16-7-8-39-14(16)2)40-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)27(31)38/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,35)(H2,31,38)(H,32,36,37)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306164
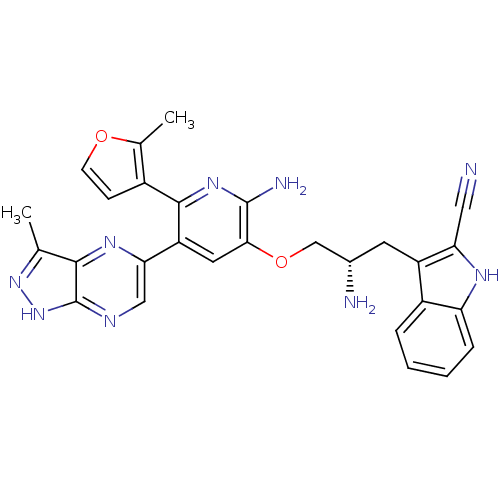
(3-((2S)-2-amino-3-(2-amino-5-(3-methyl-1H-pyrazolo...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c([nH]c3ccccc23)C#N)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H25N9O2/c1-14-25-28(37-36-14)32-12-23(34-25)20-10-24(27(31)35-26(20)17-7-8-38-15(17)2)39-13-16(30)9-19-18-5-3-4-6-21(18)33-22(19)11-29/h3-8,10,12,16,33H,9,13,30H2,1-2H3,(H2,31,35)(H,32,36,37)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306155
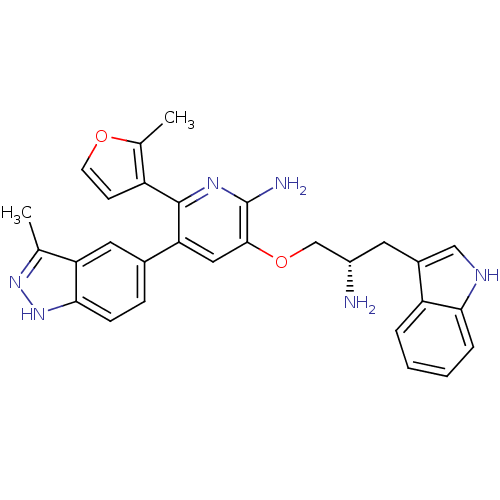
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C29H28N6O2/c1-16-23-12-18(7-8-26(23)35-34-16)24-13-27(29(31)33-28(24)21-9-10-36-17(21)2)37-15-20(30)11-19-14-32-25-6-4-3-5-22(19)25/h3-10,12-14,20,32H,11,15,30H2,1-2H3,(H2,31,33)(H,34,35)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50305883
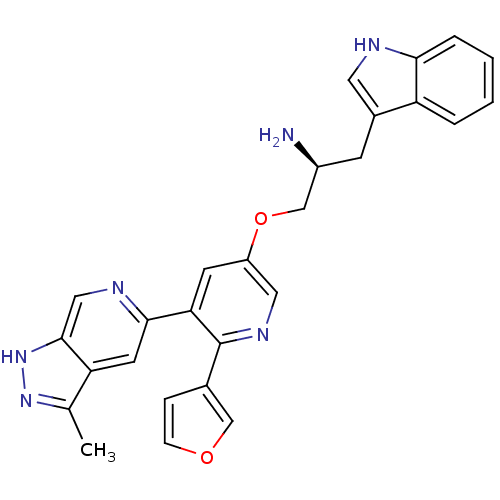
((S)-1-(6-(furan-3-yl)-5-(3-methyl-1H-pyrazolo[3,4-...)Show SMILES Cc1n[nH]c2cnc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)cnc1-c1ccoc1 |r| Show InChI InChI=1S/C27H24N6O2/c1-16-22-10-25(30-13-26(22)33-32-16)23-9-20(12-31-27(23)17-6-7-34-14-17)35-15-19(28)8-18-11-29-24-5-3-2-4-21(18)24/h2-7,9-14,19,29H,8,15,28H2,1H3,(H,32,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 20: 679-83 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.060
BindingDB Entry DOI: 10.7270/Q298873F |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50305878
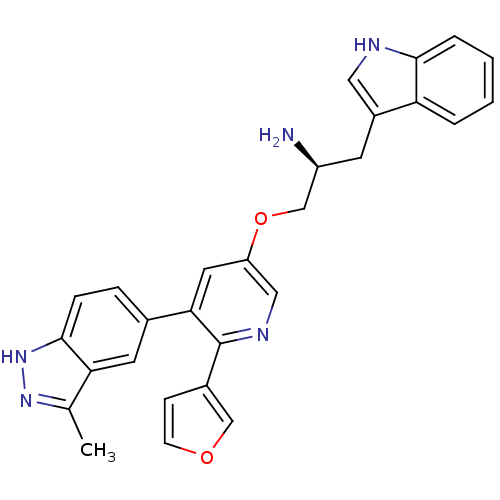
((2S)-1-{[6-furan-3-yl-5-(3-methyl-2H-indazol-5-yl)...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)cnc1-c1ccoc1 |r| Show InChI InChI=1S/C28H25N5O2/c1-17-24-11-18(6-7-27(24)33-32-17)25-12-22(14-31-28(25)19-8-9-34-15-19)35-16-21(29)10-20-13-30-26-5-3-2-4-23(20)26/h2-9,11-15,21,30H,10,16,29H2,1H3,(H,32,33)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 20: 679-83 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.060
BindingDB Entry DOI: 10.7270/Q298873F |
More data for this
Ligand-Target Pair | |
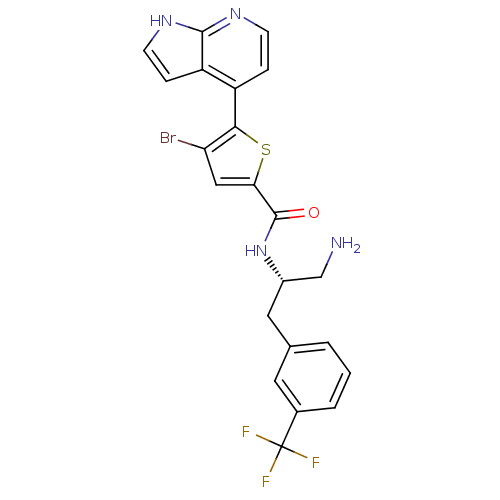
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099
(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192
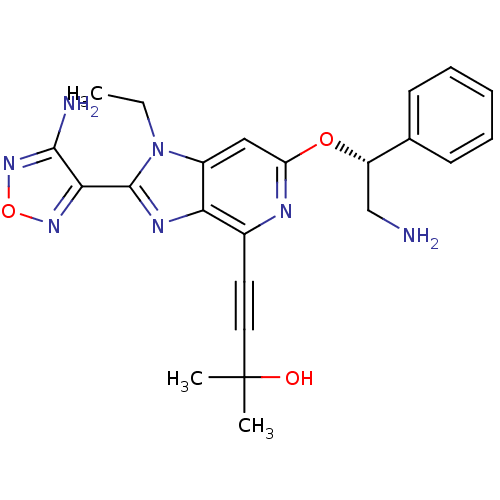
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50398382
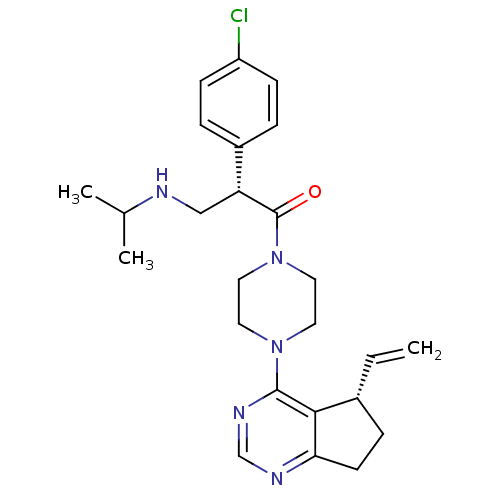
(CHEMBL2177387)Show SMILES CC(C)NC[C@@H](C(=O)N1CCN(CC1)c1ncnc2CC[C@@H](C=C)c12)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C25H32ClN5O/c1-4-18-7-10-22-23(18)24(29-16-28-22)30-11-13-31(14-12-30)25(32)21(15-27-17(2)3)19-5-8-20(26)9-6-19/h4-6,8-9,16-18,21,27H,1,7,10-15H2,2-3H3/t18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of wild-type full-length amino-terminal polyhistidine-tagged human Akt3 expressed in recombinant baculovirus system using fluo... |
J Med Chem 55: 8110-27 (2012)
Article DOI: 10.1021/jm301024w
BindingDB Entry DOI: 10.7270/Q20P1160 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606312
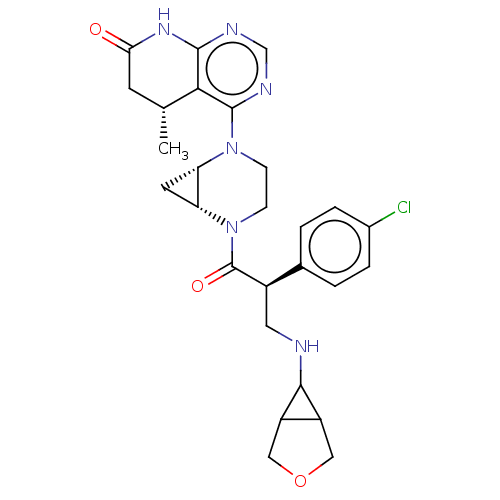
(CHEMBL5199199)Show SMILES [H][C@@]12C[C@]1([H])N(CCN2C(=O)[C@H](CNC1C2COCC12)c1ccc(Cl)cc1)c1ncnc2NC(=O)C[C@@H](C)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606327
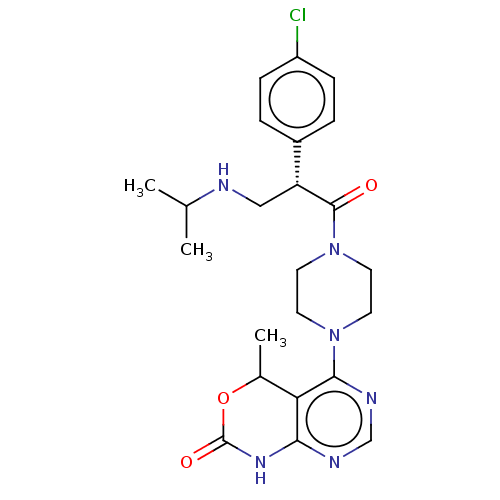
(CHEMBL5192188)Show SMILES CC(C)NC[C@@H](C(=O)N1CCN(CC1)c1ncnc2NC(=O)OC(C)c12)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170284
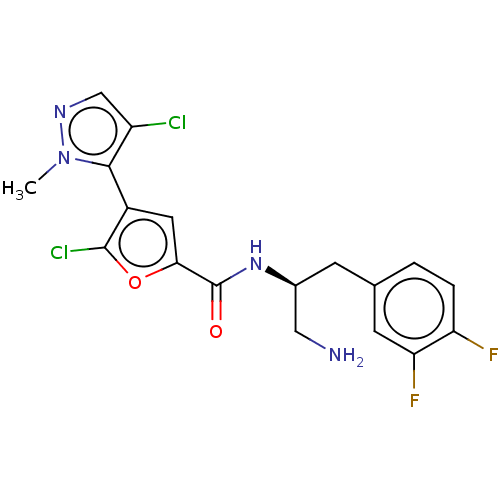
(GSK2141795 | GSK2141795C | Uprosertib)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@H](CN)Cc1ccc(F)c(F)c1 |r,wD:16.18,(2.37,-.54,;3.52,.49,;3.36,2.02,;4.77,2.65,;5.8,1.51,;7.33,1.67,;5.03,.17,;5.65,-1.23,;7.16,-1.55,;7.32,-3.09,;5.91,-3.71,;4.88,-2.57,;3.35,-2.73,;8.65,-3.86,;9.99,-3.09,;8.65,-5.4,;9.99,-6.17,;11.32,-5.4,;12.65,-6.17,;9.99,-7.71,;11.32,-8.48,;12.65,-7.71,;13.99,-8.48,;13.99,-10.02,;15.32,-10.79,;12.65,-10.79,;12.65,-12.33,;11.32,-10.02,)| Show InChI InChI=1S/C18H16Cl2F2N4O2/c1-26-16(12(19)8-24-26)11-6-15(28-17(11)20)18(27)25-10(7-23)4-9-2-3-13(21)14(22)5-9/h2-3,5-6,8,10H,4,7,23H2,1H3,(H,25,27)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Akt3 (unknown origin) by mobile shift assay |
J Med Chem 62: 7264-7288 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00891
BindingDB Entry DOI: 10.7270/Q2XW4P7W |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | VEVORISERTIB
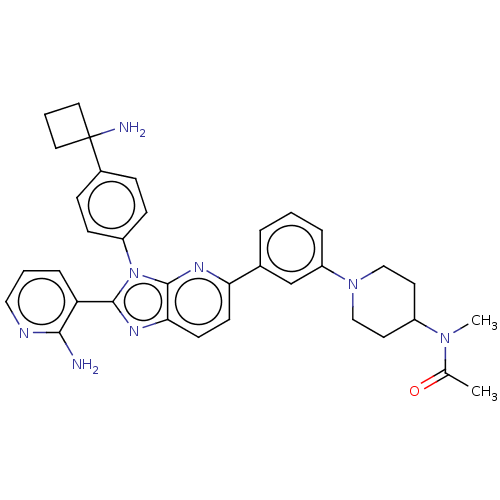
(Vevorisertib)Show InChI InChI=1S/C13H21NO2/c1-11(2)14(3)9-12(15)10-16-13-7-5-4-6-8-13/h4-8,11-12,15H,9-10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50170284

(GSK2141795 | GSK2141795C | Uprosertib)Show SMILES Cn1ncc(Cl)c1-c1cc(oc1Cl)C(=O)N[C@H](CN)Cc1ccc(F)c(F)c1 |r,wD:16.18,(2.37,-.54,;3.52,.49,;3.36,2.02,;4.77,2.65,;5.8,1.51,;7.33,1.67,;5.03,.17,;5.65,-1.23,;7.16,-1.55,;7.32,-3.09,;5.91,-3.71,;4.88,-2.57,;3.35,-2.73,;8.65,-3.86,;9.99,-3.09,;8.65,-5.4,;9.99,-6.17,;11.32,-5.4,;12.65,-6.17,;9.99,-7.71,;11.32,-8.48,;12.65,-7.71,;13.99,-8.48,;13.99,-10.02,;15.32,-10.79,;12.65,-10.79,;12.65,-12.33,;11.32,-10.02,)| Show InChI InChI=1S/C18H16Cl2F2N4O2/c1-26-16(12(19)8-24-26)11-6-15(28-17(11)20)18(27)25-10(7-23)4-9-2-3-13(21)14(22)5-9/h2-3,5-6,8,10H,4,7,23H2,1H3,(H,25,27)/t10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of choline acetyltransferase (ChAT) activity |
Citation and Details
|
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50606318
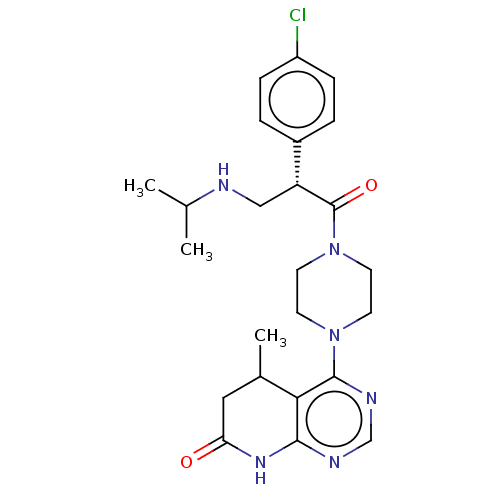
(CHEMBL5187287)Show SMILES CC(C)NC[C@@H](C(=O)N1CCN(CC1)c1ncnc2NC(=O)CC(C)c12)c1ccc(Cl)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00527
BindingDB Entry DOI: 10.7270/Q2TX3KGT |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50528416
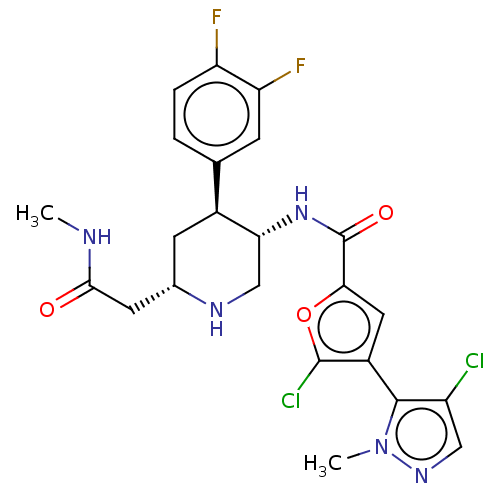
(CHEMBL4440965)Show SMILES CNC(=O)C[C@@H]1C[C@H]([C@@H](CN1)NC(=O)c1cc(c(Cl)o1)-c1c(Cl)cnn1C)c1ccc(F)c(F)c1 |r,wU:8.11,5.4,wD:7.29,(76.34,-11.8,;75,-12.56,;73.67,-11.79,;73.68,-10.25,;72.34,-12.55,;71.01,-11.78,;71.01,-10.23,;69.68,-9.47,;68.34,-10.24,;68.34,-11.77,;69.67,-12.55,;67.01,-9.46,;65.68,-10.23,;65.67,-11.77,;64.34,-9.46,;62.93,-10.08,;61.9,-8.93,;62.68,-7.6,;62.05,-6.19,;64.18,-7.92,;60.37,-9.09,;59.59,-10.42,;60.22,-11.83,;58.09,-10.1,;57.93,-8.57,;59.34,-7.94,;59.66,-6.44,;69.68,-7.94,;68.35,-7.17,;68.35,-5.62,;69.68,-4.85,;69.67,-3.31,;71.01,-5.62,;72.34,-4.84,;71.02,-7.17,)| Show InChI InChI=1S/C23H23Cl2F2N5O3/c1-28-20(33)7-12-6-13(11-3-4-16(26)17(27)5-11)18(10-29-12)31-23(34)19-8-14(22(25)35-19)21-15(24)9-30-32(21)2/h3-5,8-9,12-13,18,29H,6-7,10H2,1-2H3,(H,28,33)(H,31,34)/t12-,13-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Akt3 (unknown origin) by mobile shift assay |
J Med Chem 62: 7264-7288 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00891
BindingDB Entry DOI: 10.7270/Q2XW4P7W |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278770

(CHEMBL470597 | N-((S)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306163
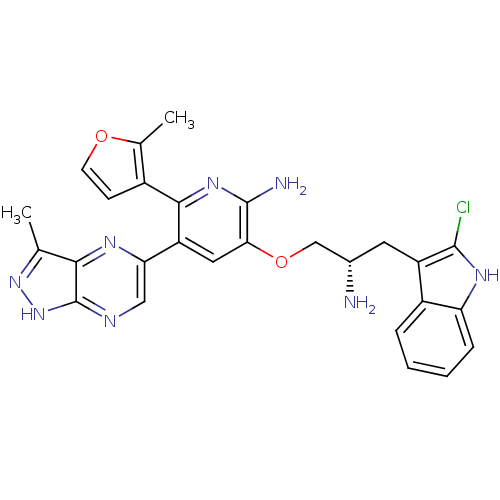
(3-((S)-2-amino-3-(2-chloro-1H-indol-3-yl)propoxy)-...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c(Cl)[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H25ClN8O2/c1-13-23-27(36-35-13)31-11-21(32-23)19-10-22(26(30)34-24(19)16-7-8-37-14(16)2)38-12-15(29)9-18-17-5-3-4-6-20(17)33-25(18)28/h3-8,10-11,15,33H,9,12,29H2,1-2H3,(H2,30,34)(H,31,35,36)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306157
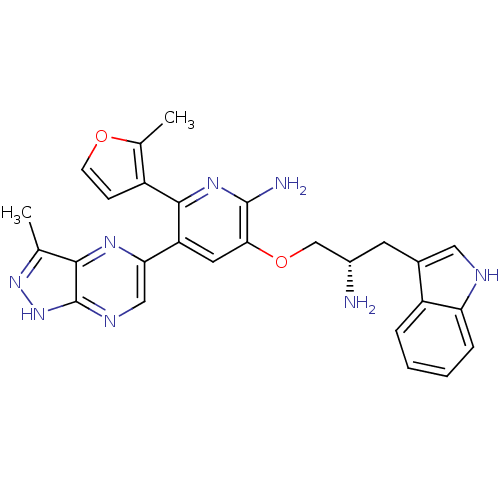
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(nc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C27H26N8O2/c1-14-24-27(35-34-14)31-12-22(32-24)20-10-23(26(29)33-25(20)18-7-8-36-15(18)2)37-13-17(28)9-16-11-30-21-6-4-3-5-19(16)21/h3-8,10-12,17,30H,9,13,28H2,1-2H3,(H2,29,33)(H,31,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50306156
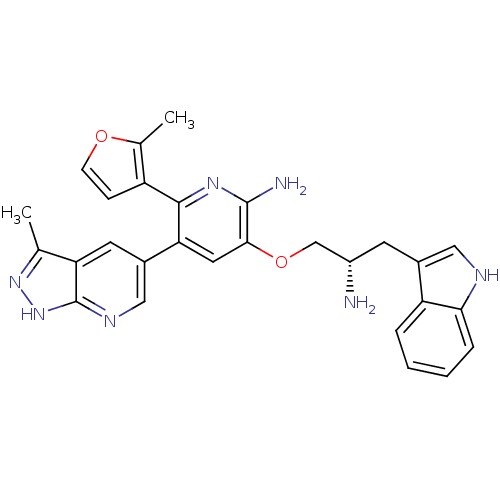
(3-((S)-2-amino-3-(1H-indol-3-yl)propoxy)-5-(3-meth...)Show SMILES Cc1n[nH]c2ncc(cc12)-c1cc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c(N)nc1-c1ccoc1C |r| Show InChI InChI=1S/C28H27N7O2/c1-15-22-10-18(13-32-28(22)35-34-15)23-11-25(27(30)33-26(23)20-7-8-36-16(20)2)37-14-19(29)9-17-12-31-24-6-4-3-5-21(17)24/h3-8,10-13,19,31H,9,14,29H2,1-2H3,(H2,30,33)(H,32,34,35)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human AKT3 |
Bioorg Med Chem Lett 20: 684-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.061
BindingDB Entry DOI: 10.7270/Q20V8CXX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278835
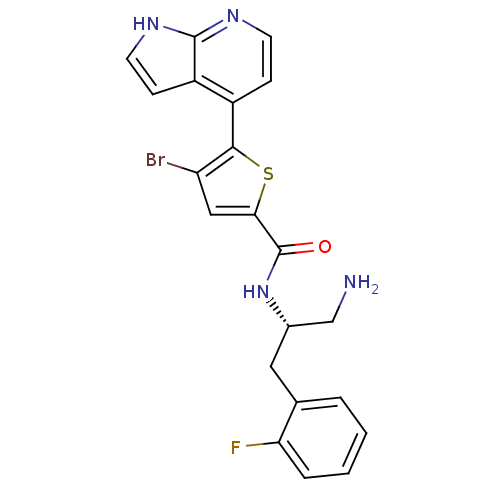
(CHEMBL498052 | N-((S)-1-amino-3-(2-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1ccccc1F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-16-10-18(29-19(16)14-5-7-25-20-15(14)6-8-26-20)21(28)27-13(11-24)9-12-3-1-2-4-17(12)23/h1-8,10,13H,9,11,24H2,(H,25,26)(H,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098
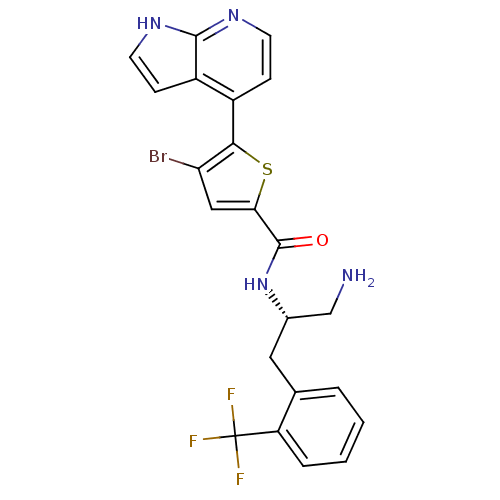
(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50398358
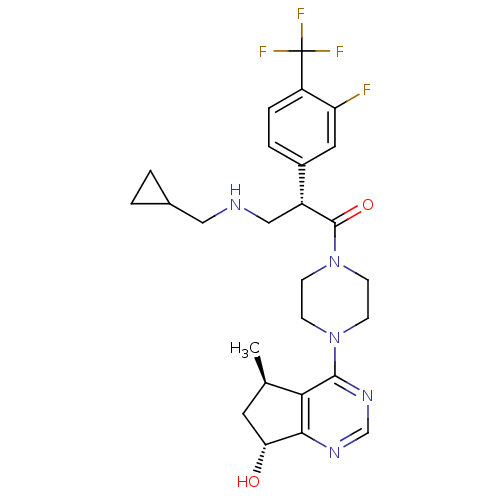
(CHEMBL2177361)Show SMILES C[C@@H]1C[C@@H](O)c2ncnc(N3CCN(CC3)C(=O)[C@H](CNCC3CC3)c3ccc(c(F)c3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C26H31F4N5O2/c1-15-10-21(36)23-22(15)24(33-14-32-23)34-6-8-35(9-7-34)25(37)18(13-31-12-16-2-3-16)17-4-5-19(20(27)11-17)26(28,29)30/h4-5,11,14-16,18,21,31,36H,2-3,6-10,12-13H2,1H3/t15-,18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of wild-type full-length amino-terminal polyhistidine-tagged human Akt3 expressed in recombinant baculovirus system using fluo... |
J Med Chem 55: 8110-27 (2012)
Article DOI: 10.1021/jm301024w
BindingDB Entry DOI: 10.7270/Q20P1160 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50398359
(CHEMBL2178598)Show SMILES C[C@@H]1C[C@@H](O)c2ncnc(N3CCN(CC3)C(=O)[C@H](CNC(C)(C)CO)c3ccc(c(F)c3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C26H33F4N5O3/c1-15-10-20(37)22-21(15)23(32-14-31-22)34-6-8-35(9-7-34)24(38)17(12-33-25(2,3)13-36)16-4-5-18(19(27)11-16)26(28,29)30/h4-5,11,14-15,17,20,33,36-37H,6-10,12-13H2,1-3H3/t15-,17-,20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Array BioPharma Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of wild-type full-length amino-terminal polyhistidine-tagged human Akt3 expressed in recombinant baculovirus system using fluo... |
J Med Chem 55: 8110-27 (2012)
Article DOI: 10.1021/jm301024w
BindingDB Entry DOI: 10.7270/Q20P1160 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50311411
((R)-2-(6-acetamidohexanamido)-6-amino-N-(6-((R)-1-...)Show SMILES [#6]-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C32H63N13O6/c1-22(46)39-18-8-2-4-15-26(47)43-24(12-6-7-17-33)29(50)40-19-9-3-5-16-27(48)44-25(14-11-21-42-32(37)38)30(51)45-23(28(34)49)13-10-20-41-31(35)36/h23-25H,2-21,33H2,1H3,(H2,34,49)(H,39,46)(H,40,50)(H,43,47)(H,44,48)(H,45,51)(H4,35,36,41)(H4,37,38,42)/t23-,24-,25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tartu
Curated by ChEMBL
| Assay Description
Inhibition of PKBgamma in presence of 100 uM ATP |
Bioorg Med Chem Lett 19: 6098-101 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.026
BindingDB Entry DOI: 10.7270/Q2JM29R4 |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278834
(CHEMBL524998 | N-((S)-1-amino-3-(4-methoxyphenyl)p...)Show SMILES COc1ccc(C[C@@H](CN)NC(=O)c2cc(Br)c(s2)-c2ccnc3[nH]ccc23)cc1 |r| Show InChI InChI=1S/C22H21BrN4O2S/c1-29-15-4-2-13(3-5-15)10-14(12-24)27-22(28)19-11-18(23)20(30-19)16-6-8-25-21-17(16)7-9-26-21/h2-9,11,14H,10,12,24H2,1H3,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data