 Found 5422 hits of ic50 data for polymerid = 2128
Found 5422 hits of ic50 data for polymerid = 2128 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50029559
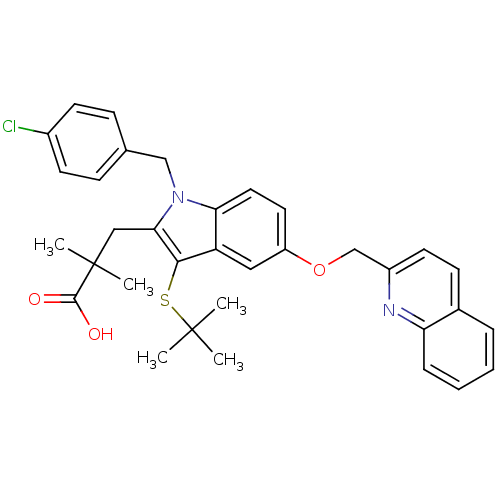
(2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50200841

(CHEMBL220360 | MK-0364 | MK-0634 | N-((2S,3S)-4-(4...)Show SMILES C[C@H](NC(=O)C(C)(C)Oc1ccc(cn1)C(F)(F)F)[C@@H](Cc1ccc(Cl)cc1)c1cccc(c1)C#N |r| Show InChI InChI=1S/C27H25ClF3N3O2/c1-17(34-25(35)26(2,3)36-24-12-9-21(16-33-24)27(29,30)31)23(14-18-7-10-22(28)11-8-18)20-6-4-5-19(13-20)15-32/h4-13,16-17,23H,14H2,1-3H3,(H,34,35)/t17-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes after 30 mins |
Bioorg Med Chem 18: 6377-88 (2010)
Article DOI: 10.1016/j.bmc.2010.07.013
BindingDB Entry DOI: 10.7270/Q2XG9RBT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50352206
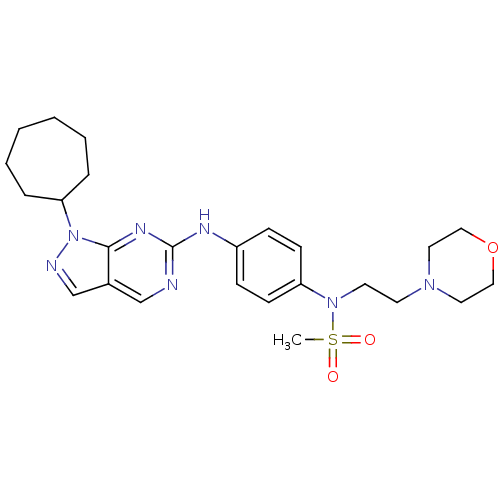
(CHEMBL1825089)Show SMILES CS(=O)(=O)N(CCN1CCOCC1)c1ccc(Nc2ncc3cnn(C4CCCCCC4)c3n2)cc1 Show InChI InChI=1S/C25H35N7O3S/c1-36(33,34)31(13-12-30-14-16-35-17-15-30)22-10-8-21(9-11-22)28-25-26-18-20-19-27-32(24(20)29-25)23-6-4-2-3-5-7-23/h8-11,18-19,23H,2-7,12-17H2,1H3,(H,26,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 using a fluorescent probe 7-methoxy-4-trifluoromethylcoumarin |
Bioorg Med Chem Lett 21: 5633-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.129
BindingDB Entry DOI: 10.7270/Q2HM58VW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50080250
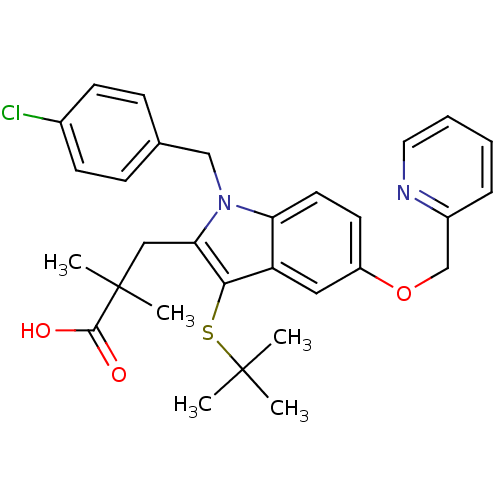
(3-(3-(tert-butylthio)-1-(4-chlorobenzyl)-5-(pyridi...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccccn3)cc12 Show InChI InChI=1S/C30H33ClN2O3S/c1-29(2,3)37-27-24-16-23(36-19-22-8-6-7-15-32-22)13-14-25(24)33(18-20-9-11-21(31)12-10-20)26(27)17-30(4,5)28(34)35/h6-16H,17-19H2,1-5H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50359083

(CHEMBL1922663)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4ccc(F)cn4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C36H38FN3O3S/c1-23-7-13-27(38-19-23)22-43-28-14-16-31-29(17-28)33(44-35(2,3)4)32(18-36(5,6)34(41)42)40(31)21-24-8-10-25(11-9-24)30-15-12-26(37)20-39-30/h7-17,19-20H,18,21-22H2,1-6H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50325213

(2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...)Show SMILES CC(C)(C)c1nnc(s1)-c1nn(c(c1Cn1cncn1)-c1ccc(Br)cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H20BrCl2N7S/c1-24(2,3)23-31-30-22(35-23)20-17(11-33-13-28-12-29-33)21(14-4-6-15(25)7-5-14)34(32-20)19-9-8-16(26)10-18(19)27/h4-10,12-13H,11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes after 30 mins |
Bioorg Med Chem 18: 6377-88 (2010)
Article DOI: 10.1016/j.bmc.2010.07.013
BindingDB Entry DOI: 10.7270/Q2XG9RBT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM143355
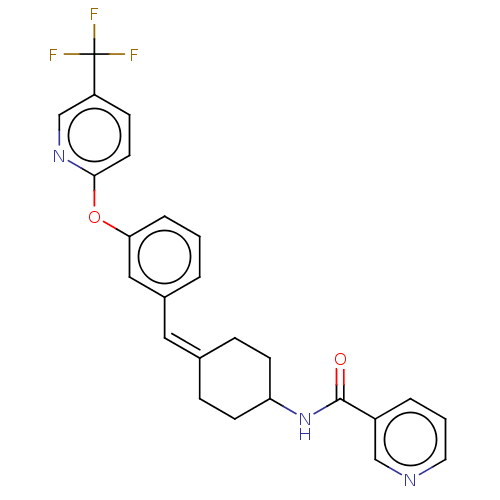
(US9682953, 20.A-1)Show SMILES CCOC(=O)Cn1nc2C(=O)N(C(c2c1C(C)C)c1ccc(Cl)cc1C)c1cc(Cl)ccc1C Show InChI InChI=1S/C25H22F3N3O2/c26-25(27,28)20-8-11-23(30-16-20)33-22-5-1-3-18(14-22)13-17-6-9-21(10-7-17)31-24(32)19-4-2-12-29-15-19/h1-5,8,11-16,21H,6-7,9-10H2,(H,31,32)/b17-13- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 5 mins followed by NADPH cofactor addition and measured... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50321353

(4-cyano-N-(2-cyclohexenyl-4-(pyridin-4-yl)phenyl)-...)Show SMILES O=C(Nc1ccc(cc1C1=CCCCC1)-c1ccncc1)c1ncc([nH]1)C#N |t:10| Show InChI InChI=1S/C22H19N5O/c23-13-18-14-25-21(26-18)22(28)27-20-7-6-17(15-8-10-24-11-9-15)12-19(20)16-4-2-1-3-5-16/h4,6-12,14H,1-3,5H2,(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 3925-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.013
BindingDB Entry DOI: 10.7270/Q22Z16HG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM143359
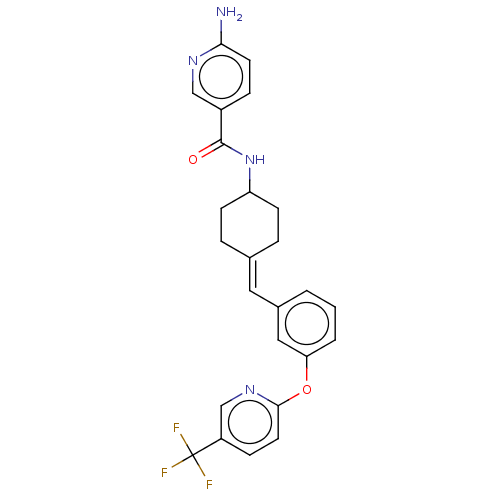
(US9682953, 20.A-3)Show SMILES Nc1ccc(cn1)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(12.65,-1.17,;11.31,-1.94,;11.31,-3.48,;9.98,-4.25,;8.65,-3.48,;8.65,-1.94,;9.98,-1.17,;7.31,-4.25,;7.31,-5.79,;5.98,-3.48,;4.65,-4.25,;4.65,-5.79,;3.31,-6.56,;1.98,-5.79,;1.98,-4.25,;3.31,-3.48,;.65,-6.56,;-.69,-5.79,;-.69,-4.25,;-2.02,-3.48,;-3.36,-4.25,;-3.36,-5.79,;-4.69,-6.56,;-6.02,-5.79,;-6.02,-4.25,;-7.36,-3.48,;-8.69,-4.25,;-8.69,-5.79,;-7.36,-6.56,;-10.02,-3.48,;-11.36,-4.25,;-10.02,-1.94,;-10.02,-5.02,;-2.02,-6.56,)| Show InChI InChI=1S/C25H23F3N4O2/c26-25(27,28)19-7-11-23(31-15-19)34-21-3-1-2-17(13-21)12-16-4-8-20(9-5-16)32-24(33)18-6-10-22(29)30-14-18/h1-3,6-7,10-15,20H,4-5,8-9H2,(H2,29,30)(H,32,33)/b16-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 5 mins followed by NADPH cofactor addition and measured... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50359088

(CHEMBL1229205)Show SMILES COc1cnc(nc1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccc(C)cn4)ccc23)cc1 Show InChI InChI=1S/C36H40N4O4S/c1-23-8-13-26(37-18-23)22-44-27-14-15-30-29(16-27)32(45-35(2,3)4)31(17-36(5,6)34(41)42)40(30)21-24-9-11-25(12-10-24)33-38-19-28(43-7)20-39-33/h8-16,18-20H,17,21-22H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes after 30 mins |
Bioorg Med Chem 18: 6377-88 (2010)
Article DOI: 10.1016/j.bmc.2010.07.013
BindingDB Entry DOI: 10.7270/Q2XG9RBT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM143373

(US9682953, 20.A-10 | US9682953, 20.A-9)Show SMILES Nc1ncc(cn1)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(11.34,2.69,;10,1.93,;10,.38,;8.67,-.38,;7.34,.38,;7.34,1.93,;8.67,2.69,;6,-.38,;6,-1.93,;4.67,.38,;3.33,-.38,;3.33,-1.93,;2,-2.69,;.67,-1.93,;.67,-.38,;2,.38,;-.67,-2.69,;-2,-1.93,;-2,-.38,;-3.33,.38,;-4.67,-.38,;-4.67,-1.93,;-6,-2.69,;-7.34,-1.93,;-7.34,-.38,;-8.67,.38,;-10,-.38,;-10,-1.93,;-8.67,-2.69,;-11.34,.38,;-12.67,-.39,;-11.34,1.93,;-11.34,-1.16,;-3.33,-2.69,)| Show InChI InChI=1S/C24H22F3N5O2/c25-24(26,27)18-6-9-21(29-14-18)34-20-3-1-2-16(11-20)10-15-4-7-19(8-5-15)32-22(33)17-12-30-23(28)31-13-17/h1-3,6,9-14,19H,4-5,7-8H2,(H,32,33)(H2,28,30,31)/b15-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 5 mins followed by NADPH cofactor addition and measured... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50440019
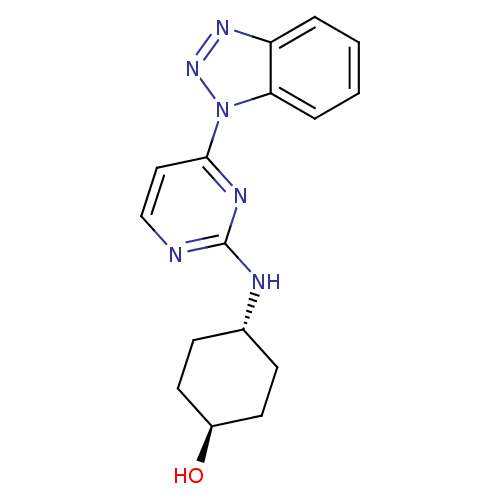
(CHEMBL2425654)Show SMILES O[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1nnc2ccccc12 |r,wU:4.7,wD:1.0,(2.77,-16.29,;4.12,-17.05,;4.13,-18.6,;5.48,-19.36,;6.8,-18.58,;6.79,-17.04,;5.47,-16.28,;8.15,-19.35,;9.49,-18.57,;9.49,-17.03,;10.83,-16.26,;12.15,-17.02,;12.16,-18.57,;10.83,-19.34,;13.49,-19.34,;14.91,-18.78,;15.89,-19.97,;15.06,-21.27,;15.46,-22.75,;14.39,-23.83,;12.88,-23.42,;12.49,-21.97,;13.58,-20.87,)| Show InChI InChI=1S/C16H18N6O/c23-12-7-5-11(6-8-12)18-16-17-10-9-15(19-16)22-14-4-2-1-3-13(14)20-21-22/h1-4,9-12,23H,5-8H2,(H,17,18,19)/t11-,12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50297370

(3-(3-(tert-butylthio)-5-(pyridin-2-ylmethoxy)-1-(4...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(cc2)-c2nccs2)c2ccc(OCc3ccccn3)cc12 Show InChI InChI=1S/C33H35N3O3S2/c1-32(2,3)41-29-26-18-25(39-21-24-8-6-7-15-34-24)13-14-27(26)36(28(29)19-33(4,5)31(37)38)20-22-9-11-23(12-10-22)30-35-16-17-40-30/h6-18H,19-21H2,1-5H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM143362
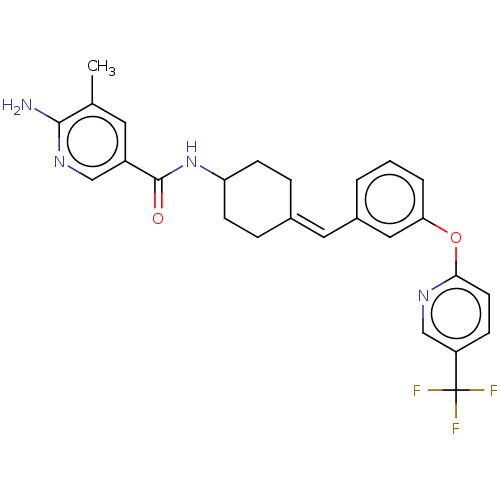
(US9682953, 20.A-5)Show SMILES Cc1cc(cnc1N)C(=O)NC1CCC(CC1)=Cc1cccc(Oc2ccc(cn2)C(F)(F)F)c1 |(11.34,-.39,;10,.38,;8.67,-.38,;7.34,.38,;7.34,1.93,;8.67,2.69,;10,1.93,;11.34,2.69,;6,-.38,;6,-1.93,;4.67,.38,;3.33,-.38,;3.33,-1.93,;2,-2.69,;.67,-1.93,;.67,-.38,;2,.38,;-.67,-2.69,;-2,-1.93,;-2,-.38,;-3.33,.38,;-4.67,-.38,;-4.67,-1.93,;-6,-2.69,;-7.34,-1.93,;-7.34,-.38,;-8.67,.38,;-10,-.38,;-10,-1.93,;-8.67,-2.69,;-11.34,.38,;-12.67,-.39,;-11.34,1.93,;-11.34,-1.16,;-3.33,-2.69,)| Show InChI InChI=1S/C26H25F3N4O2/c1-16-11-19(14-32-24(16)30)25(34)33-21-8-5-17(6-9-21)12-18-3-2-4-22(13-18)35-23-10-7-20(15-31-23)26(27,28)29/h2-4,7,10-15,21H,5-6,8-9H2,1H3,(H2,30,32)(H,33,34)/b17-12- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Limited
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 5 mins followed by NADPH cofactor addition and measured... |
Bioorg Med Chem Lett 29: 238-243 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.048
BindingDB Entry DOI: 10.7270/Q2125X14 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50297377

(3-[3-tert-Butylsulfanyl-1-[4-(6-methylpyridin-3-yl...)Show SMILES Cc1ccc(cn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccccn4)ccc23)cc1 Show InChI InChI=1S/C36H39N3O3S/c1-24-10-13-27(21-38-24)26-14-11-25(12-15-26)22-39-31-17-16-29(42-23-28-9-7-8-18-37-28)19-30(31)33(43-35(2,3)4)32(39)20-36(5,6)34(40)41/h7-19,21H,20,22-23H2,1-6H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50391744

(CHEMBL2146710)Show SMILES Clc1ccc(C(=O)NC[C@@]2(CC3CC3)CC[C@@H](CC2)S(=O)(=O)c2cnn[nH]2)c(Cl)c1 |r,wU:9.9,16.20,wD:9.8,(38.69,-13.12,;37.37,-12.33,;36.02,-13.08,;34.7,-12.29,;34.73,-10.76,;33.41,-9.97,;33.43,-8.43,;32.06,-10.72,;30.69,-10.03,;29.4,-10.87,;28.33,-9.74,;26.83,-10.09,;25.36,-9.65,;25.71,-11.15,;28.07,-11.64,;28.07,-13.18,;29.4,-13.95,;30.73,-13.18,;30.73,-11.64,;29.4,-15.49,;27.85,-15.48,;28.61,-16.82,;30.57,-16.47,;32.02,-15.96,;32.96,-17.18,;32.08,-18.45,;30.61,-18.01,;36.06,-10,;36.07,-8.46,;37.39,-10.78,)| Show InChI InChI=1S/C20H24Cl2N4O3S/c21-14-3-4-16(17(22)9-14)19(27)23-12-20(10-13-1-2-13)7-5-15(6-8-20)30(28,29)18-11-24-26-25-18/h3-4,9,11,13,15H,1-2,5-8,10,12H2,(H,23,27)(H,24,25,26)/t15-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
ACS Med Chem Lett 1: 350-354 (2010)
Article DOI: 10.1021/ml1001085
BindingDB Entry DOI: 10.7270/Q2W09700 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50297384
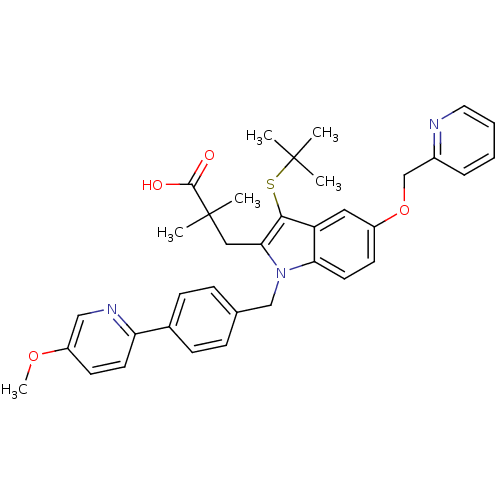
(3-(3-(tert-butylthio)-1-(4-(5-methoxypyridin-2-yl)...)Show SMILES COc1ccc(nc1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccccn4)ccc23)cc1 Show InChI InChI=1S/C36H39N3O4S/c1-35(2,3)44-33-29-19-27(43-23-26-9-7-8-18-37-26)15-17-31(29)39(32(33)20-36(4,5)34(40)41)22-24-10-12-25(13-11-24)30-16-14-28(42-6)21-38-30/h7-19,21H,20,22-23H2,1-6H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50297371
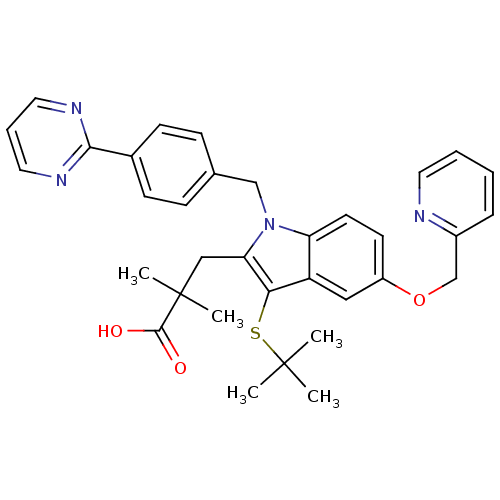
(3-(3-(tert-butylthio)-5-(pyridin-2-ylmethoxy)-1-(4...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(cc2)-c2ncccn2)c2ccc(OCc3ccccn3)cc12 Show InChI InChI=1S/C34H36N4O3S/c1-33(2,3)42-30-27-19-26(41-22-25-9-6-7-16-35-25)14-15-28(27)38(29(30)20-34(4,5)32(39)40)21-23-10-12-24(13-11-23)31-36-17-8-18-37-31/h6-19H,20-22H2,1-5H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50359082

(CHEMBL1922662)Show SMILES Cc1ccc(COc2ccc3n(Cc4ccc(cc4)-c4ccc(C)cn4)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3c2)nc1 Show InChI InChI=1S/C37H41N3O3S/c1-24-8-14-28(38-20-24)23-43-29-15-17-32-30(18-29)34(44-36(3,4)5)33(19-37(6,7)35(41)42)40(32)22-26-10-12-27(13-11-26)31-16-9-25(2)21-39-31/h8-18,20-21H,19,22-23H2,1-7H3,(H,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 54: 8013-29 (2011)
Article DOI: 10.1021/jm2008369
BindingDB Entry DOI: 10.7270/Q269740W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50297374

(3-(3-(tert-butylthio)-5-(pyridin-2-ylmethoxy)-1-(4...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(cc2)-c2cccnc2)c2ccc(OCc3ccccn3)cc12 Show InChI InChI=1S/C35H37N3O3S/c1-34(2,3)42-32-29-19-28(41-23-27-10-6-7-18-37-27)15-16-30(29)38(31(32)20-35(4,5)33(39)40)22-24-11-13-25(14-12-24)26-9-8-17-36-21-26/h6-19,21H,20,22-23H2,1-5H3,(H,39,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50201124
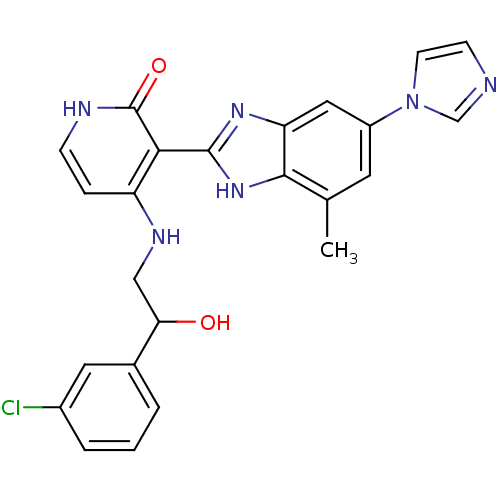
(3-(6-(1H-imidazol-1-yl)-4-methyl-1H-benzo[d]imidaz...)Show SMILES Cc1cc(cc2nc([nH]c12)-c1c(NCC(O)c2cccc(Cl)c2)cc[nH]c1=O)-n1ccnc1 Show InChI InChI=1S/C24H21ClN6O2/c1-14-9-17(31-8-7-26-13-31)11-19-22(14)30-23(29-19)21-18(5-6-27-24(21)33)28-12-20(32)15-3-2-4-16(25)10-15/h2-11,13,20,32H,12H2,1H3,(H,29,30)(H2,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co
Curated by ChEMBL
| Assay Description
Concentration required to inhibit Cytochrome P450 2C9 in vitro by 50% |
J Med Chem 48: 5639-43 (2005)
Article DOI: 10.1021/jm050392q
BindingDB Entry DOI: 10.7270/Q2MW2GPP |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50437838
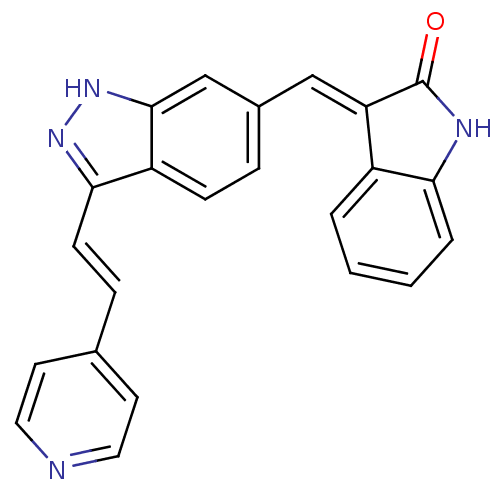
(CHEMBL2407749)Show SMILES O=C1Nc2ccccc2\C1=C/c1ccc2c(\C=C\c3ccncc3)n[nH]c2c1 Show InChI InChI=1S/C23H16N4O/c28-23-19(17-3-1-2-4-20(17)25-23)13-16-5-7-18-21(26-27-22(18)14-16)8-6-15-9-11-24-12-10-15/h1-14H,(H,25,28)(H,26,27)/b8-6+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) using MFC as substrate after 45 mins by fluorescence assay |
J Med Chem 56: 6069-87 (2013)
Article DOI: 10.1021/jm400380m
BindingDB Entry DOI: 10.7270/Q2HM59V9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50325211
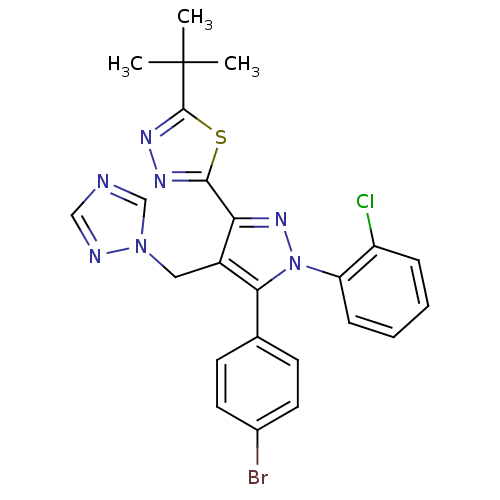
(2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophe...)Show SMILES CC(C)(C)c1nnc(s1)-c1nn(c(c1Cn1cncn1)-c1ccc(Br)cc1)-c1ccccc1Cl Show InChI InChI=1S/C24H21BrClN7S/c1-24(2,3)23-30-29-22(34-23)20-17(12-32-14-27-13-28-32)21(15-8-10-16(25)11-9-15)33(31-20)19-7-5-4-6-18(19)26/h4-11,13-14H,12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes after 30 mins |
Bioorg Med Chem 18: 6377-88 (2010)
Article DOI: 10.1016/j.bmc.2010.07.013
BindingDB Entry DOI: 10.7270/Q2XG9RBT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50325212
(2-(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-chloroph...)Show SMILES FC(F)(F)C1(CCC1)c1nnc(s1)-c1nn(c(c1Cn1cncn1)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H17Cl3F3N7S/c26-15-4-2-14(3-5-15)21-17(11-37-13-32-12-33-37)20(36-38(21)19-7-6-16(27)10-18(19)28)22-34-35-23(39-22)24(8-1-9-24)25(29,30)31/h2-7,10,12-13H,1,8-9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes after 30 mins |
Bioorg Med Chem 18: 6377-88 (2010)
Article DOI: 10.1016/j.bmc.2010.07.013
BindingDB Entry DOI: 10.7270/Q2XG9RBT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50437838

(CHEMBL2407749)Show SMILES O=C1Nc2ccccc2\C1=C/c1ccc2c(\C=C\c3ccncc3)n[nH]c2c1 Show InChI InChI=1S/C23H16N4O/c28-23-19(17-3-1-2-4-20(17)25-23)13-16-5-7-18-21(26-27-22(18)14-16)8-6-15-9-11-24-12-10-15/h1-14H,(H,25,28)(H,26,27)/b8-6+,19-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) using MFC substrate after 45 mins by fluorescence assay |
J Med Chem 58: 130-46 (2015)
Article DOI: 10.1021/jm500537u
BindingDB Entry DOI: 10.7270/Q2125V9W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50297376
(3-(3-(tert-butylthio)-1-(4-(6-methoxypyridazin-3-y...)Show SMILES COc1ccc(nn1)-c1ccc(Cn2c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c3cc(OCc4ccccn4)ccc23)cc1 Show InChI InChI=1S/C35H38N4O4S/c1-34(2,3)44-32-27-19-26(43-22-25-9-7-8-18-36-25)14-16-29(27)39(30(32)20-35(4,5)33(40)41)21-23-10-12-24(13-11-23)28-15-17-31(42-6)38-37-28/h7-19H,20-22H2,1-6H3,(H,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Amira Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 52: 5803-15 (2009)
Article DOI: 10.1021/jm900945d
BindingDB Entry DOI: 10.7270/Q2G44QB1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM247370
(US9447092, 3)Show SMILES Cc1nc(CN2CCN(CC2)c2c(Cl)cnc3[nH]c(nc23)-c2cn(C)nc2C)no1 Show InChI InChI=1S/C19H22ClN9O/c1-11-13(9-27(3)25-11)18-23-16-17(14(20)8-21-19(16)24-18)29-6-4-28(5-7-29)10-15-22-12(2)30-26-15/h8-9H,4-7,10H2,1-3H3,(H,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50044666
(CHEMBL3353340 | US10358436, Example A22)Show SMILES O=C1Nc2ccccc2[C@]11C[C@H]1c1ccc2c(\C=C\c3ccncc3)n[nH]c2c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
EntreMed Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) using MFC substrate after 45 mins by fluorescence assay |
J Med Chem 58: 130-46 (2015)
Article DOI: 10.1021/jm500537u
BindingDB Entry DOI: 10.7270/Q2125V9W |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50303662

(3-(1-(2,4-dichlorobenzyl)-5-fluoro-3-methyl-1H-ind...)Show SMILES Cc1cn(Cc2ccc(Cl)cc2Cl)c2c(\C=C\C(=O)NS(=O)(=O)c3cc(Cl)c(Cl)s3)cc(F)cc12 Show InChI InChI=1S/C23H15Cl4FN2O3S2/c1-12-10-30(11-14-2-4-15(24)7-18(14)25)22-13(6-16(28)8-17(12)22)3-5-20(31)29-35(32,33)21-9-19(26)23(27)34-21/h2-10H,11H2,1H3,(H,29,31)/b5-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM103346
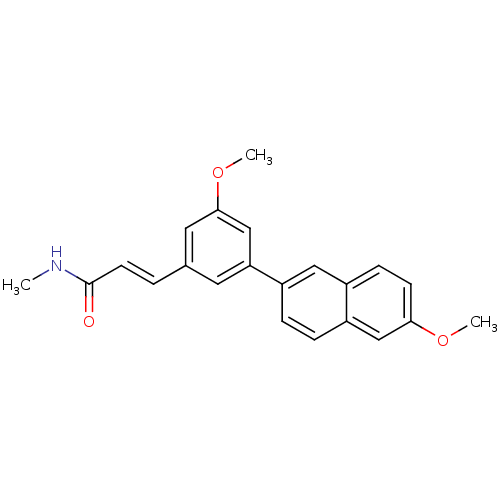
(US8546392, 70)Show SMILES CNC(=O)\C=C\c1cc(OC)cc(c1)-c1ccc2cc(OC)ccc2c1 Show InChI InChI=1S/C22H21NO3/c1-23-22(24)9-4-15-10-19(14-21(11-15)26-3)17-5-6-18-13-20(25-2)8-7-16(18)12-17/h4-14H,1-3H3,(H,23,24)/b9-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitaet des Saarlandes
US Patent
| Assay Description
Inhibition assay using P450 CYP enzymes. |
US Patent US8546392 (2013)
BindingDB Entry DOI: 10.7270/Q2WW7G8P |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM247369
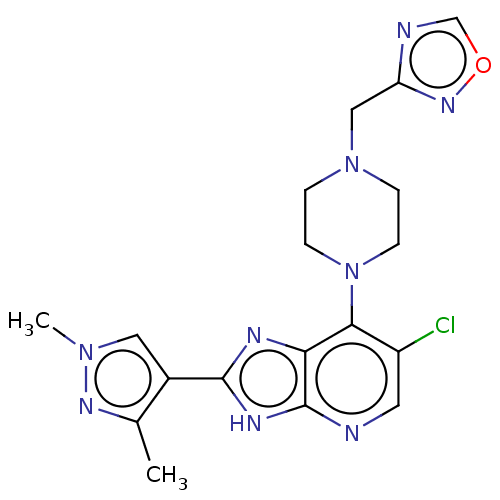
(US9447092, 2)Show SMILES Cc1nn(C)cc1-c1nc2c(N3CCN(Cc4ncon4)CC3)c(Cl)cnc2[nH]1 Show InChI InChI=1S/C18H20ClN9O/c1-11-12(8-26(2)24-11)17-22-15-16(13(19)7-20-18(15)23-17)28-5-3-27(4-6-28)9-14-21-10-29-25-14/h7-8,10H,3-6,9H2,1-2H3,(H,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM247371
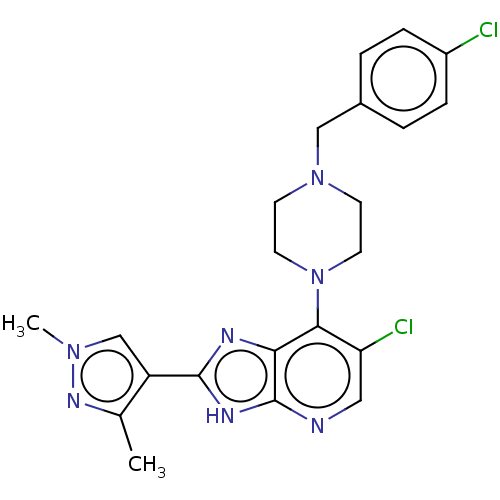
(US9447092, 1)Show SMILES Cc1nn(C)cc1-c1nc2c(N3CCN(Cc4ccc(Cl)cc4)CC3)c(Cl)cnc2[nH]1 Show InChI InChI=1S/C22H23Cl2N7/c1-14-17(13-29(2)28-14)21-26-19-20(18(24)11-25-22(19)27-21)31-9-7-30(8-10-31)12-15-3-5-16(23)6-4-15/h3-6,11,13H,7-10,12H2,1-2H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM247368
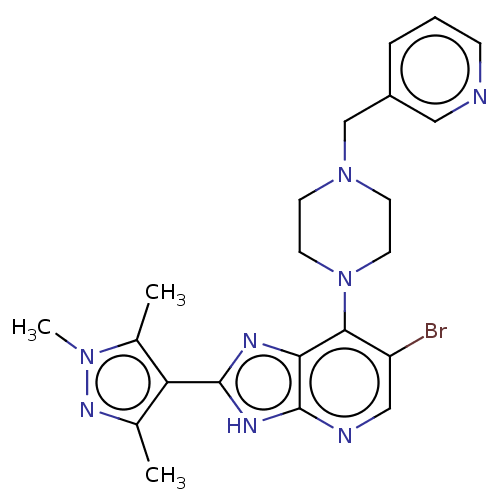
(US9447092, Comparator 2, Example 57)Show SMILES Cc1nn(C)c(C)c1-c1nc2c(N3CCN(Cc4cccnc4)CC3)c(Br)cnc2[nH]1 Show InChI InChI=1S/C22H25BrN8/c1-14-18(15(2)29(3)28-14)21-26-19-20(17(23)12-25-22(19)27-21)31-9-7-30(8-10-31)13-16-5-4-6-24-11-16/h4-6,11-12H,7-10,13H2,1-3H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM247367
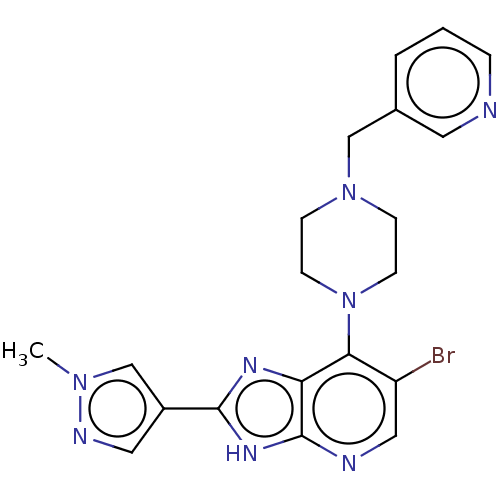
(US9447092, Comparator 1, Example 56)Show SMILES Cn1cc(cn1)-c1nc2c(N3CCN(Cc4cccnc4)CC3)c(Br)cnc2[nH]1 Show InChI InChI=1S/C20H21BrN8/c1-27-13-15(10-24-27)19-25-17-18(16(21)11-23-20(17)26-19)29-7-5-28(6-8-29)12-14-3-2-4-22-9-14/h2-4,9-11,13H,5-8,12H2,1H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CANCER RESEARCH TECHNOLOGY LIMITED
US Patent
| Assay Description
Inhibition of CYP isozymes was determined using a mixture of probe substrates. The samples were incubated for 10 minutes followed by protein precipit... |
US Patent US9447092 (2016)
BindingDB Entry DOI: 10.7270/Q2PV6J9C |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50175297
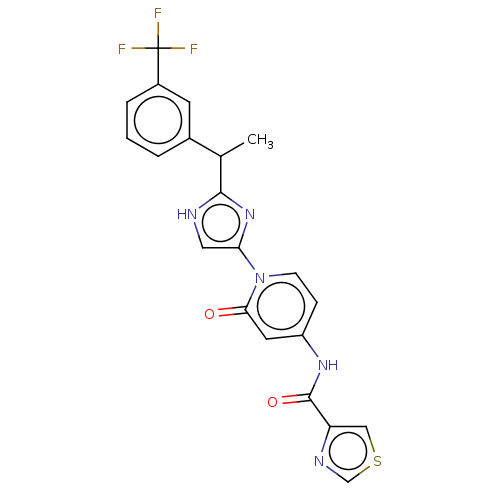
(CHEMBL3810245)Show SMILES CC(c1nc(c[nH]1)-n1ccc(NC(=O)c2cscn2)cc1=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H16F3N5O2S/c1-12(13-3-2-4-14(7-13)21(22,23)24)19-25-9-17(28-19)29-6-5-15(8-18(29)30)27-20(31)16-10-32-11-26-16/h2-12H,1H3,(H,25,28)(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
ACS Med Chem Lett 7: 525-30 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00064
BindingDB Entry DOI: 10.7270/Q24B337T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
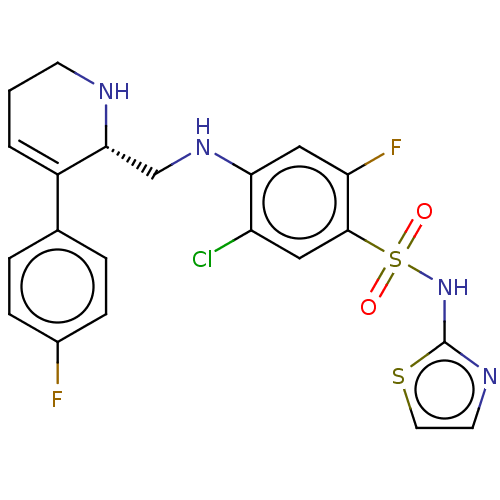
(Homo sapiens (Human)) | BDBM50240240
(CHEMBL4102132)Show SMILES Fc1ccc(cc1)C1=CCCN[C@@H]1CNc1cc(F)c(cc1Cl)S(=O)(=O)Nc1nccs1 |r,t:8| Show InChI InChI=1S/C21H19ClF2N4O2S2/c22-16-10-20(32(29,30)28-21-26-8-9-31-21)17(24)11-18(16)27-12-19-15(2-1-7-25-19)13-3-5-14(23)6-4-13/h2-6,8-11,19,25,27H,1,7,12H2,(H,26,28)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
WuXi AppTec (Shanghai) Co., Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 10 mins followed by substrate addition measured after 1... |
Bioorg Med Chem Lett 27: 2210-2215 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.043
BindingDB Entry DOI: 10.7270/Q2JH3PBN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50257213

(CHEMBL2325016)Show SMILES Clc1ccc(Oc2ccc(cc2C#N)S(=O)(=O)Nc2nccs2)c(c1)-c1cn[nH]c1 Show InChI InChI=1S/C19H12ClN5O3S2/c20-14-1-3-18(16(8-14)13-10-23-24-11-13)28-17-4-2-15(7-12(17)9-21)30(26,27)25-19-22-5-6-29-19/h1-8,10-11H,(H,22,25)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C9
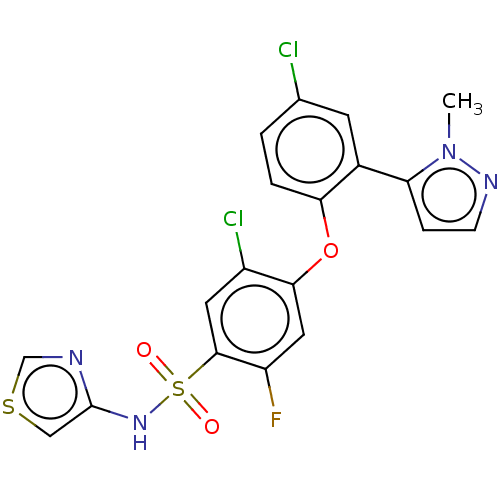
(Homo sapiens (Human)) | BDBM50257164
(CHEMBL2324405)Show SMILES Cn1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1Cl)S(=O)(=O)Nc1cscn1 Show InChI InChI=1S/C19H13Cl2FN4O3S2/c1-26-15(4-5-24-26)12-6-11(20)2-3-16(12)29-17-8-14(22)18(7-13(17)21)31(27,28)25-19-9-30-10-23-19/h2-10,25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50142256

(4-(4-tert-Butyl-benzenesulfonyl)-1-(3H-imidazol-4-...)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1CC(CCc2ccccc2)N(Cc2cnc[nH]2)c2ccccc2C1 Show InChI InChI=1S/C31H36N4O2S/c1-31(2,3)26-14-17-29(18-15-26)38(36,37)34-20-25-11-7-8-12-30(25)35(21-27-19-32-23-33-27)28(22-34)16-13-24-9-5-4-6-10-24/h4-12,14-15,17-19,23,28H,13,16,20-22H2,1-3H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against ccytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 1031-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.052
BindingDB Entry DOI: 10.7270/Q2D50MCF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50257216
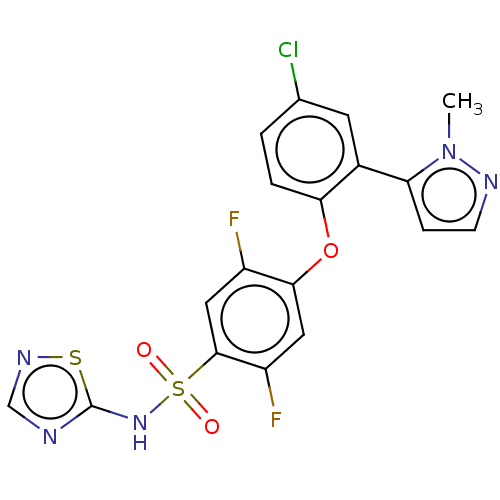
(CHEMBL2325350)Show SMILES Cn1nccc1-c1cc(Cl)ccc1Oc1cc(F)c(cc1F)S(=O)(=O)Nc1ncns1 Show InChI InChI=1S/C18H12ClF2N5O3S2/c1-26-14(4-5-23-26)11-6-10(19)2-3-15(11)29-16-7-13(21)17(8-12(16)20)31(27,28)25-18-22-9-24-30-18/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 60: 7029-7042 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00598
BindingDB Entry DOI: 10.7270/Q21G0PQF |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
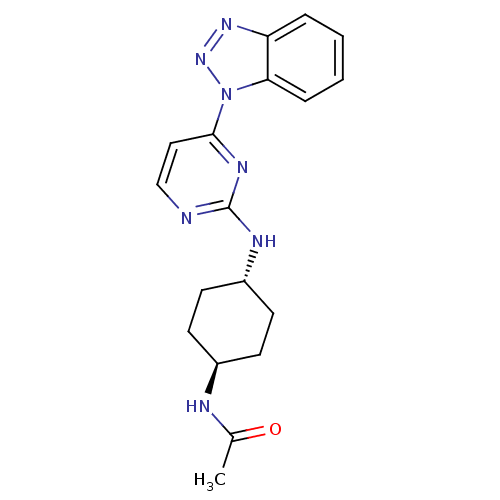
(Homo sapiens (Human)) | BDBM50440017
(CHEMBL2425651)Show SMILES CC(=O)N[C@H]1CC[C@@H](CC1)Nc1nccc(n1)-n1nnc2ccccc12 |r,wU:7.10,wD:4.3,(22.14,-19.72,;22.13,-18.18,;20.79,-17.42,;23.46,-17.4,;24.81,-18.17,;24.83,-19.72,;26.17,-20.47,;27.5,-19.7,;27.49,-18.16,;26.16,-17.39,;28.85,-20.47,;30.19,-19.68,;30.19,-18.14,;31.53,-17.37,;32.86,-18.13,;32.86,-19.68,;31.53,-20.45,;34.2,-20.45,;35.62,-19.9,;36.6,-21.09,;35.77,-22.38,;36.17,-23.87,;35.1,-24.95,;33.59,-24.54,;33.19,-23.09,;34.28,-21.99,)| Show InChI InChI=1S/C18H21N7O/c1-12(26)20-13-6-8-14(9-7-13)21-18-19-11-10-17(22-18)25-16-5-3-2-4-15(16)23-24-25/h2-5,10-11,13-14H,6-9H2,1H3,(H,20,26)(H,19,21,22)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 23: 1486-92 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.047
BindingDB Entry DOI: 10.7270/Q20003HK |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
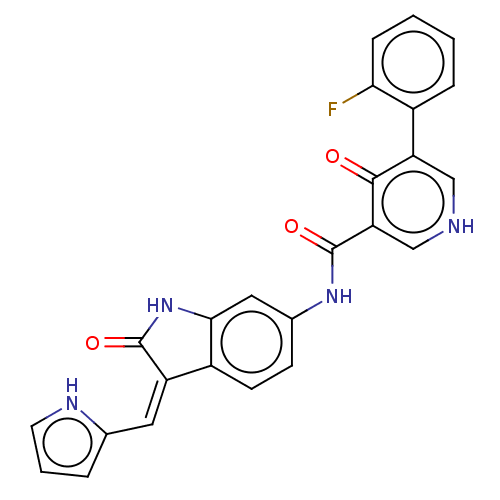
(Homo sapiens (Human)) | BDBM50051939
(CHEMBL3322590)Show SMILES Fc1ccccc1-c1c[nH]cc(C(=O)Nc2ccc3\C(=C\c4ccc[nH]4)C(=O)Nc3c2)c1=O Show InChI InChI=1S/C25H17FN4O3/c26-21-6-2-1-5-16(21)19-12-27-13-20(23(19)31)25(33)29-15-7-8-17-18(10-14-4-3-9-28-14)24(32)30-22(17)11-15/h1-13,28H,(H,27,31)(H,29,33)(H,30,32)/b18-10- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) using Vivid OOMR substrate |
Eur J Med Chem 84: 312-34 (2014)
Article DOI: 10.1016/j.ejmech.2014.07.033
BindingDB Entry DOI: 10.7270/Q2RV0QB7 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50158460
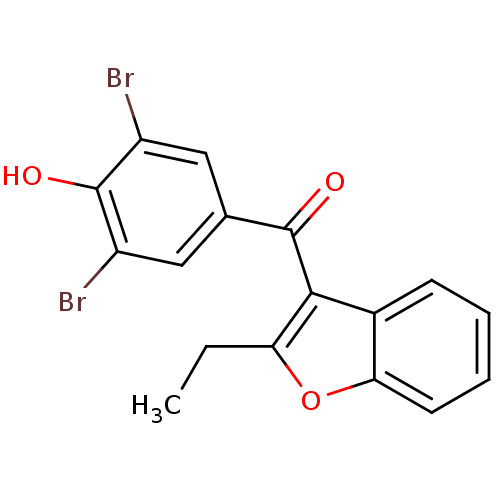
((3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran...)Show InChI InChI=1S/C17H12Br2O3/c1-2-13-15(10-5-3-4-6-14(10)22-13)16(20)9-7-11(18)17(21)12(19)8-9/h3-8,21H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00176
BindingDB Entry DOI: 10.7270/Q2TQ6587 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50158460

((3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran...)Show InChI InChI=1S/C17H12Br2O3/c1-2-13-15(10-5-3-4-6-14(10)22-13)16(20)9-7-11(18)17(21)12(19)8-9/h3-8,21H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127900
BindingDB Entry DOI: 10.7270/Q2P55S6N |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50136043
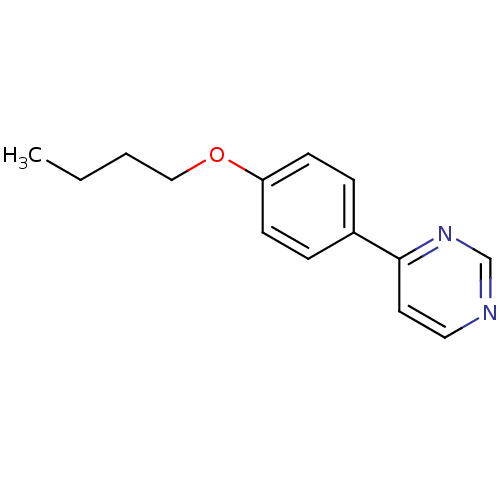
(4-(4-Butoxy-phenyl)-pyrimidine | CHEMBL151974)Show InChI InChI=1S/C14H16N2O/c1-2-3-10-17-13-6-4-12(5-7-13)14-8-9-15-11-16-14/h4-9,11H,2-3,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >46 | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Concentration required to inhibit cytochrome P450 2C9. |
J Med Chem 46: 5416-27 (2003)
Article DOI: 10.1021/jm020557k
BindingDB Entry DOI: 10.7270/Q2QF8S96 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50325805
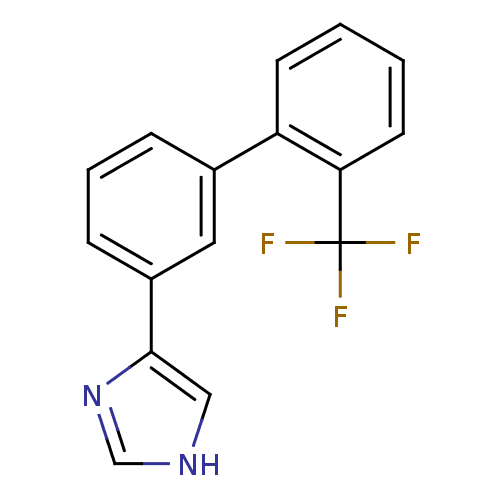
(4-(2'-(trifluoromethyl)biphenyl-3-yl)-1H-imidazole...)Show InChI InChI=1S/C16H11F3N2/c17-16(18,19)14-7-2-1-6-13(14)11-4-3-5-12(8-11)15-9-20-10-21-15/h1-10H,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
Bioorg Med Chem Lett 20: 5536-40 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.064
BindingDB Entry DOI: 10.7270/Q2FB535G |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50166305
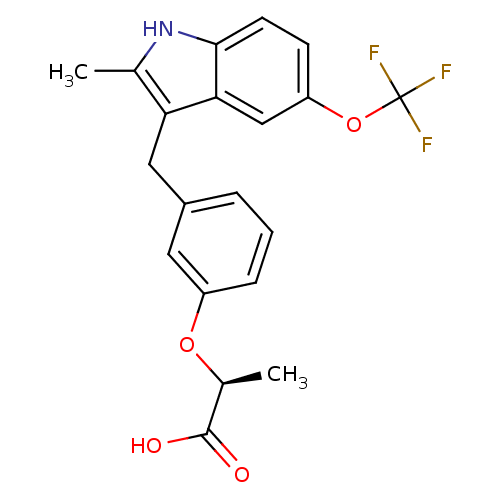
((S)-2-[3-(2-Methyl-5-trifluoromethoxy-1H-indol-3-y...)Show SMILES C[C@H](Oc1cccc(Cc2c(C)[nH]c3ccc(OC(F)(F)F)cc23)c1)C(O)=O Show InChI InChI=1S/C20H18F3NO4/c1-11-16(9-13-4-3-5-14(8-13)27-12(2)19(25)26)17-10-15(28-20(21,22)23)6-7-18(17)24-11/h3-8,10,12,24H,9H2,1-2H3,(H,25,26)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Cytochrome P450 2C9 in rats |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50123453
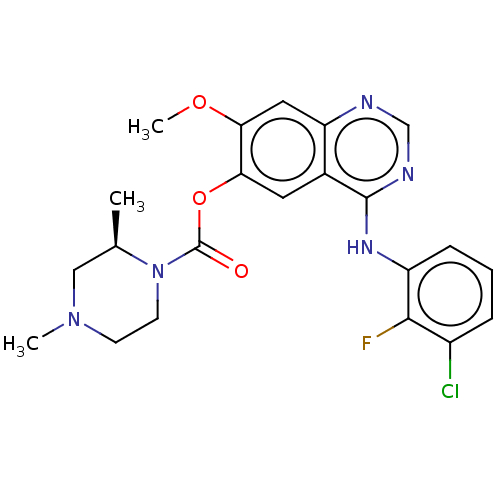
(CHEMBL3623290)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC(=O)N1CCN(C)C[C@H]1C |r| Show InChI InChI=1S/C22H23ClFN5O3/c1-13-11-28(2)7-8-29(13)22(30)32-19-9-14-17(10-18(19)31-3)25-12-26-21(14)27-16-6-4-5-15(23)20(16)24/h4-6,9-10,12-13H,7-8,11H2,1-3H3,(H,25,26,27)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 58: 8200-15 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01073
BindingDB Entry DOI: 10.7270/Q29P33FH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50256712
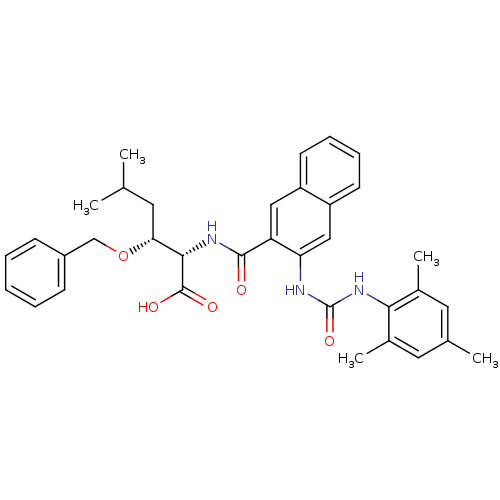
((2S,3R)-3-(benzyloxy)-2-(3-(3-mesitylureido)-2-nap...)Show SMILES CC(C)C[C@@H](OCc1ccccc1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C35H39N3O5/c1-21(2)15-30(43-20-25-11-7-6-8-12-25)32(34(40)41)37-33(39)28-18-26-13-9-10-14-27(26)19-29(28)36-35(42)38-31-23(4)16-22(3)17-24(31)5/h6-14,16-19,21,30,32H,15,20H2,1-5H3,(H,37,39)(H,40,41)(H2,36,38,42)/t30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data