

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170371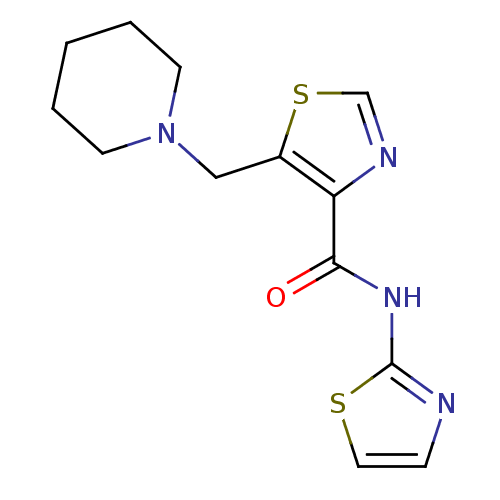 (5-Piperidin-1-ylmethyl-thiazole-4-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50267199 (CHEMBL4079583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado Mesa University Curated by ChEMBL | Assay Description Inhibition of human MetAP1 | J Nat Prod 80: 740-755 (2017) Article DOI: 10.1021/acs.jnatprod.6b00970 BindingDB Entry DOI: 10.7270/Q2SN0CG4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50169672 (5-(Cyclopentanecarbonyl-amino)-thiazole-4-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration required against Saccharomyces cerevisiae MetAP1 (330 nM) | Bioorg Med Chem Lett 15: 3732-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.055 BindingDB Entry DOI: 10.7270/Q2T1534B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129648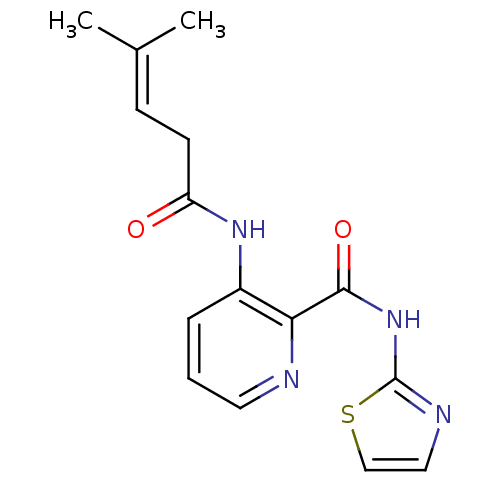 (3-(4-Methyl-pent-3-enoylamino)-pyridine-2-carboxyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170369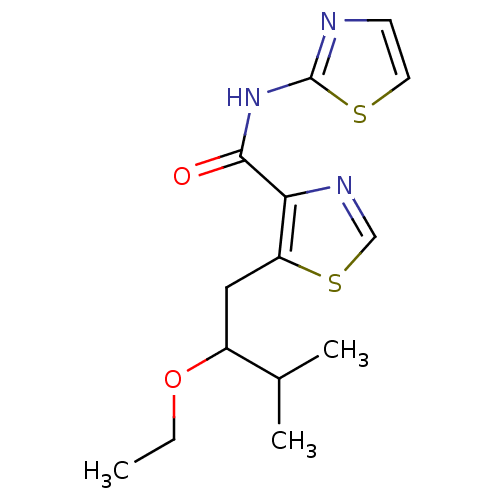 (5-(2-Ethoxy-3-methyl-butyl)-thiazole-4-carboxylic ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129642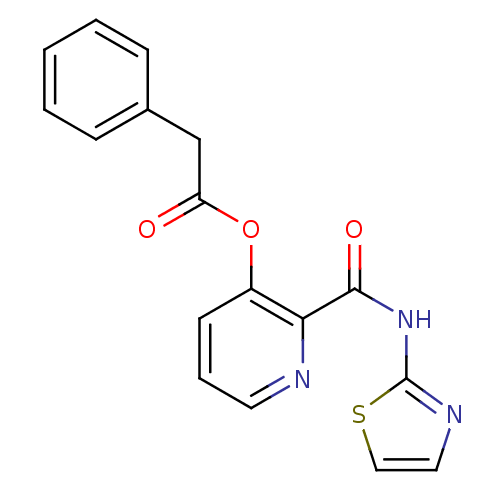 (CHEMBL86427 | Phenyl-acetic acid 2-(thiazol-2-ylca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50169671 (5-Propionylamino-thiazole-4-carboxylic acid thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration required against Saccharomyces cerevisiae MetAP1 (330 nM) | Bioorg Med Chem Lett 15: 3732-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.055 BindingDB Entry DOI: 10.7270/Q2T1534B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170363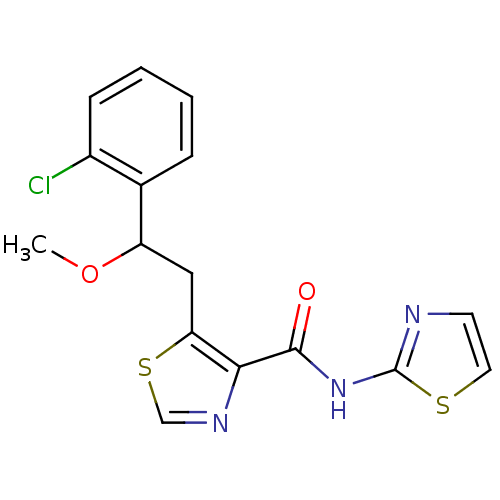 (5-[2-(2-Chloro-phenyl)-2-methoxy-ethyl]-thiazole-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50169669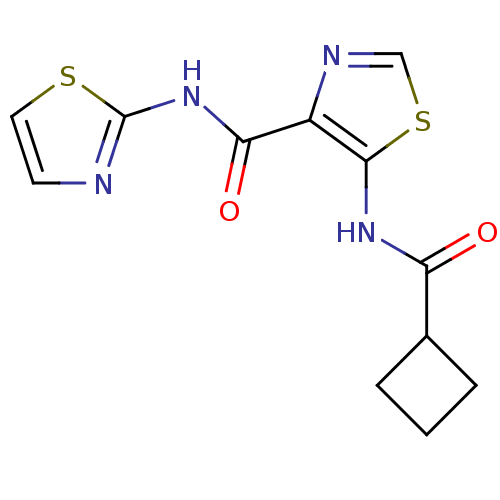 (5-(Cyclobutanecarbonyl-amino)-thiazole-4-carboxyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration required against Saccharomyces cerevisiae MetAP1 (330 nM) | Bioorg Med Chem Lett 15: 3732-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.055 BindingDB Entry DOI: 10.7270/Q2T1534B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170383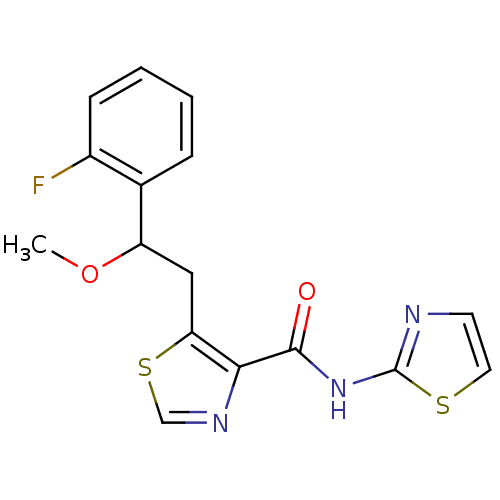 (5-[2-(2-Fluoro-phenyl)-2-methoxy-ethyl]-thiazole-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170370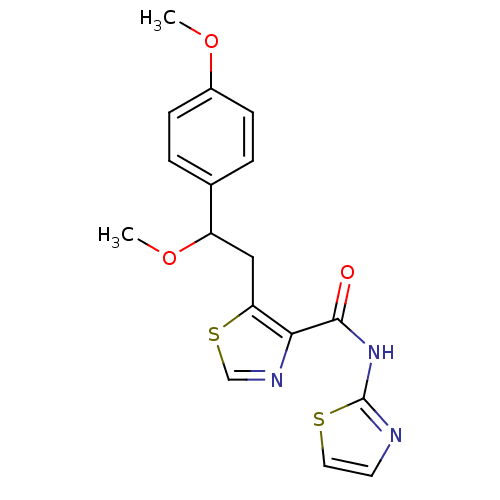 (5-[2-Methoxy-2-(4-methoxy-phenyl)-ethyl]-thiazole-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129669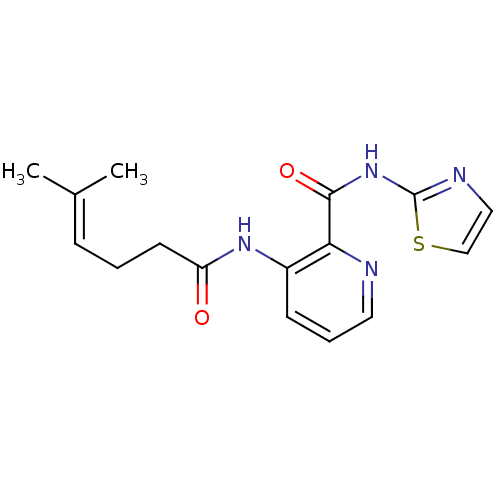 (3-(5-Methyl-hex-4-enoylamino)-pyridine-2-carboxyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129635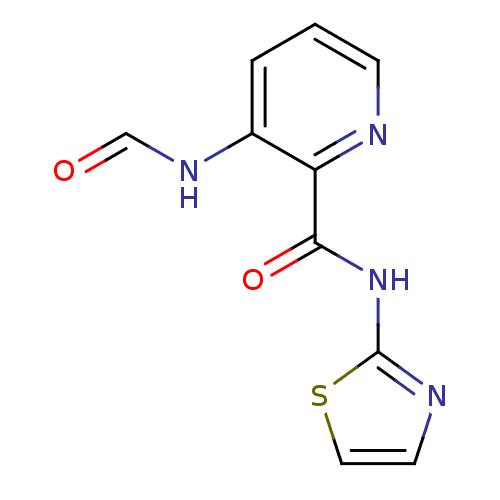 (3-Formylamino-pyridine-2-carboxylic acid thiazol-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129649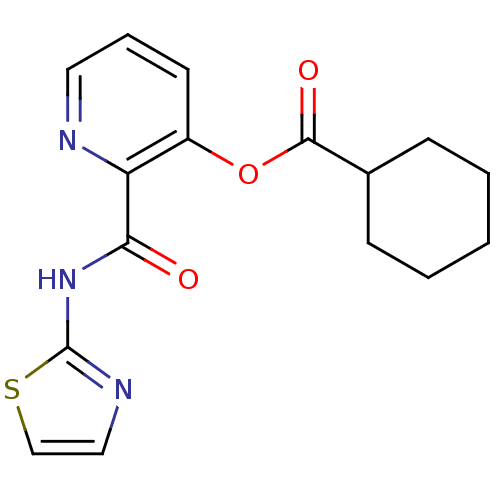 (CHEMBL315098 | Cyclohexanecarboxylic acid 2-(thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170358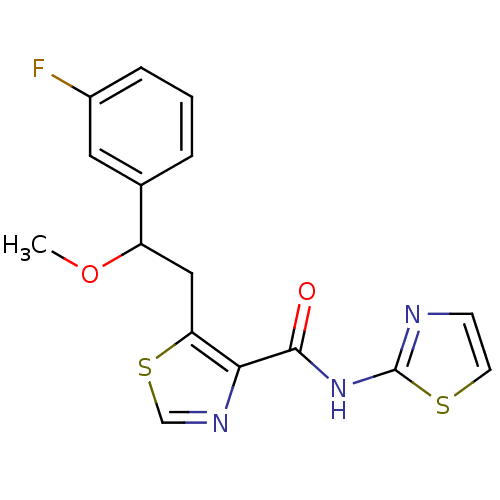 (5-[2-(3-Fluoro-phenyl)-2-methoxy-ethyl]-thiazole-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM17849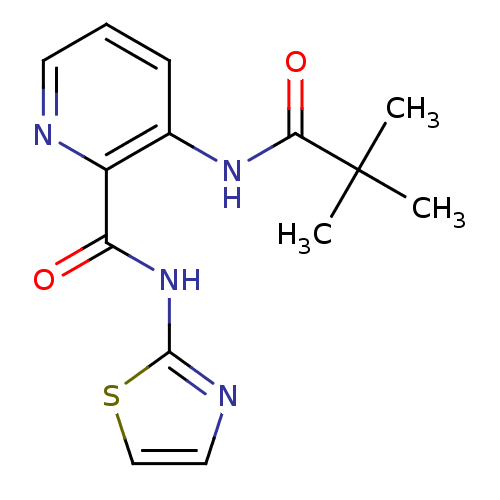 (3-(2,2-dimethylpropanamido)-N-(1,3-thiazol-2-yl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129672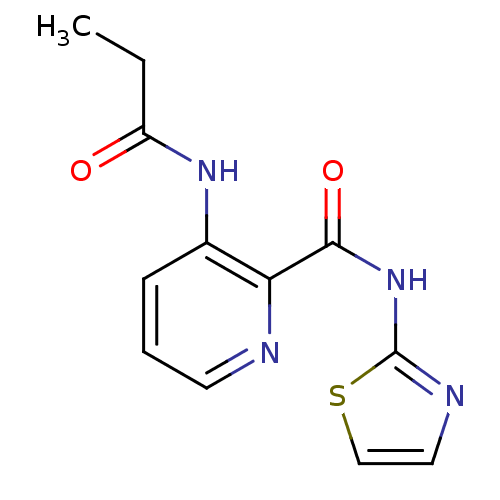 (3-Propionylamino-pyridine-2-carboxylic acid thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against Saccharomyces cerevisiae methionine aminopeptidase 1 | Bioorg Med Chem Lett 15: 635-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.034 BindingDB Entry DOI: 10.7270/Q2222T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129672 (3-Propionylamino-pyridine-2-carboxylic acid thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170365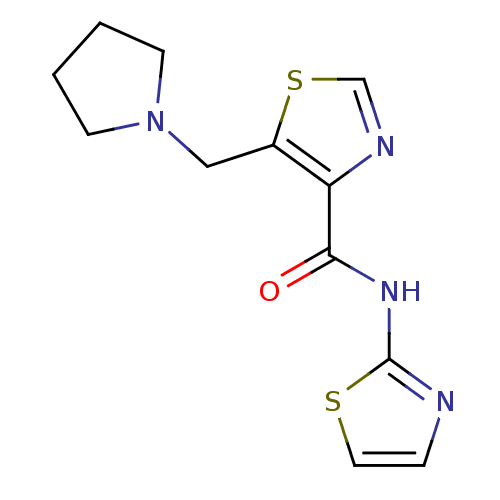 (5-Pyrrolidin-1-ylmethyl-thiazole-4-carboxylic acid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50435227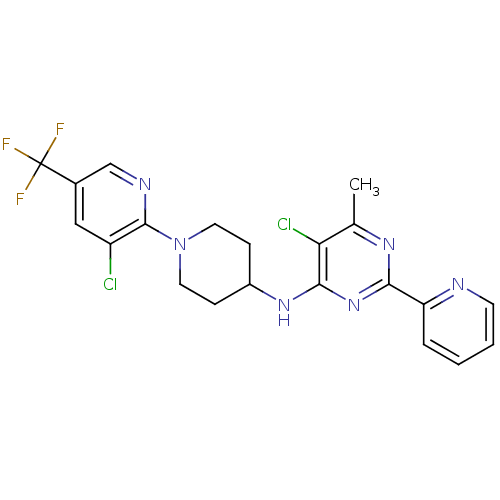 (CHEMBL2392772) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... | Bioorg Med Chem 21: 2600-17 (2013) Article DOI: 10.1016/j.bmc.2013.02.023 BindingDB Entry DOI: 10.7270/Q290256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM17589 ((2S,3R)-3-amino-5-(ethylsulfanyl)-2-hydroxy-N-[(1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Laboratories | Assay Description A coupled enzyme chromogenic assay was developed to measure methionine aminopeptidase activity by monitoring the production of free methionine with L... | Cancer Res 63: 7861-9 (2003) BindingDB Entry DOI: 10.7270/Q2Z31WXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50169656 (5-(2-Methyl-but-3-enoylamino)-thiazole-4-carboxyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration required against Saccharomyces cerevisiae MetAP1 (330 nM) | Bioorg Med Chem Lett 15: 3732-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.055 BindingDB Entry DOI: 10.7270/Q2T1534B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM17589 ((2S,3R)-3-amino-5-(ethylsulfanyl)-2-hydroxy-N-[(1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against human methionine aminopeptidase-1 | Bioorg Med Chem Lett 14: 865-8 (2004) Article DOI: 10.1016/j.bmcl.2003.12.031 BindingDB Entry DOI: 10.7270/Q2Z31Z3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129663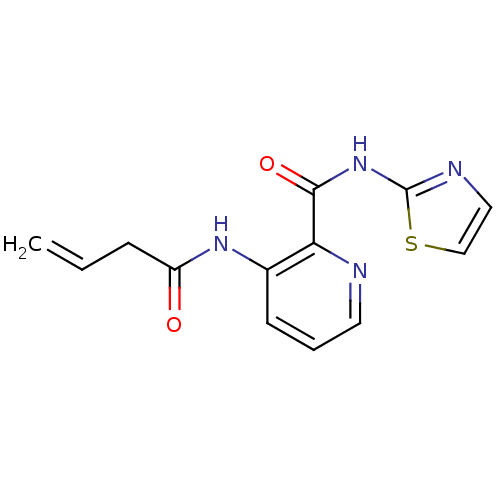 (3-But-3-enoylamino-pyridine-2-carboxylic acid thia...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129650 (CHEMBL314448 | Propionic acid 2-(thiazol-2-ylcarba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170366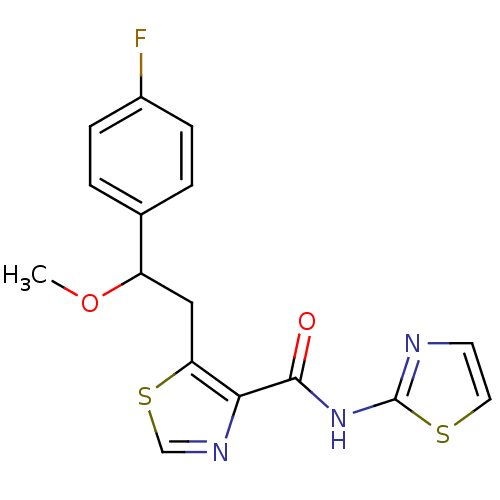 (5-[2-(4-Fluoro-phenyl)-2-methoxy-ethyl]-thiazole-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129641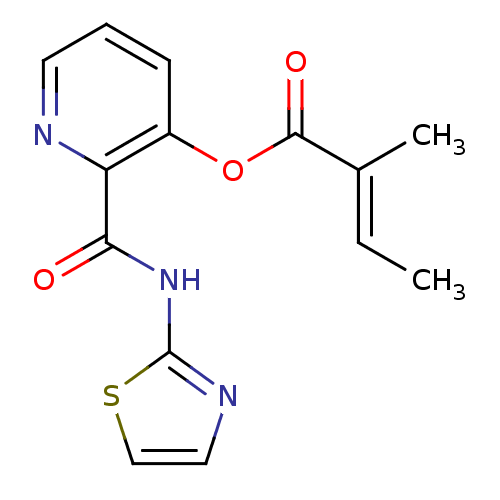 (2-Methyl-but-2-enoic acid 2-(thiazol-2-ylcarbamoyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM17847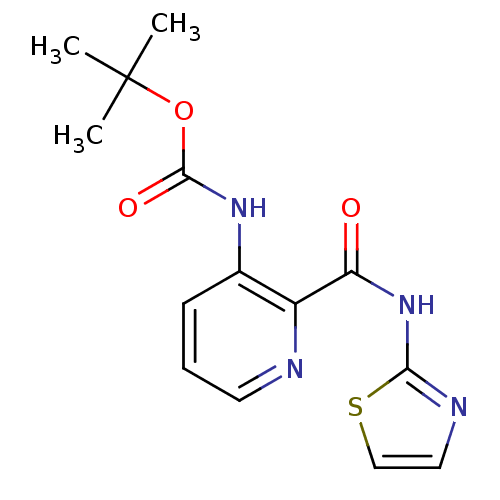 (CHEMBL327579 | pyridine-2-carboxylic acid inhibito...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Colorado Mesa University Curated by ChEMBL | Assay Description Inhibition of human MetAP1 | J Nat Prod 80: 740-755 (2017) Article DOI: 10.1021/acs.jnatprod.6b00970 BindingDB Entry DOI: 10.7270/Q2SN0CG4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170389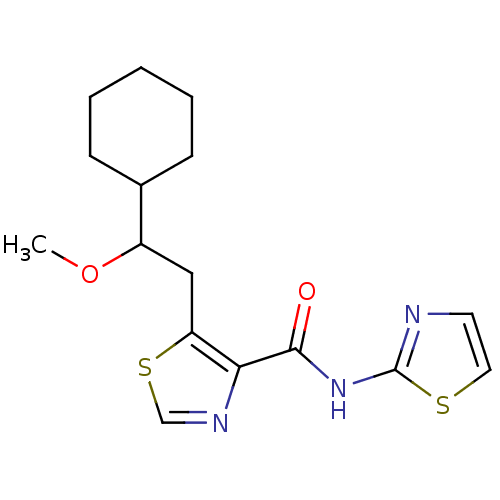 (5-(2-Cyclohexyl-2-methoxy-ethyl)-thiazole-4-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170361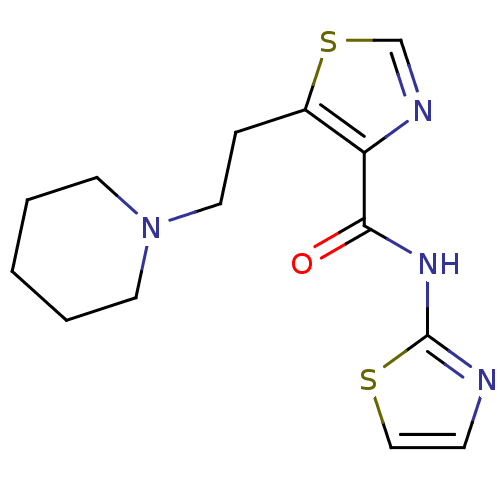 (5-(2-Piperidin-1-yl-ethyl)-thiazole-4-carboxylic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170382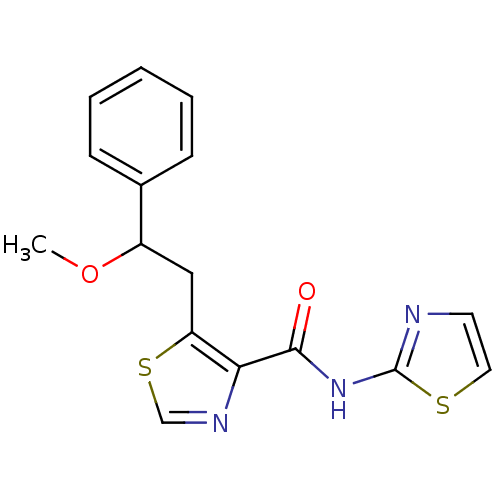 (5-(2-Methoxy-2-phenyl-ethyl)-thiazole-4-carboxylic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170381 (5-(2-Methoxy-hexyl)-thiazole-4-carboxylic acid thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170384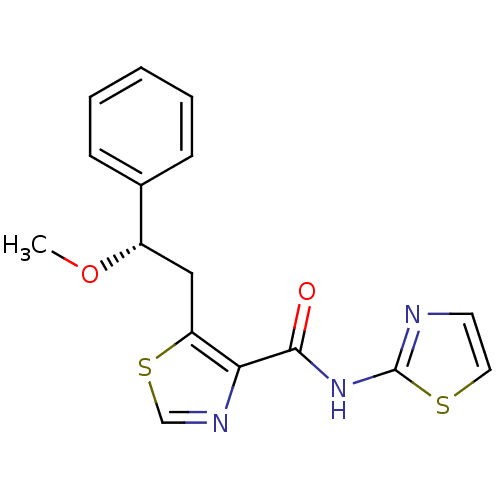 (5-((S)-2-Methoxy-2-phenyl-ethyl)-thiazole-4-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170388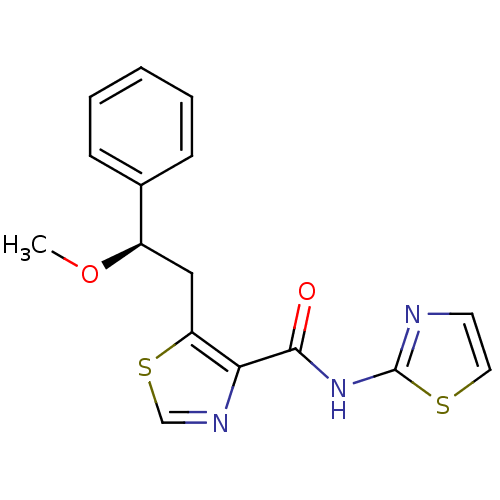 (5-((R)-2-Methoxy-2-phenyl-ethyl)-thiazole-4-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50169686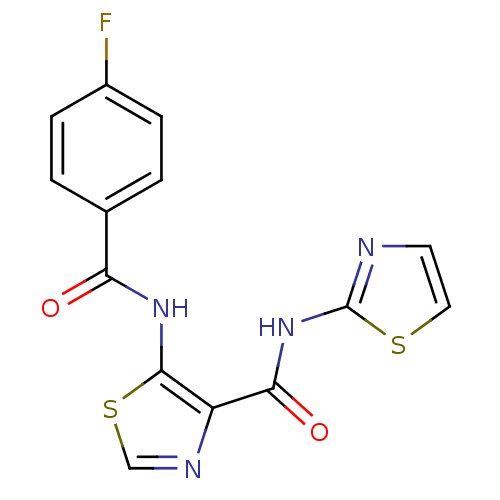 (5-(4-Fluoro-benzoylamino)-thiazole-4-carboxylic ac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration required against Saccharomyces cerevisiae MetAP1 (330 nM) | Bioorg Med Chem Lett 15: 3732-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.055 BindingDB Entry DOI: 10.7270/Q2T1534B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129646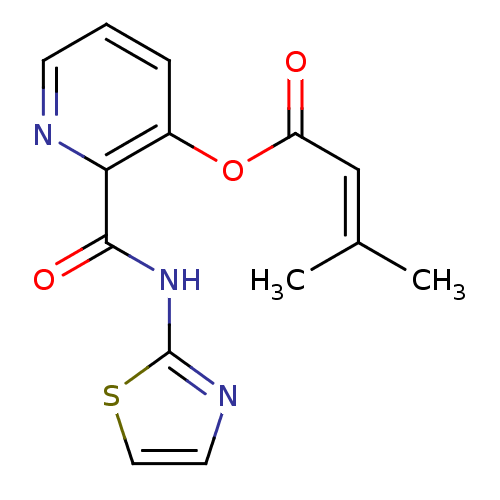 (3-Methyl-but-2-enoic acid 2-(thiazol-2-ylcarbamoyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170360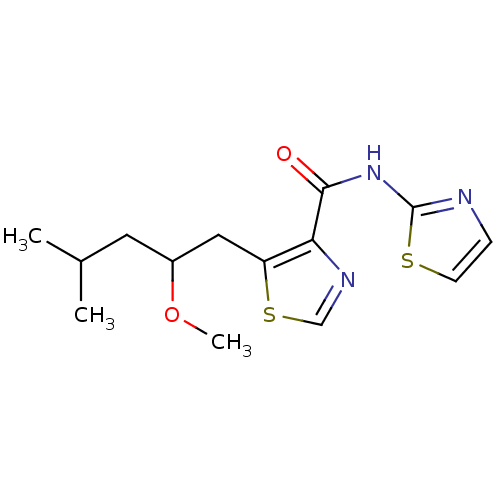 (5-(2-Methoxy-4-methyl-pentyl)-thiazole-4-carboxyli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50169679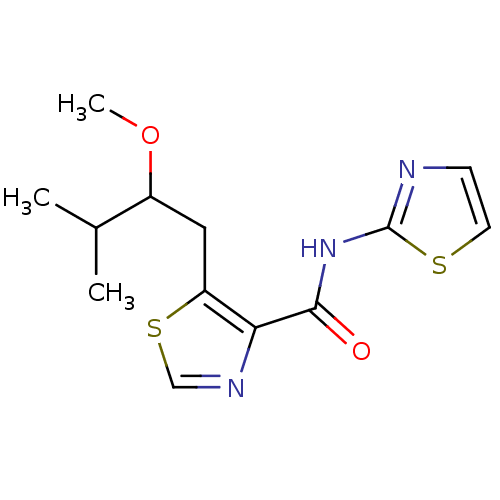 (5-(2-Methoxy-3-methyl-butyl)-thiazole-4-carboxylic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50169679 (5-(2-Methoxy-3-methyl-butyl)-thiazole-4-carboxylic...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration required against Saccharomyces cerevisiae MetAP1 (330 nM) | Bioorg Med Chem Lett 15: 3732-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.055 BindingDB Entry DOI: 10.7270/Q2T1534B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170374 (5-[2-(3-Chloro-phenyl)-2-methoxy-ethyl]-thiazole-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129675 (2-Methoxy-benzoic acid 2-(thiazol-2-ylcarbamoyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170380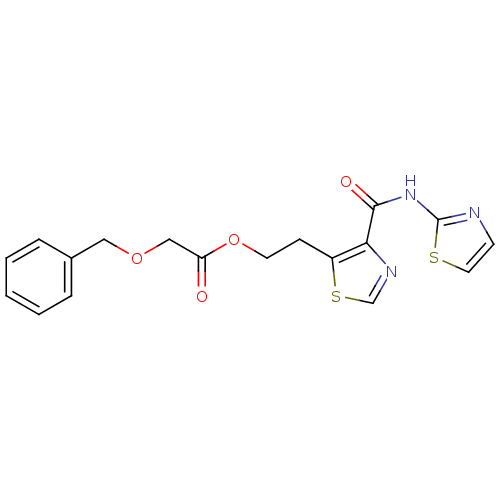 (Benzyloxy-acetic acid 2-[4-(thiazol-2-ylcarbamoyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170378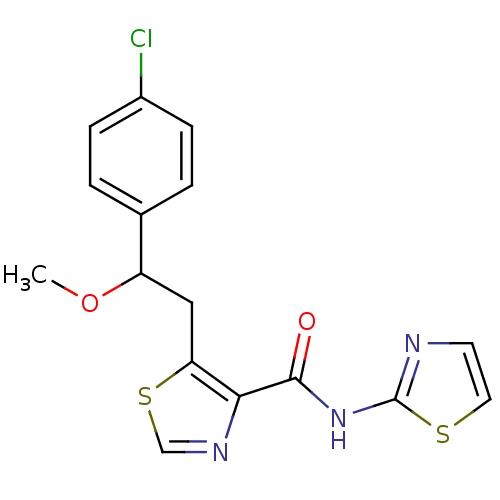 (5-[2-(4-Chloro-phenyl)-2-methoxy-ethyl]-thiazole-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50170367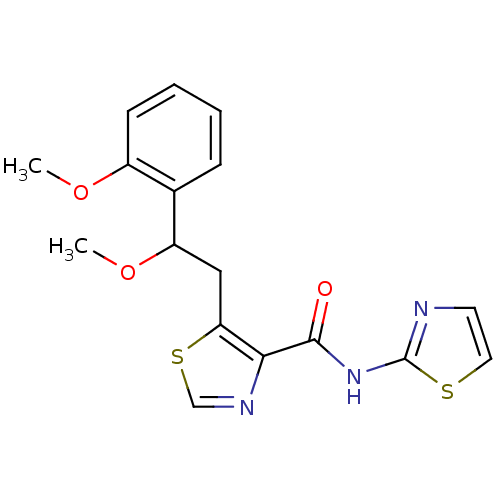 (5-[2-Methoxy-2-(2-methoxy-phenyl)-ethyl]-thiazole-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against ScMetAP1 enzyme activity | Bioorg Med Chem Lett 15: 4130-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.005 BindingDB Entry DOI: 10.7270/Q2FN15RH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50435224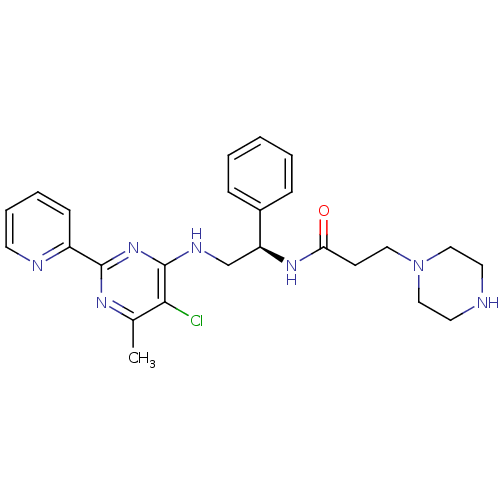 (CHEMBL2392893) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... | Bioorg Med Chem 21: 2600-17 (2013) Article DOI: 10.1016/j.bmc.2013.02.023 BindingDB Entry DOI: 10.7270/Q290256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Homo sapiens (Human)) | BDBM50435217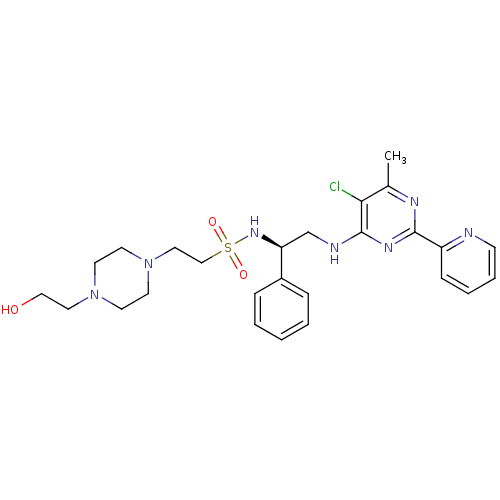 (CHEMBL2392900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant full-length cytosolic METAP1 expressed in Escherichia coli BL21(DE3) using Met-Pro-p-nitroanilide as substrate after ... | Bioorg Med Chem 21: 2600-17 (2013) Article DOI: 10.1016/j.bmc.2013.02.023 BindingDB Entry DOI: 10.7270/Q290256X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129656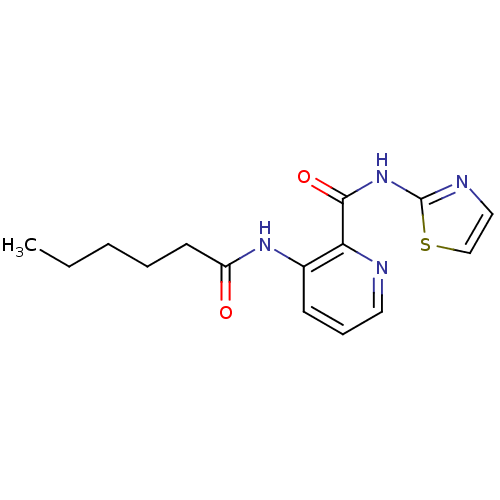 (3-Hexanoylamino-pyridine-2-carboxylic acid thiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration against Saccharomyces cerevisiae methionine aminopeptidase 1 | Bioorg Med Chem Lett 15: 635-8 (2005) Article DOI: 10.1016/j.bmcl.2004.11.034 BindingDB Entry DOI: 10.7270/Q2222T7R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50129656 (3-Hexanoylamino-pyridine-2-carboxylic acid thiazol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity against type I methionine aminopeptidase from Saccharomyces cerevisiae | J Med Chem 46: 2631-40 (2003) Article DOI: 10.1021/jm0300532 BindingDB Entry DOI: 10.7270/Q2CF9QTC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50169670 (5-Phenethyl-thiazole-4-carboxylic acid thiazol-2-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration required against Saccharomyces cerevisiae MetAP1 (330 nM) | Bioorg Med Chem Lett 15: 3732-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.055 BindingDB Entry DOI: 10.7270/Q2T1534B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 1 (Saccharomyces cerevisiae) | BDBM50169685 (CHEMBL360353 | N-[4-(Thiazol-2-ylcarbamoyl)-thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Graduate School of the Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory concentration required against Saccharomyces cerevisiae MetAP1 (330 nM) | Bioorg Med Chem Lett 15: 3732-6 (2005) Article DOI: 10.1016/j.bmcl.2005.05.055 BindingDB Entry DOI: 10.7270/Q2T1534B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 386 total ) | Next | Last >> |