 Found 332 hits of ic50 data for polymerid = 1733,1733,4481
Found 332 hits of ic50 data for polymerid = 1733,1733,4481 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM15459

(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1cc(ccn1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C29H30F3N5/c1-19(20-7-4-3-5-8-20)35-25-18-23(13-16-34-25)27-26(22-9-6-10-24(17-22)29(30,31)32)36-28(37(27)2)21-11-14-33-15-12-21/h3-10,13,16-19,21,33H,11-12,14-15H2,1-2H3,(H,34,35)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type p38beta (unknown origin) |
J Biol Chem 282: 34663-71 (2007)
Article DOI: 10.1074/jbc.M704236200
BindingDB Entry DOI: 10.7270/Q2G44Q2F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50605782

(CHEMBL5174953)Show SMILES C[C@H]1CCC[C@@H](C)N1c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4cnn(CCN(C)C)c4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50605748
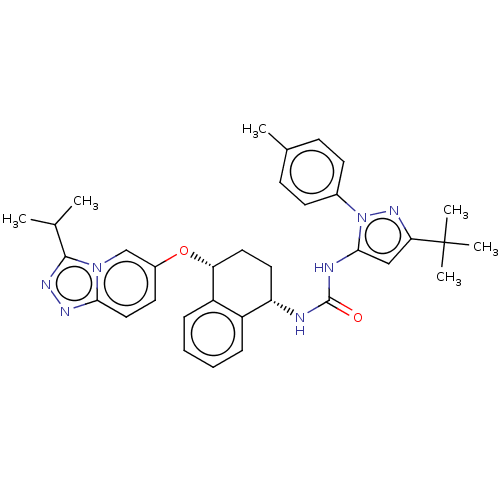
(CHEMBL5206328)Show SMILES CC(C)c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4ccc(C)cc4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50605781

(CHEMBL5202475)Show SMILES C[C@H]1CCCCN1c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4cnn(CCN(C)C)c4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50175745
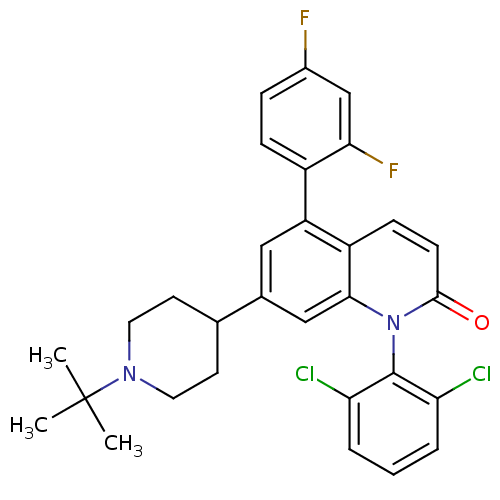
(7-(1-tert-butylpiperidin-4-yl)-1-(2,6-dichlorophen...)Show SMILES CC(C)(C)N1CCC(CC1)c1cc(-c2ccc(F)cc2F)c2ccc(=O)n(-c3c(Cl)cccc3Cl)c2c1 |(5.3,.54,;3.97,-.23,;4.75,-1.56,;3.2,1.1,;2.64,-1,;2.64,-2.54,;1.31,-3.31,;-.03,-2.54,;-.04,-1.01,;1.3,-.23,;-1.36,-3.31,;-1.35,-4.86,;-2.69,-5.64,;-2.69,-7.17,;-4.03,-7.94,;-4.03,-9.48,;-2.69,-10.25,;-2.69,-11.79,;-1.35,-9.47,;-1.36,-7.94,;-.03,-7.16,;-4.03,-4.86,;-5.36,-5.63,;-6.69,-4.85,;-6.69,-3.31,;-8.02,-2.53,;-5.34,-2.55,;-5.33,-1.01,;-6.66,-.24,;-8,-1,;-6.66,1.3,;-5.31,2.07,;-3.98,1.29,;-3.99,-.25,;-2.67,-1.03,;-4.02,-3.32,;-2.69,-2.55,)| Show InChI InChI=1S/C30H28Cl2F2N2O/c1-30(2,3)35-13-11-18(12-14-35)19-15-23(21-8-7-20(33)17-26(21)34)22-9-10-28(37)36(27(22)16-19)29-24(31)5-4-6-25(29)32/h4-10,15-18H,11-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type p38beta (unknown origin) |
J Biol Chem 282: 34663-71 (2007)
Article DOI: 10.1074/jbc.M704236200
BindingDB Entry DOI: 10.7270/Q2G44Q2F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50605756

(CHEMBL5184542)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)N[C@H]1CC[C@@H](Oc2ccc3nnc(N4CCCCC4)n3c2)c2ccccc12)C(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50605761

(CHEMBL5197888)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)N[C@H]1CC[C@@H](Oc2ccc3nnc(N4CCC(CO)CC4)n3c2)c2ccccc12)C(C)(C)C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50605772

(CHEMBL5208103)Show SMILES C[C@H]1CCCCN1c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4cccc(OCCN(C)C)c4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50236473
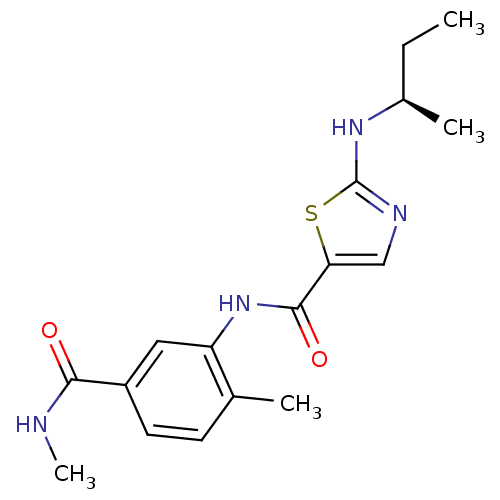
((R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbam...)Show SMILES CC[C@@H](C)Nc1ncc(s1)C(=O)Nc1cc(ccc1C)C(=O)NC Show InChI InChI=1S/C17H22N4O2S/c1-5-11(3)20-17-19-9-14(24-17)16(23)21-13-8-12(15(22)18-4)7-6-10(13)2/h6-9,11H,5H2,1-4H3,(H,18,22)(H,19,20)(H,21,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human p38beta |
Bioorg Med Chem Lett 18: 1762-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.031
BindingDB Entry DOI: 10.7270/Q2057FNW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM16530
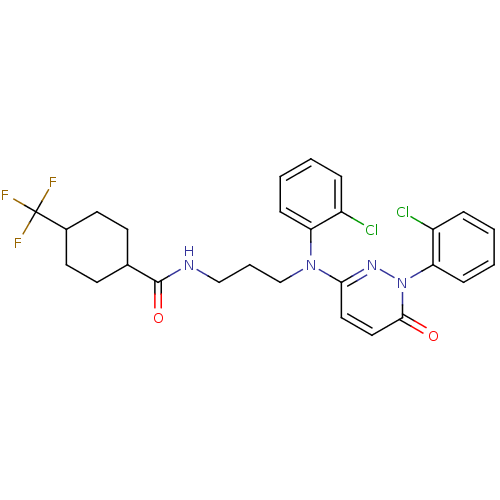
(2-arylpyridazin-3-one, 36 | N-{3-[(2-chlorophenyl)...)Show SMILES FC(F)(F)C1CCC(CC1)C(=O)NCCCN(c1ccc(=O)n(n1)-c1ccccc1Cl)c1ccccc1Cl |(13.52,-3.17,;12.75,-4.5,;14.08,-5.27,;11.98,-5.84,;11.41,-3.73,;11.41,-2.19,;10.08,-1.42,;8.74,-2.19,;8.74,-3.73,;10.08,-4.5,;7.41,-1.42,;7.41,.12,;6.08,-2.19,;4.74,-1.42,;3.41,-2.19,;2.08,-1.42,;.74,-2.19,;-.59,-1.42,;-1.92,-2.19,;-3.26,-1.42,;-3.26,.12,;-4.59,.89,;-1.92,.89,;-.59,.12,;-1.92,2.43,;-.59,3.2,;-.59,4.74,;-1.92,5.51,;-3.26,4.74,;-3.26,3.2,;-4.59,2.43,;.74,-3.73,;-.59,-4.5,;-.59,-6.04,;.74,-6.81,;2.08,-6.04,;2.08,-4.5,;3.41,-3.73,)| Show InChI InChI=1S/C27H27Cl2F3N4O2/c28-20-6-1-3-8-22(20)35(24-14-15-25(37)36(34-24)23-9-4-2-7-21(23)29)17-5-16-33-26(38)18-10-12-19(13-11-18)27(30,31)32/h1-4,6-9,14-15,18-19H,5,10-13,16-17H2,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 5809-13 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.074
BindingDB Entry DOI: 10.7270/Q28P5XSC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50236473

((R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbam...)Show SMILES CC[C@@H](C)Nc1ncc(s1)C(=O)Nc1cc(ccc1C)C(=O)NC Show InChI InChI=1S/C17H22N4O2S/c1-5-11(3)20-17-19-9-14(24-17)16(23)21-13-8-12(15(22)18-4)7-6-10(13)2/h6-9,11H,5H2,1-4H3,(H,18,22)(H,19,20)(H,21,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38beta (unknown origin) |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50236473

((R)-2-(sec-butylamino)-N-(2-methyl-5-(methylcarbam...)Show SMILES CC[C@@H](C)Nc1ncc(s1)C(=O)Nc1cc(ccc1C)C(=O)NC Show InChI InChI=1S/C17H22N4O2S/c1-5-11(3)20-17-19-9-14(24-17)16(23)21-13-8-12(15(22)18-4)7-6-10(13)2/h6-9,11H,5H2,1-4H3,(H,18,22)(H,19,20)(H,21,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38beta expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after ... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50492386
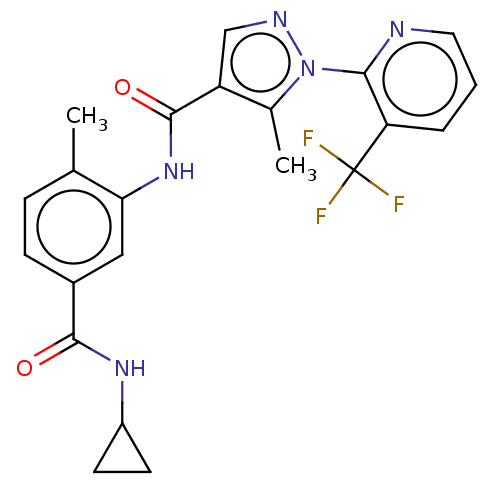
(CHEMBL2401979)Show SMILES Cc1c(cnn1-c1ncccc1C(F)(F)F)C(=O)Nc1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C22H20F3N5O2/c1-12-5-6-14(20(31)28-15-7-8-15)10-18(12)29-21(32)16-11-27-30(13(16)2)19-17(22(23,24)25)4-3-9-26-19/h3-6,9-11,15H,7-8H2,1-2H3,(H,28,31)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38beta expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after ... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50313107
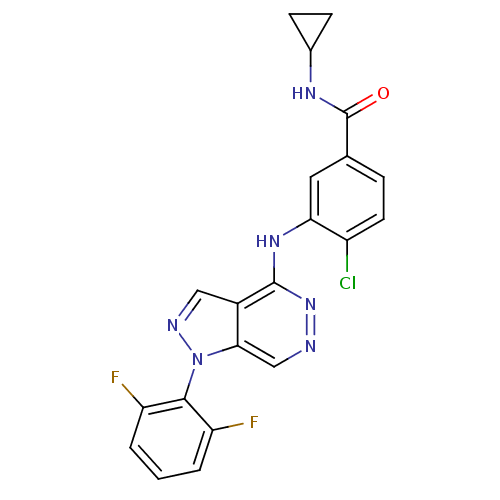
(4-chloro-N-cyclopropyl-3-(1-(2,6-difluorophenyl)-1...)Show SMILES Fc1cccc(F)c1-n1ncc2c(Nc3cc(ccc3Cl)C(=O)NC3CC3)nncc12 |(-6.01,-8.16,;-5.23,-9.48,;-5.98,-10.82,;-5.21,-12.15,;-3.66,-12.13,;-2.9,-10.79,;-1.37,-10.77,;-3.69,-9.47,;-2.93,-8.14,;-3.84,-6.9,;-2.95,-5.64,;-1.48,-6.1,;-.16,-5.33,;-.1,-3.79,;1.27,-3.07,;2.56,-3.9,;3.92,-3.18,;3.98,-1.64,;2.68,-.83,;1.32,-1.54,;.02,-.73,;5.22,-4.01,;5.16,-5.55,;6.59,-3.29,;7.89,-4.12,;8.6,-5.48,;9.43,-4.18,;1.18,-6.08,;1.19,-7.63,;-.14,-8.4,;-1.47,-7.64,)| Show InChI InChI=1S/C21H15ClF2N6O/c22-14-7-4-11(21(31)27-12-5-6-12)8-17(14)28-20-13-9-26-30(18(13)10-25-29-20)19-15(23)2-1-3-16(19)24/h1-4,7-10,12H,5-6H2,(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38beta |
Bioorg Med Chem Lett 20: 1680-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.059
BindingDB Entry DOI: 10.7270/Q2348KG8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50492386

(CHEMBL2401979)Show SMILES Cc1c(cnn1-c1ncccc1C(F)(F)F)C(=O)Nc1cc(ccc1C)C(=O)NC1CC1 Show InChI InChI=1S/C22H20F3N5O2/c1-12-5-6-14(20(31)28-15-7-8-15)10-18(12)29-21(32)16-11-27-30(13(16)2)19-17(22(23,24)25)4-3-9-26-19/h3-6,9-11,15H,7-8H2,1-2H3,(H,28,31)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38beta (unknown origin) |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of P38beta2 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM50421141
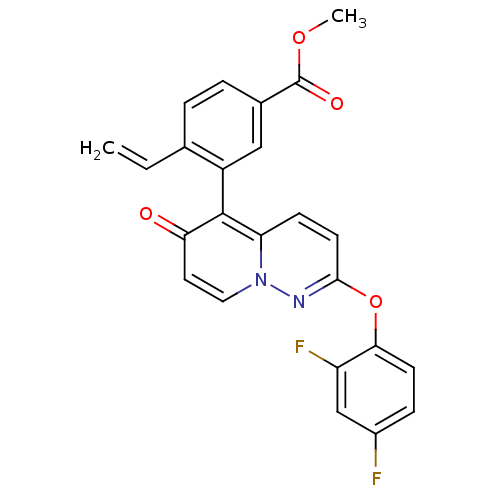
(CHEMBL2088578)Show SMILES COC(=O)c1ccc(C=C)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(46,-11.48,;44.67,-12.26,;43.33,-11.49,;43.33,-9.95,;42,-12.27,;40.67,-11.5,;39.34,-12.27,;39.34,-13.82,;38,-14.59,;36.67,-13.82,;40.67,-14.59,;42.01,-13.82,;40.67,-16.12,;42,-16.89,;43.32,-16.12,;44.65,-16.88,;44.65,-18.42,;45.99,-19.18,;46,-20.72,;44.66,-21.49,;44.67,-23.03,;46.01,-23.8,;46.01,-25.34,;47.34,-23.01,;47.33,-21.48,;48.66,-20.7,;43.33,-19.18,;42,-18.42,;40.67,-19.2,;39.33,-18.43,;39.34,-16.89,;38,-16.12,)| Show InChI InChI=1S/C24H16F2N2O4/c1-3-14-4-5-15(24(30)31-2)12-17(14)23-19-7-9-22(27-28(19)11-10-20(23)29)32-21-8-6-16(25)13-18(21)26/h3-13H,1H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38beta assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50071873

(4-[2-(4-Fluoro-phenyl)-5-(4-methanesulfinyl-phenyl...)Show SMILES CS(=O)c1ccc(cc1)-c1cc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C22H17FN2OS/c1-27(26)19-8-4-16(5-9-19)21-14-20(15-10-12-24-13-11-15)22(25-21)17-2-6-18(23)7-3-17/h2-14,25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 beta |
Bioorg Med Chem Lett 8: 2689-94 (1999)
BindingDB Entry DOI: 10.7270/Q27D2T9N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM50421140
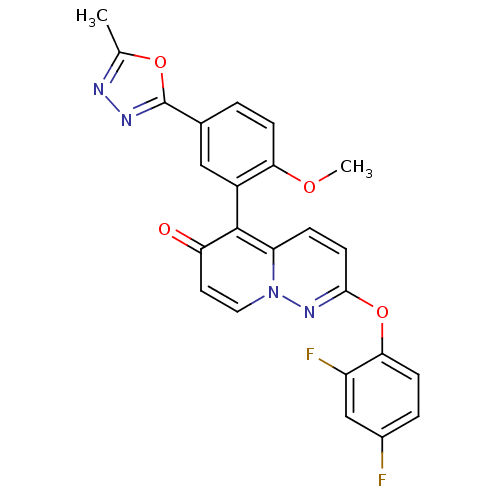
(CHEMBL2088591)Show SMILES COc1ccc(cc1-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O)-c1nnc(C)o1 |(33.77,-46.54,;35.1,-47.32,;36.43,-46.55,;36.44,-45,;37.77,-44.23,;39.1,-45,;39.11,-46.55,;37.77,-47.32,;37.77,-48.85,;39.1,-49.62,;40.42,-48.85,;41.75,-49.61,;41.75,-51.14,;43.09,-51.91,;43.1,-53.45,;41.76,-54.22,;41.77,-55.76,;43.1,-56.53,;43.11,-58.07,;44.44,-55.74,;44.43,-54.21,;45.76,-53.43,;40.43,-51.91,;39.1,-51.15,;37.77,-51.93,;36.43,-51.16,;36.43,-49.62,;35.1,-48.85,;40.36,-44.1,;41.82,-44.58,;42.73,-43.34,;41.83,-42.09,;42.31,-40.63,;40.36,-42.56,)| Show InChI InChI=1S/C24H16F2N4O4/c1-13-27-28-24(33-13)14-3-6-20(32-2)16(11-14)23-18-5-8-22(29-30(18)10-9-19(23)31)34-21-7-4-15(25)12-17(21)26/h3-12H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38beta assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM50317579

(6-(2,4-difluorophenyl)-1-(2,6-difluorophenyl)-2H-q...)Show SMILES Fc1ccc(c(F)c1)-c1cccc2c(-c3c(F)cccc3F)c(=O)ccn12 |(29.65,-6.7,;29.65,-5.16,;28.31,-4.39,;28.31,-2.85,;29.65,-2.08,;30.98,-2.84,;32.31,-2.07,;30.99,-4.38,;29.65,-.54,;30.98,.23,;30.98,1.77,;29.65,2.53,;28.33,1.77,;27,2.55,;27,4.09,;28.33,4.85,;29.67,4.08,;28.33,6.39,;27,7.17,;25.66,6.39,;25.67,4.85,;24.33,4.08,;25.67,1.77,;24.33,2.53,;25.67,.23,;27,-.54,;28.32,.23,)| Show InChI InChI=1S/C21H11F4NO/c22-12-7-8-13(16(25)11-12)17-5-2-6-18-21(19(27)9-10-26(17)18)20-14(23)3-1-4-15(20)24/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38beta assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM50421137
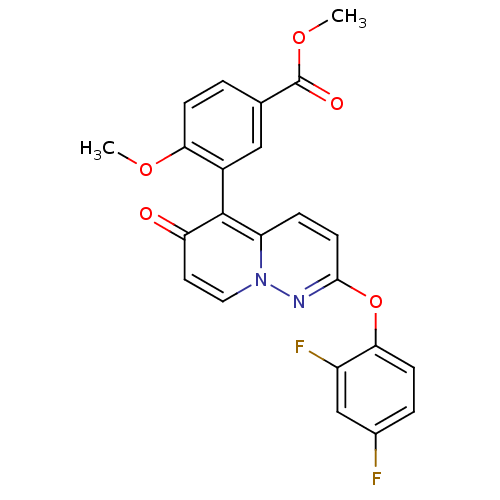
(CHEMBL2088588)Show SMILES COC(=O)c1ccc(OC)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(7.25,-42.41,;5.92,-43.19,;4.58,-42.42,;4.58,-40.88,;3.25,-43.2,;1.92,-42.43,;.59,-43.21,;.59,-44.75,;-.75,-45.52,;-2.08,-44.75,;1.92,-45.52,;3.26,-44.75,;1.92,-47.06,;3.25,-47.82,;4.57,-47.05,;5.9,-47.81,;5.91,-49.35,;7.24,-50.11,;7.25,-51.65,;5.91,-52.42,;5.92,-53.96,;7.26,-54.73,;7.26,-56.27,;8.59,-53.95,;8.58,-52.41,;9.91,-51.63,;4.58,-50.12,;3.25,-49.35,;1.92,-50.14,;.59,-49.36,;.59,-47.83,;-.75,-47.05,)| Show InChI InChI=1S/C23H16F2N2O5/c1-30-19-6-3-13(23(29)31-2)11-15(19)22-17-5-8-21(26-27(17)10-9-18(22)28)32-20-7-4-14(24)12-16(20)25/h3-12H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38beta assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50044784
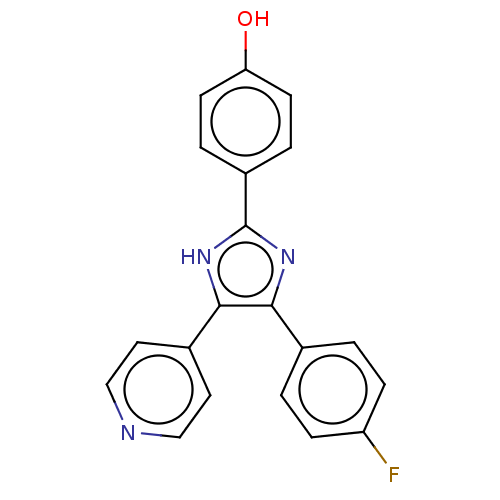
(CHEBI:79090 | SB-202190 | US10865384, Compound SB2...)Show SMILES Oc1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H14FN3O/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(25)8-4-15/h1-12,25H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of p38beta (unknown origin) |
Nat Rev Drug Discov 16: 424-440 (2017)
Article DOI: 10.1038/nrd.2016.266
BindingDB Entry DOI: 10.7270/Q2125VNC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50071867
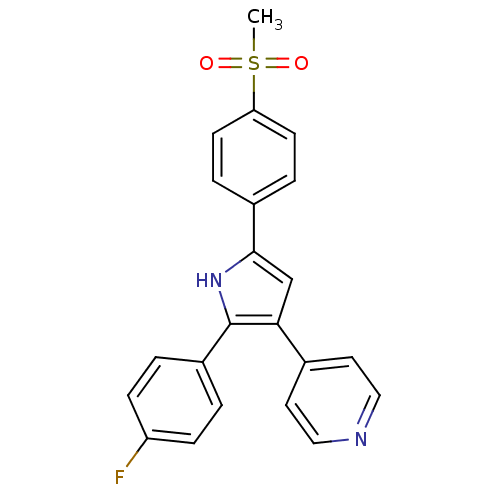
(4-[2-(4-Fluoro-phenyl)-5-(4-methanesulfonyl-phenyl...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C22H17FN2O2S/c1-28(26,27)19-8-4-16(5-9-19)21-14-20(15-10-12-24-13-11-15)22(25-21)17-2-6-18(23)7-3-17/h2-14,25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratory
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase p38 beta |
Bioorg Med Chem Lett 8: 2689-94 (1999)
BindingDB Entry DOI: 10.7270/Q27D2T9N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50331622
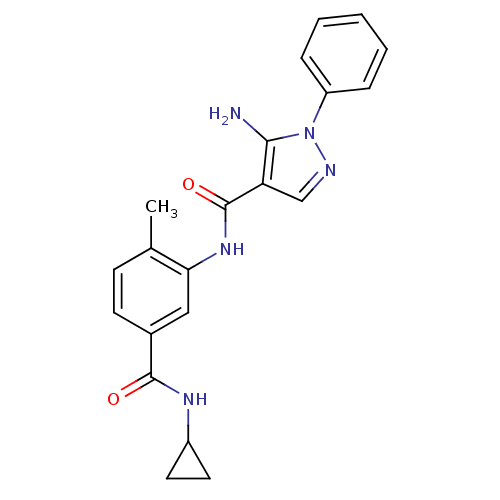
(5-amino-N-(5-(cyclopropylcarbamoyl)-2-methylphenyl...)Show SMILES Cc1ccc(cc1NC(=O)c1cnn(c1N)-c1ccccc1)C(=O)NC1CC1 Show InChI InChI=1S/C21H21N5O2/c1-13-7-8-14(20(27)24-15-9-10-15)11-18(13)25-21(28)17-12-23-26(19(17)22)16-5-3-2-4-6-16/h2-8,11-12,15H,9-10,22H2,1H3,(H,24,27)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged P38beta MAPK (1 to 364 residues) expressed in Escherichia coli incubated for 60 mins by [gamma-33P]ATP bas... |
J Med Chem 62: 10757-10782 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01227
BindingDB Entry DOI: 10.7270/Q2NV9NJK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50505859
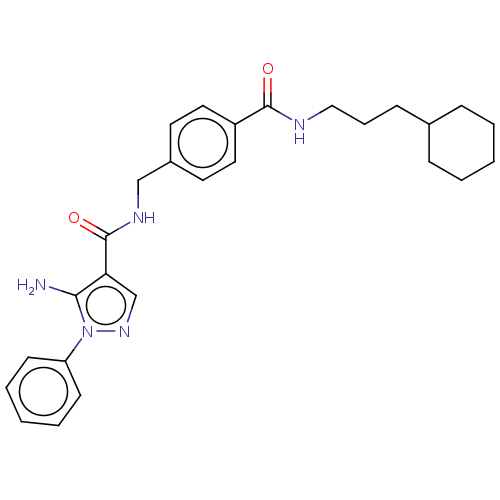
(CHEMBL4436748)Show SMILES Nc1c(cnn1-c1ccccc1)C(=O)NCc1ccc(cc1)C(=O)NCCCC1CCCCC1 Show InChI InChI=1S/C27H33N5O2/c28-25-24(19-31-32(25)23-11-5-2-6-12-23)27(34)30-18-21-13-15-22(16-14-21)26(33)29-17-7-10-20-8-3-1-4-9-20/h2,5-6,11-16,19-20H,1,3-4,7-10,17-18,28H2,(H,29,33)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-University
Curated by ChEMBL
| Assay Description
Inhibition of p38-beta (unknown origin) expressed in human HEK293 cells incubated for 2 hrs followed by NanoBRET NanoGlo Substrate addition by NanoBR... |
J Med Chem 62: 10757-10782 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01227
BindingDB Entry DOI: 10.7270/Q2NV9NJK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50045333
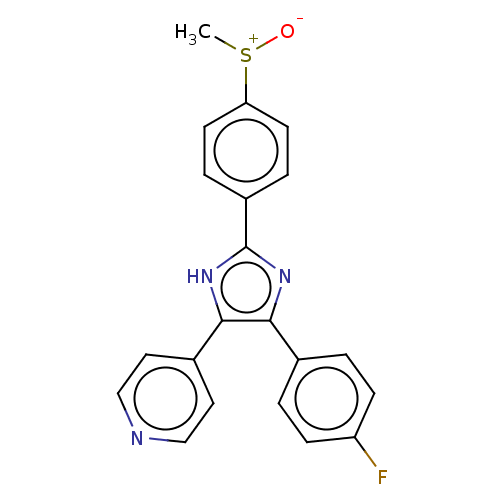
(CHEBI:90705 | SB-203580)Show SMILES C[S+]([O-])c1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney
Curated by ChEMBL
| Assay Description
Inhibition of p38beta (unknown origin) |
Nat Rev Drug Discov 16: 424-440 (2017)
Article DOI: 10.1038/nrd.2016.266
BindingDB Entry DOI: 10.7270/Q2125VNC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50376227
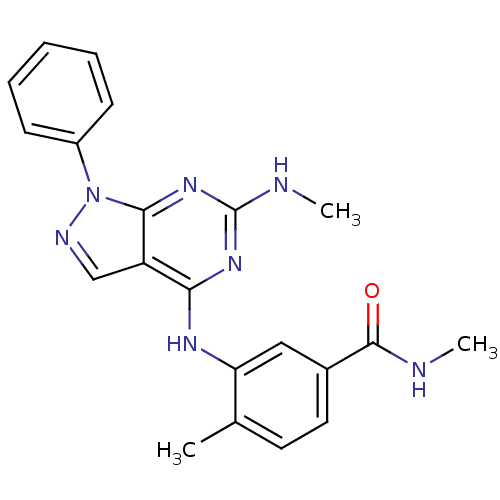
(CHEMBL412281)Show SMILES CNC(=O)c1ccc(C)c(Nc2nc(NC)nc3n(ncc23)-c2ccccc2)c1 Show InChI InChI=1S/C21H21N7O/c1-13-9-10-14(20(29)22-2)11-17(13)25-18-16-12-24-28(15-7-5-4-6-8-15)19(16)27-21(23-3)26-18/h4-12H,1-3H3,(H,22,29)(H2,23,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of p38beta |
Bioorg Med Chem Lett 18: 2652-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.019
BindingDB Entry DOI: 10.7270/Q2C53MR6 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50099349
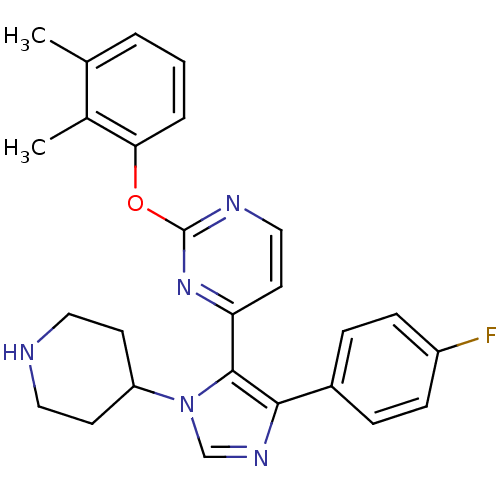
(2-(2,3-Dimethyl-phenoxy)-4-[5-(4-fluoro-phenyl)-3-...)Show SMILES Cc1cccc(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)c1C Show InChI InChI=1S/C26H26FN5O/c1-17-4-3-5-23(18(17)2)33-26-29-15-12-22(31-26)25-24(19-6-8-20(27)9-7-19)30-16-32(25)21-10-13-28-14-11-21/h3-9,12,15-16,21,28H,10-11,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM16524
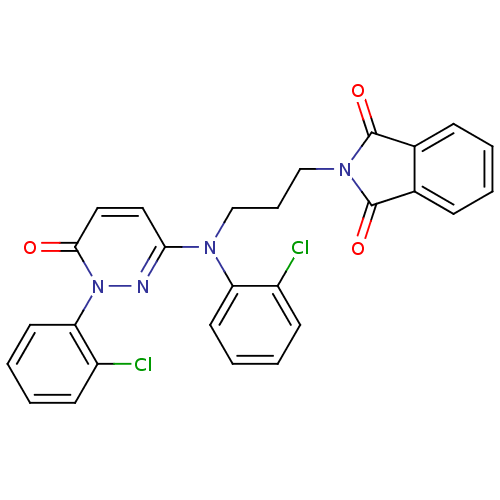
(2-arylpyridazin-3-one, 30 | 2-{3-[(2-chlorophenyl)...)Show SMILES Clc1ccccc1N(CCCN1C(=O)c2ccccc2C1=O)c1ccc(=O)n(n1)-c1ccccc1Cl Show InChI InChI=1S/C27H20Cl2N4O3/c28-20-10-3-5-12-22(20)31(16-7-17-32-26(35)18-8-1-2-9-19(18)27(32)36)24-14-15-25(34)33(30-24)23-13-6-4-11-21(23)29/h1-6,8-15H,7,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 5809-13 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.074
BindingDB Entry DOI: 10.7270/Q28P5XSC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM50317588
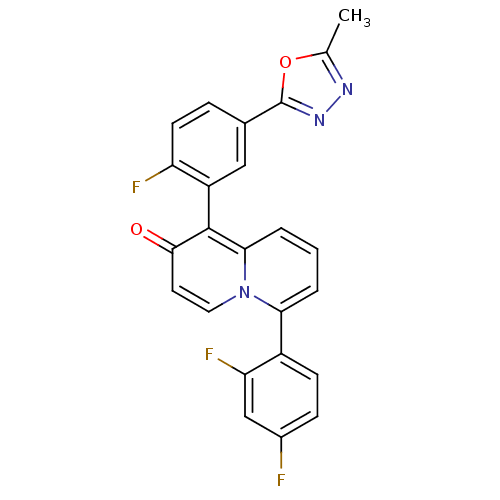
(6-(2,4-difluorophenyl)-1-(2-fluoro-5-(5-methyl-1,3...)Show SMILES Cc1nnc(o1)-c1ccc(F)c(c1)-c1c2cccc(-c3ccc(F)cc3F)n2ccc1=O |(31.09,6.57,;30.64,5.09,;31.57,3.86,;30.68,2.6,;29.21,3.05,;29.18,4.59,;27.88,2.28,;26.54,3.06,;25.2,2.28,;25.21,.74,;23.87,-.03,;26.54,-.02,;27.87,.74,;26.54,-1.56,;27.87,-2.34,;29.19,-1.58,;30.52,-2.34,;30.52,-3.88,;29.19,-4.65,;29.19,-6.19,;27.85,-6.96,;27.85,-8.5,;29.19,-9.27,;29.19,-10.81,;30.53,-8.49,;30.52,-6.95,;31.85,-6.18,;27.86,-3.88,;26.54,-4.65,;25.21,-3.88,;25.21,-2.34,;23.87,-1.58,)| Show InChI InChI=1S/C24H14F3N3O2/c1-13-28-29-24(32-13)14-5-8-18(26)17(11-14)23-21-4-2-3-20(30(21)10-9-22(23)31)16-7-6-15(25)12-19(16)27/h2-12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38beta assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50556597
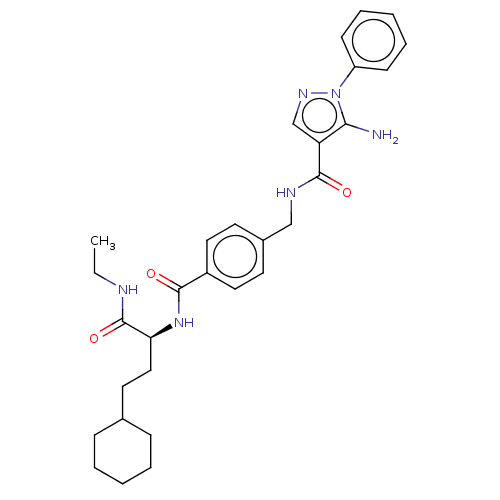
(CHEMBL4756586)Show SMILES CCNC(=O)[C@H](CCC1CCCCC1)NC(=O)c1ccc(CNC(=O)c2cnn(c2N)-c2ccccc2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of C-terminal NanoLuc-fused full length p38beta (unknown origin) expressed in HEK293T cells after 2 hrs by NanoBRET assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112721
BindingDB Entry DOI: 10.7270/Q24X5CGW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM50317583
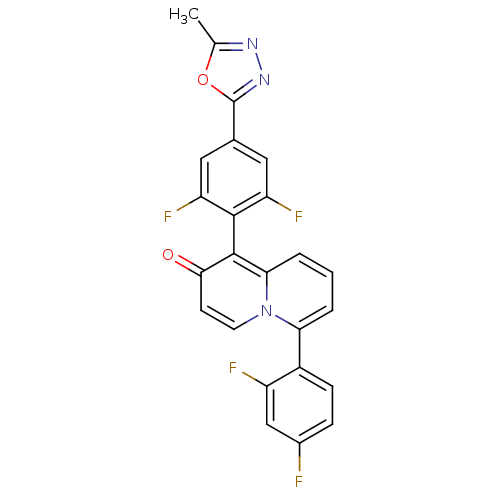
(1-(2,6-difluoro-4-(5-methyl-1,3,4-oxadiazol-2-yl)p...)Show SMILES Cc1nnc(o1)-c1cc(F)c(c(F)c1)-c1c2cccc(-c3ccc(F)cc3F)n2ccc1=O |(-7.52,-29.92,;-6.62,-31.17,;-5.08,-31.17,;-4.6,-32.63,;-5.84,-33.54,;-7.09,-32.63,;-5.85,-35.08,;-4.51,-35.86,;-4.51,-37.39,;-3.18,-38.17,;-5.85,-38.16,;-7.18,-37.4,;-8.51,-38.17,;-7.19,-35.86,;-5.85,-39.7,;-4.52,-40.48,;-3.2,-39.71,;-1.87,-40.47,;-1.87,-42.02,;-3.2,-42.78,;-3.2,-44.32,;-4.53,-45.09,;-4.54,-46.63,;-3.2,-47.4,;-3.2,-48.94,;-1.86,-46.62,;-1.87,-45.09,;-.54,-44.31,;-4.52,-42.02,;-5.85,-42.78,;-7.18,-42.02,;-7.18,-40.48,;-8.52,-39.71,)| Show InChI InChI=1S/C24H13F4N3O2/c1-12-29-30-24(33-12)13-9-17(27)22(18(28)10-13)23-20-4-2-3-19(31(20)8-7-21(23)32)15-6-5-14(25)11-16(15)26/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38beta assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
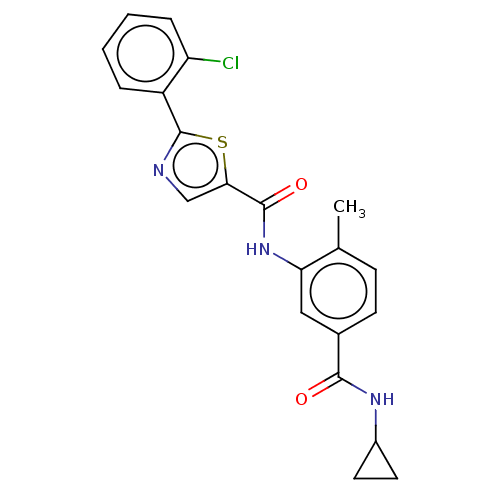
(Homo sapiens (Human)) | BDBM50492393
(CHEMBL2401978)Show SMILES Cc1ccc(cc1NC(=O)c1cnc(s1)-c1ccccc1Cl)C(=O)NC1CC1 Show InChI InChI=1S/C21H18ClN3O2S/c1-12-6-7-13(19(26)24-14-8-9-14)10-17(12)25-20(27)18-11-23-21(28-18)15-4-2-3-5-16(15)22/h2-7,10-11,14H,8-9H2,1H3,(H,24,26)(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human p38beta expressed in Escherichia coli using MBP as substrate preincubated for 10 mins prior to substrate addition measured after ... |
Bioorg Med Chem Lett 23: 4120-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.047
BindingDB Entry DOI: 10.7270/Q2FN193N |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
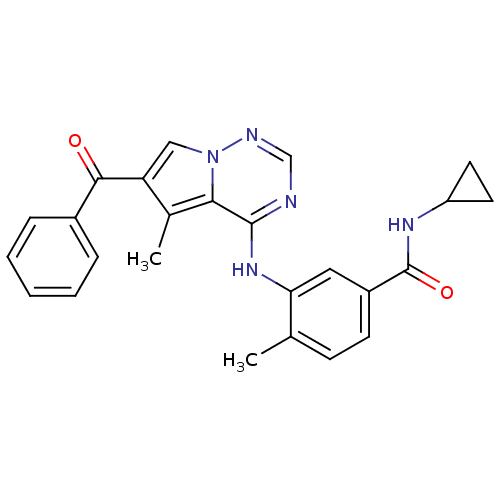
(Homo sapiens (Human)) | BDBM50348880
(CHEMBL1807446)Show SMILES Cc1c(cn2ncnc(Nc3cc(ccc3C)C(=O)NC3CC3)c12)C(=O)c1ccccc1 Show InChI InChI=1S/C25H23N5O2/c1-15-8-9-18(25(32)28-19-10-11-19)12-21(15)29-24-22-16(2)20(13-30(22)27-14-26-24)23(31)17-6-4-3-5-7-17/h3-9,12-14,19H,10-11H2,1-2H3,(H,28,32)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of p38beta |
Bioorg Med Chem Lett 21: 4633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.091
BindingDB Entry DOI: 10.7270/Q2WS8TMN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM16525
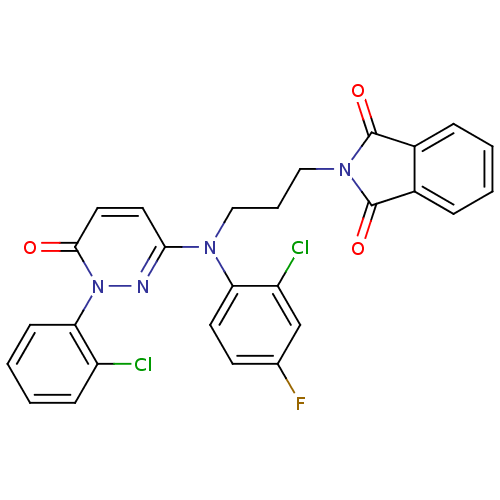
(2-arylpyridazin-3-one, 31 | 2-{3-[(2-chloro-4-fluo...)Show SMILES Fc1ccc(N(CCCN2C(=O)c3ccccc3C2=O)c2ccc(=O)n(n2)-c2ccccc2Cl)c(Cl)c1 Show InChI InChI=1S/C27H19Cl2FN4O3/c28-20-8-3-4-9-23(20)34-25(35)13-12-24(31-34)32(22-11-10-17(30)16-21(22)29)14-5-15-33-26(36)18-6-1-2-7-19(18)27(33)37/h1-4,6-13,16H,5,14-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 5809-13 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.074
BindingDB Entry DOI: 10.7270/Q28P5XSC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
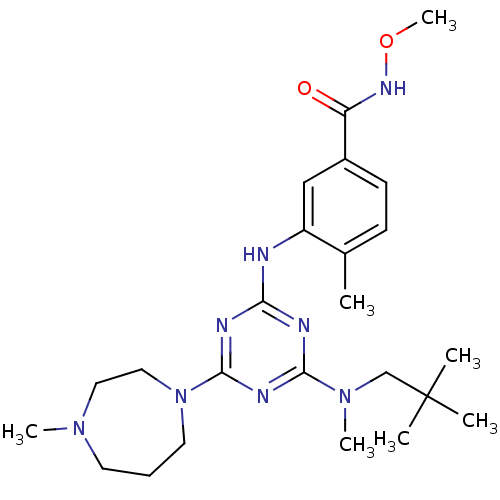
(Homo sapiens (Human)) | BDBM16316
(3-({4-[(2,2-dimethylpropyl)(methyl)amino]-6-(4-met...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(nc(n2)N2CCCN(C)CC2)N(C)CC(C)(C)C)c1 Show InChI InChI=1S/C24H38N8O2/c1-17-9-10-18(20(33)29-34-7)15-19(17)25-21-26-22(31(6)16-24(2,3)4)28-23(27-21)32-12-8-11-30(5)13-14-32/h9-10,15H,8,11-14,16H2,1-7H3,(H,29,33)(H,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human p38beta |
J Med Chem 47: 6283-91 (2004)
Article DOI: 10.1021/jm049521d
BindingDB Entry DOI: 10.7270/Q2WS8SR1 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50361467
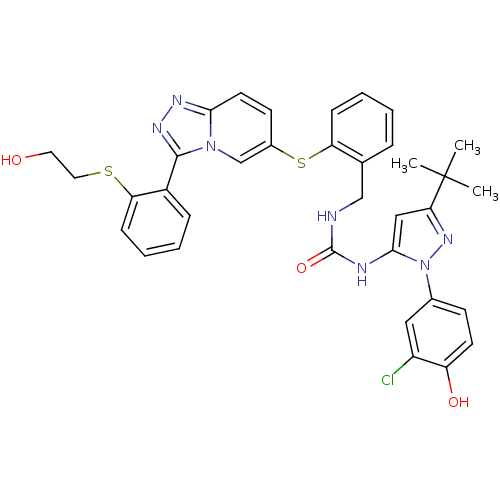
(CHEMBL1938400)Show SMILES CC(C)(C)c1cc(NC(=O)NCc2ccccc2Sc2ccc3nnc(-c4ccccc4SCCO)n3c2)n(n1)-c1ccc(O)c(Cl)c1 Show InChI InChI=1S/C35H34ClN7O3S2/c1-35(2,3)30-19-32(43(41-30)23-12-14-27(45)26(36)18-23)38-34(46)37-20-22-8-4-6-10-28(22)48-24-13-15-31-39-40-33(42(31)21-24)25-9-5-7-11-29(25)47-17-16-44/h4-15,18-19,21,44-45H,16-17,20H2,1-3H3,(H2,37,38,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38beta MAPK |
J Med Chem 54: 7797-814 (2011)
Article DOI: 10.1021/jm200677b
BindingDB Entry DOI: 10.7270/Q2HX1D4Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50099334
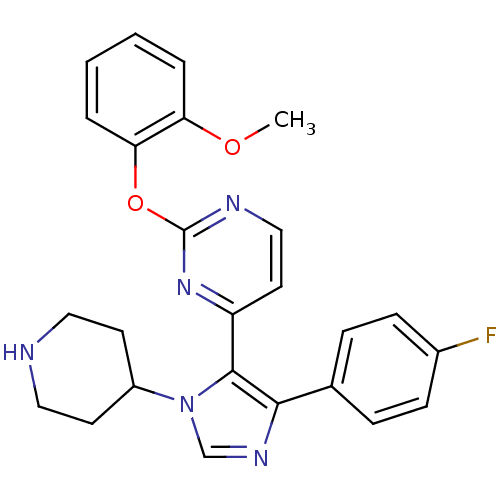
(4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...)Show SMILES COc1ccccc1Oc1nccc(n1)-c1c(ncn1C1CCNCC1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H24FN5O2/c1-32-21-4-2-3-5-22(21)33-25-28-15-12-20(30-25)24-23(17-6-8-18(26)9-7-17)29-16-31(24)19-10-13-27-14-11-19/h2-9,12,15-16,19,27H,10-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM50421127

(CHEMBL2088582)Show SMILES COC(=O)c1ccc(F)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(41.55,3.13,;41.54,4.67,;40.21,5.43,;40.2,6.97,;38.87,4.66,;37.54,5.42,;36.21,4.65,;36.21,3.11,;34.87,2.34,;37.54,2.34,;38.88,3.11,;37.55,.8,;38.88,.04,;40.19,.8,;41.52,.05,;41.53,-1.49,;42.86,-2.26,;42.87,-3.8,;41.54,-4.57,;41.54,-6.11,;42.88,-6.87,;42.89,-8.41,;44.21,-6.09,;44.2,-4.55,;45.53,-3.78,;40.2,-2.26,;38.88,-1.5,;37.54,-2.28,;36.21,-1.51,;36.21,.03,;34.88,.8,)| Show InChI InChI=1S/C22H13F3N2O4/c1-30-22(29)12-2-4-15(24)14(10-12)21-17-5-7-20(26-27(17)9-8-18(21)28)31-19-6-3-13(23)11-16(19)25/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38beta assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM50122916
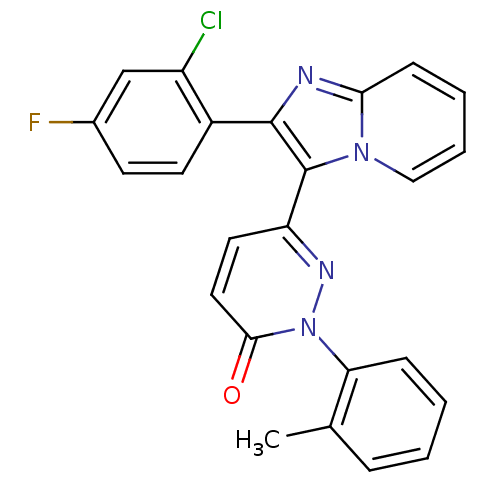
(6-[2-(2-Chloro-4-fluoro-phenyl)-imidazo[1,2-a]pyri...)Show SMILES Cc1ccccc1-n1nc(ccc1=O)-c1c(nc2ccccn12)-c1ccc(F)cc1Cl Show InChI InChI=1S/C24H16ClFN4O/c1-15-6-2-3-7-20(15)30-22(31)12-11-19(28-30)24-23(17-10-9-16(26)14-18(17)25)27-21-8-4-5-13-29(21)24/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [gamma-33P]-ATP binding from active recombinant murine FLAG-Mitogen-activated protein kinase p38 beta fusion protein (GST-ATF2) |
J Med Chem 46: 349-52 (2003)
Article DOI: 10.1021/jm025585h
BindingDB Entry DOI: 10.7270/Q27P8XRR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM16529
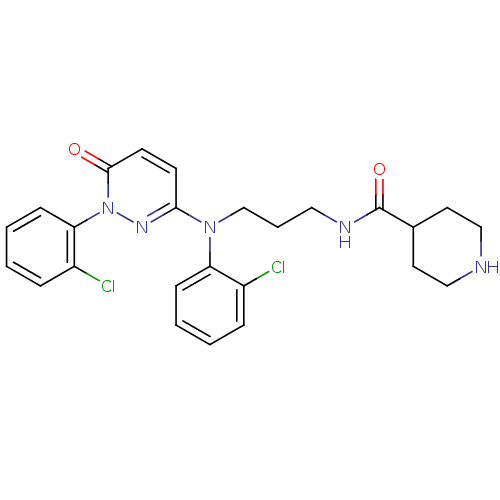
(2-arylpyridazin-3-one, 35 | N-{3-[(2-chlorophenyl)...)Show SMILES Clc1ccccc1N(CCCNC(=O)C1CCNCC1)c1ccc(=O)n(n1)-c1ccccc1Cl Show InChI InChI=1S/C25H27Cl2N5O2/c26-19-6-1-3-8-21(19)31(17-5-14-29-25(34)18-12-15-28-16-13-18)23-10-11-24(33)32(30-23)22-9-4-2-7-20(22)27/h1-4,6-11,18,28H,5,12-17H2,(H,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 5809-13 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.074
BindingDB Entry DOI: 10.7270/Q28P5XSC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM16527
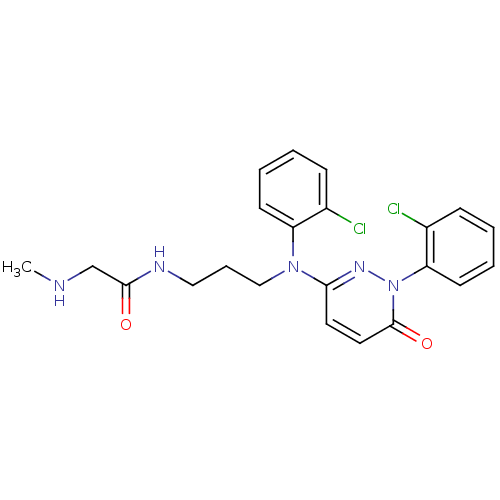
(2-arylpyridazin-3-one, 33 | N-{3-[(2-chlorophenyl)...)Show SMILES CNCC(=O)NCCCN(c1ccc(=O)n(n1)-c1ccccc1Cl)c1ccccc1Cl Show InChI InChI=1S/C22H23Cl2N5O2/c1-25-15-21(30)26-13-6-14-28(18-9-4-2-7-16(18)23)20-11-12-22(31)29(27-20)19-10-5-3-8-17(19)24/h2-5,7-12,25H,6,13-15H2,1H3,(H,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 5809-13 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.074
BindingDB Entry DOI: 10.7270/Q28P5XSC |
More data for this
Ligand-Target Pair | |
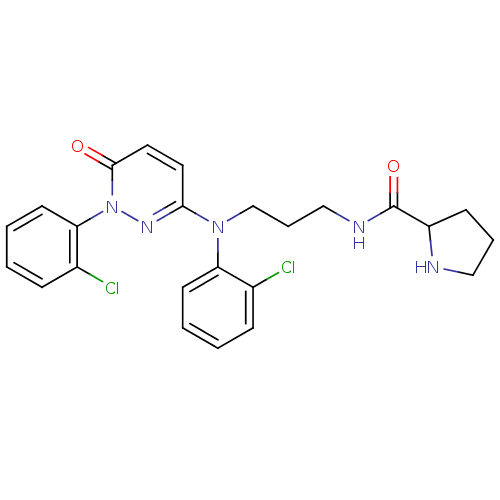
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM16528
(2-arylpyridazin-3-one, 34 | N-{3-[(2-chlorophenyl)...)Show SMILES Clc1ccccc1N(CCCNC(=O)C1CCCN1)c1ccc(=O)n(n1)-c1ccccc1Cl Show InChI InChI=1S/C24H25Cl2N5O2/c25-17-7-1-3-10-20(17)30(16-6-15-28-24(33)19-9-5-14-27-19)22-12-13-23(32)31(29-22)21-11-4-2-8-18(21)26/h1-4,7-8,10-13,19,27H,5-6,9,14-16H2,(H,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 5809-13 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.074
BindingDB Entry DOI: 10.7270/Q28P5XSC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50505859

(CHEMBL4436748)Show SMILES Nc1c(cnn1-c1ccccc1)C(=O)NCc1ccc(cc1)C(=O)NCCCC1CCCCC1 Show InChI InChI=1S/C27H33N5O2/c28-25-24(19-31-32(25)23-11-5-2-6-12-23)27(34)30-18-21-13-15-22(16-14-21)26(33)29-17-7-10-20-8-3-1-4-9-20/h2,5-6,11-16,19-20H,1,3-4,7-10,17-18,28H2,(H,29,33)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged P38beta MAPK (1 to 364 residues) expressed in Escherichia coli incubated for 60 mins by [gamma-33P]ATP bas... |
J Med Chem 62: 10757-10782 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01227
BindingDB Entry DOI: 10.7270/Q2NV9NJK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50044784

(CHEBI:79090 | SB-202190 | US10865384, Compound SB2...)Show SMILES Oc1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H14FN3O/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(25)8-4-15/h1-12,25H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human p38beta using MBP as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
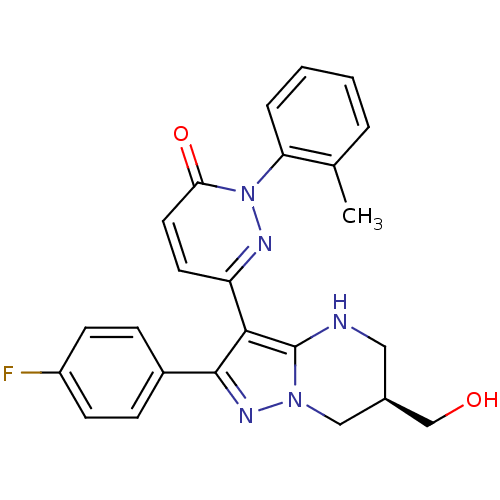
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50396845
(CHEMBL2170294)Show SMILES Cc1ccccc1-n1nc(ccc1=O)-c1c2NC[C@@H](CO)Cn2nc1-c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-4-2-3-5-20(15)30-21(32)11-10-19(27-30)22-23(17-6-8-18(25)9-7-17)28-29-13-16(14-31)12-26-24(22)29/h2-11,16,26,31H,12-14H2,1H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MAPK p38beta by Z'-LYTE assay |
J Med Chem 55: 7772-85 (2012)
Article DOI: 10.1021/jm3008008
BindingDB Entry DOI: 10.7270/Q2QN67XZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM50421133

(CHEMBL2088575)Show SMILES Fc1ccc(Oc2ccc3c(-c4cc(ccc4C=C)C(=O)NC4CC4)c(=O)ccn3n2)c(F)c1 |(13.19,-41.38,;13.18,-39.84,;11.85,-39.08,;11.84,-37.54,;13.17,-36.77,;13.17,-35.23,;11.83,-34.46,;11.82,-32.92,;10.5,-32.17,;9.18,-32.93,;7.85,-32.17,;7.85,-30.63,;9.18,-29.86,;9.18,-28.31,;7.84,-27.55,;6.52,-28.32,;6.51,-29.86,;5.18,-30.63,;3.85,-29.86,;10.51,-27.54,;10.5,-26,;11.85,-28.3,;13.18,-27.53,;14.72,-27.52,;13.94,-26.19,;6.51,-32.94,;5.18,-32.17,;6.51,-34.48,;7.85,-35.25,;9.18,-34.47,;10.51,-35.23,;14.51,-37.52,;15.84,-36.75,;14.52,-39.06,)| Show InChI InChI=1S/C26H19F2N3O3/c1-2-15-3-4-16(26(33)29-18-6-7-18)13-19(15)25-21-8-10-24(30-31(21)12-11-22(25)32)34-23-9-5-17(27)14-20(23)28/h2-5,8-14,18H,1,6-7H2,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38beta assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50284506

(4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfonyl-phenyl...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3O2S/c1-28(26,27)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01173
BindingDB Entry DOI: 10.7270/Q2P84GZF |
More data for this
Ligand-Target Pair | |
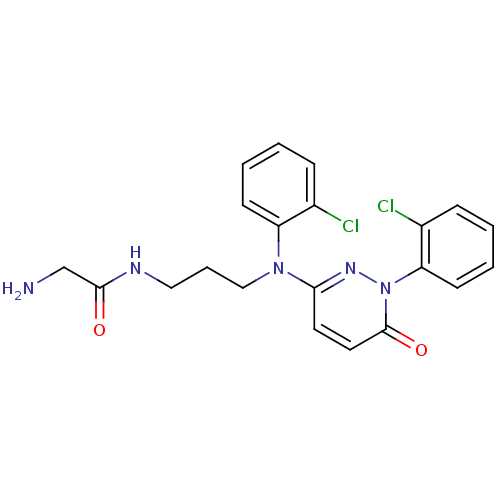
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM16526
(2-amino-N-{3-[(2-chlorophenyl)[1-(2-chlorophenyl)-...)Show SMILES NCC(=O)NCCCN(c1ccc(=O)n(n1)-c1ccccc1Cl)c1ccccc1Cl Show InChI InChI=1S/C21H21Cl2N5O2/c22-15-6-1-3-8-17(15)27(13-5-12-25-20(29)14-24)19-10-11-21(30)28(26-19)18-9-4-2-7-16(18)23/h1-4,6-11H,5,12-14,24H2,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 5809-13 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.074
BindingDB Entry DOI: 10.7270/Q28P5XSC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50105742
(4-[4-(4-Fluoro-phenyl)-5-(2-methoxy-pyrimidin-4-yl...)Show SMILES COc1nccc(n1)-c1c(ncn1[C@H]1CC[C@H](O)CC1)-c1ccc(F)cc1 |wU:16.18,wD:13.14,(3.13,-3.72,;3.16,-2.18,;4.5,-1.42,;4.53,.13,;5.87,.86,;7.2,.06,;7.17,-1.48,;5.82,-2.22,;8.5,-2.27,;8.7,-3.8,;10.21,-4.09,;10.93,-2.74,;9.88,-1.62,;10.17,-.1,;11.64,.4,;11.93,1.91,;10.77,2.91,;11.06,4.43,;9.31,2.4,;9.02,.89,;7.63,-4.91,;8.06,-6.39,;6.98,-7.48,;5.49,-7.1,;4.43,-8.22,;5.06,-5.63,;6.14,-4.53,)| Show InChI InChI=1S/C20H21FN4O2/c1-27-20-22-11-10-17(24-20)19-18(13-2-4-14(21)5-3-13)23-12-25(19)15-6-8-16(26)9-7-15/h2-5,10-12,15-16,26H,6-9H2,1H3/t15-,16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01173
BindingDB Entry DOI: 10.7270/Q2P84GZF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data