

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149598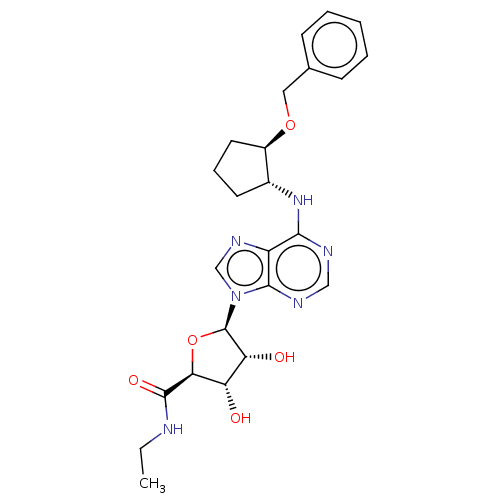 (CHEMBL3770679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0295 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474233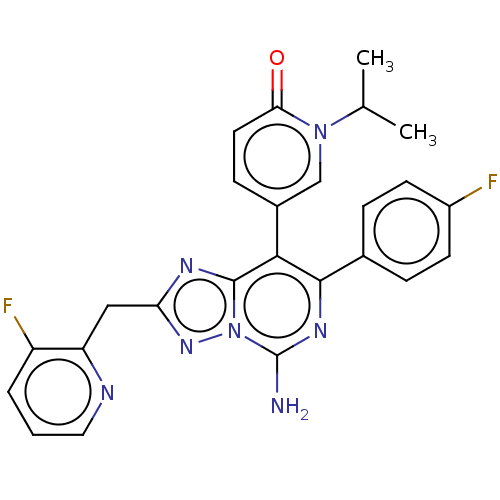 (5-[5-amino-7-(4-fluorophenyl)-2-[(3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474233 (5-[5-amino-7-(4-fluorophenyl)-2-[(3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50375517 (CHEMBL411245) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at adenosine A1 receptor assessed as inhibition of isoproterenol-stimulated cAMP accumulation in DDT1MF-2 cells | Bioorg Med Chem 16: 1861-73 (2008) Article DOI: 10.1016/j.bmc.2007.11.010 BindingDB Entry DOI: 10.7270/Q29K4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50517299 (CHEMBL4533718) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at human A1A adenosine receptor expressed in HEK cells assessed as inhibition of forskolin-stimulated cAMP production after 60 mins | J Med Chem 62: 1502-1522 (2019) Article DOI: 10.1021/acs.jmedchem.8b01662 BindingDB Entry DOI: 10.7270/Q2TM7FGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474222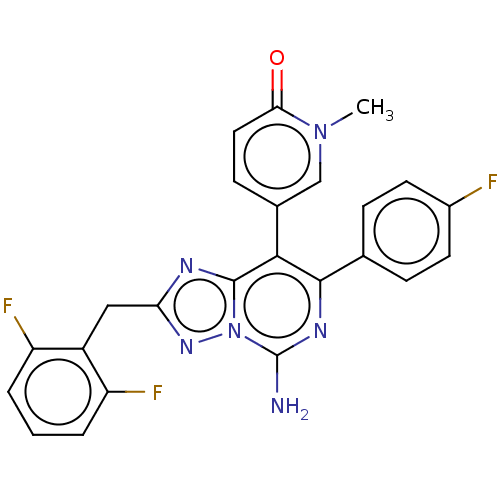 (5-(5-amino-2-(2,6-difluorobenzyl)-7-(4- fluorophen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474222 (5-(5-amino-2-(2,6-difluorobenzyl)-7-(4- fluorophen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50375516 (CHEMBL258759) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at adenosine A1 receptor assessed as inhibition of isoproterenol-stimulated cAMP accumulation in DDT1MF-2 cells | Bioorg Med Chem 16: 1861-73 (2008) Article DOI: 10.1016/j.bmc.2007.11.010 BindingDB Entry DOI: 10.7270/Q29K4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50385957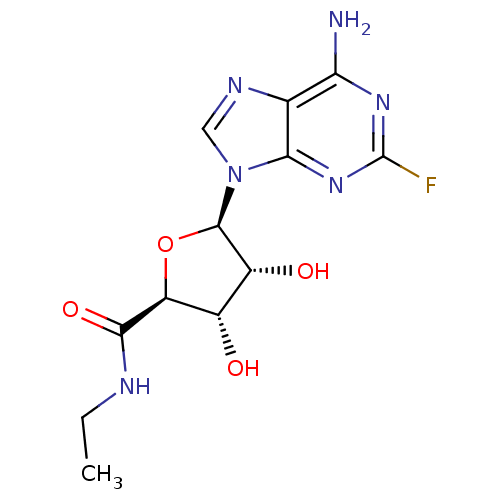 (CHEMBL2042297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | J Med Chem 55: 3521-34 (2012) Article DOI: 10.1021/jm300206u BindingDB Entry DOI: 10.7270/Q2FT8N35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149597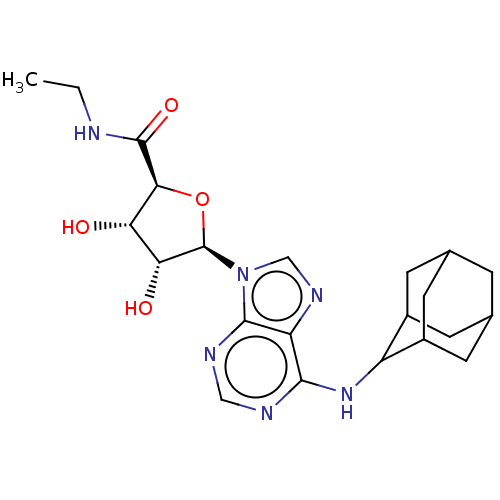 (CHEMBL3771184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human recombinant adenosine receptor A1 expressed in CHO cells measured after 60 mins by scintillation counting method | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50327343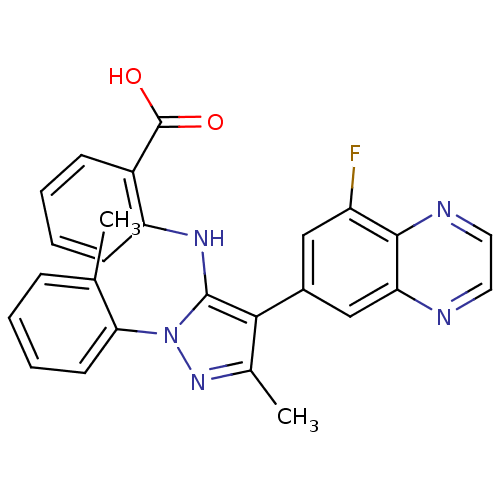 (2-(4-(8-fluoroquinoxalin-6-yl)-3-methyl-1-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Schering Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor by cAMP assay | Bioorg Med Chem Lett 20: 5891-4 (2010) Article DOI: 10.1016/j.bmcl.2010.07.095 BindingDB Entry DOI: 10.7270/Q2FQ9WV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human recombinant adenosine A1 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149594 (CHEMBL3771208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474230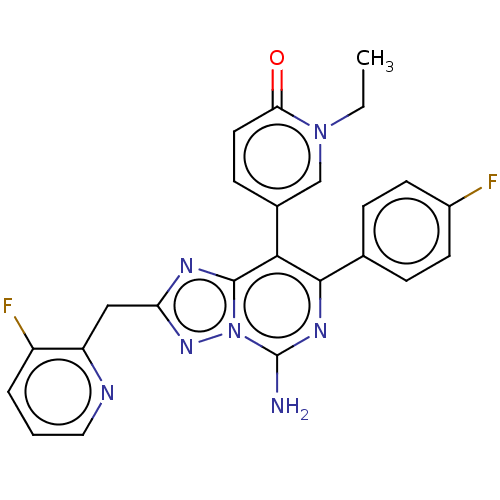 (5-(5-amino-7-(4-fluorophenyl)-2-((3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474230 (5-(5-amino-7-(4-fluorophenyl)-2-((3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474237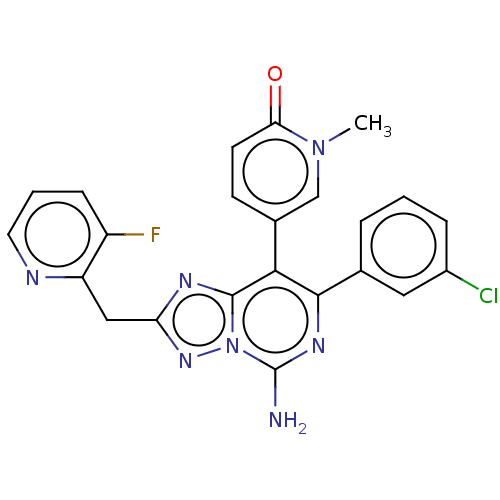 (5-(5-amino-7-(3-chlorophenyl)-2-((3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474237 (5-(5-amino-7-(3-chlorophenyl)-2-((3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM479527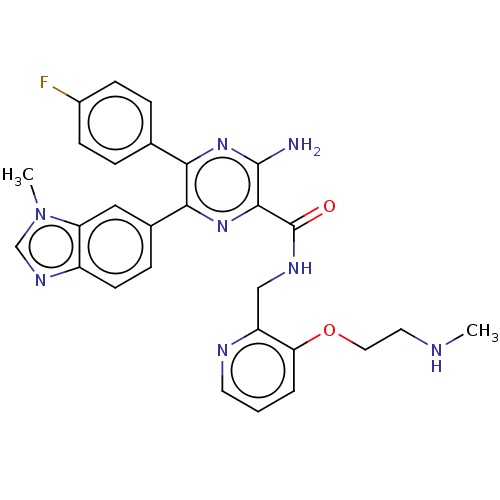 (3-amino-5-(4-fluorophenyl)-6-(3- methyl-3H-benzo[d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity and specificalities of the compounds against different subtype of human adenosine receptors (hA1, hA2a, hA2b, and hA3) were characte... | Citation and Details BindingDB Entry DOI: 10.7270/Q2TQ65GX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50375515 (CHEMBL410288) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at adenosine A1 receptor assessed as inhibition of isoproterenol-stimulated cAMP accumulation in DDT1MF-2 cells | Bioorg Med Chem 16: 1861-73 (2008) Article DOI: 10.1016/j.bmc.2007.11.010 BindingDB Entry DOI: 10.7270/Q29K4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM479527 (3-amino-5-(4-fluorophenyl)-6-(3- methyl-3H-benzo[d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10898481 (2021) BindingDB Entry DOI: 10.7270/Q2J38WPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474221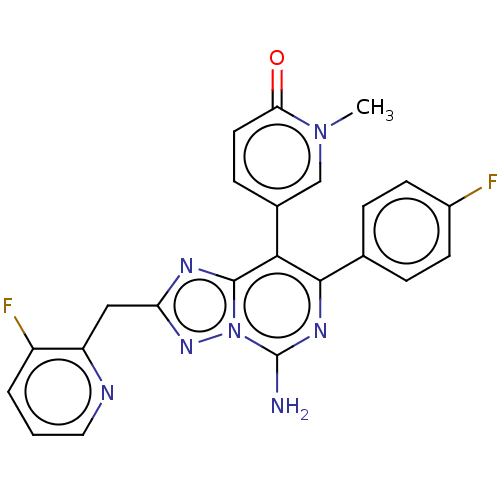 (8-(2,6-dimethylpyridin-4-yl)-7-(4-fluoropheny1)- 2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474221 (8-(2,6-dimethylpyridin-4-yl)-7-(4-fluoropheny1)- 2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01123 BindingDB Entry DOI: 10.7270/Q2KP8643 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of cAMP formation in CHO cells expressing adenosine A1 receptor | J Med Chem 46: 1492-503 (2003) Article DOI: 10.1021/jm021074j BindingDB Entry DOI: 10.7270/Q2MG7Q7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50327327 (2-(4-(4-fluorophenyl)-3-methyl-1-o-tolyl-1H-pyrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Schering Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor by cAMP assay | Bioorg Med Chem Lett 20: 5891-4 (2010) Article DOI: 10.1016/j.bmcl.2010.07.095 BindingDB Entry DOI: 10.7270/Q2FQ9WV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50591171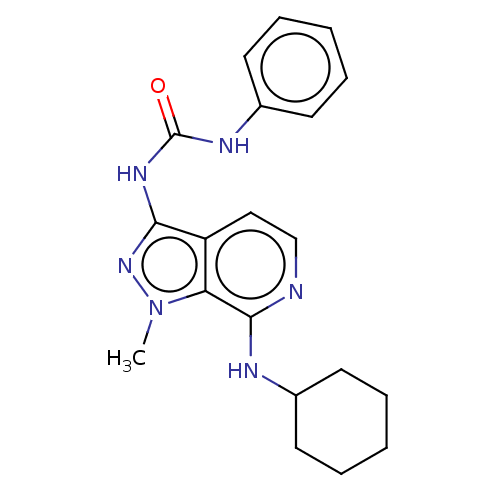 (CHEMBL5174600) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01123 BindingDB Entry DOI: 10.7270/Q2KP8643 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474238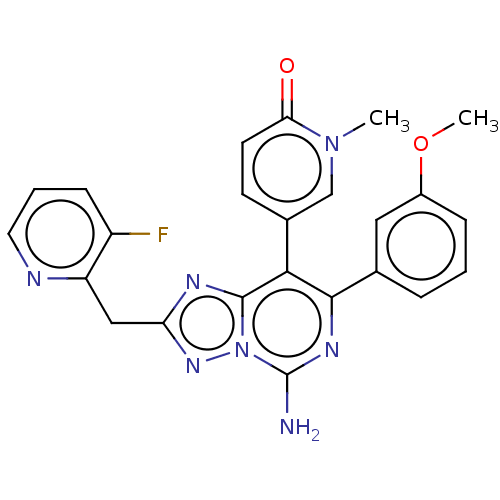 (5-(5-amino-2-((3-fluoropyridin-2-yl)methyl)-7- (3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474238 (5-(5-amino-2-((3-fluoropyridin-2-yl)methyl)-7- (3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474234 (5-(5-amino-2-((3-fluoropyridin-2-yl)methyl)-7- (4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474234 (5-(5-amino-2-((3-fluoropyridin-2-yl)methyl)-7- (4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50225877 ((2R,3S,4R,5R)-2-(hydroxymethyl)-5-(6-((R)-tetrahyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at adenosine A1 receptor expressed in DDT1 MF2 cells assessed as inhibition of (-)-isoproterenol-stimulated cAMP accumulation | Bioorg Med Chem Lett 17: 6779-84 (2007) Article DOI: 10.1016/j.bmcl.2007.10.028 BindingDB Entry DOI: 10.7270/Q24T6K64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50591163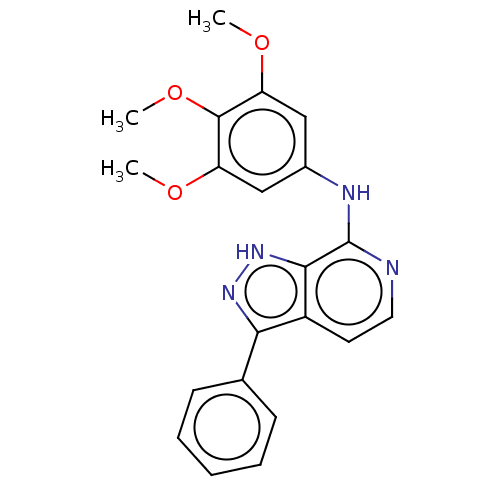 (CHEMBL3929143) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01123 BindingDB Entry DOI: 10.7270/Q2KP8643 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50199620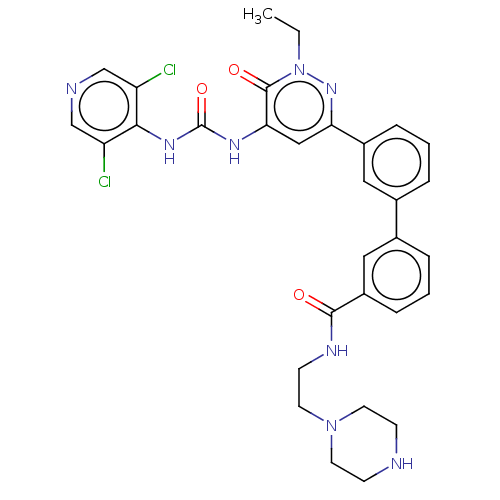 (CHEMBL3916846) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor (unknown origin) | J Med Chem 59: 10479-10497 (2016) Article DOI: 10.1021/acs.jmedchem.6b00829 BindingDB Entry DOI: 10.7270/Q21G0P75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50199719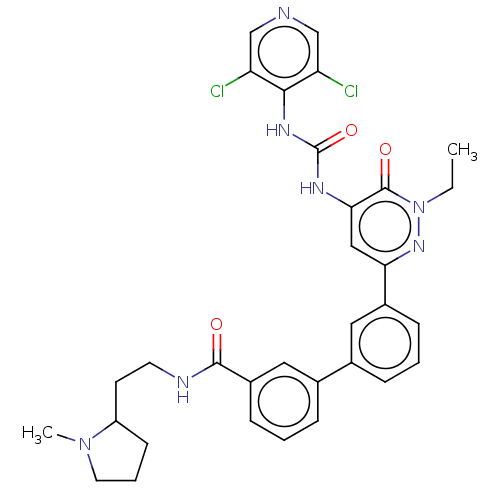 (CHEMBL3918173) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor (unknown origin) | J Med Chem 59: 10479-10497 (2016) Article DOI: 10.1021/acs.jmedchem.6b00829 BindingDB Entry DOI: 10.7270/Q21G0P75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50199697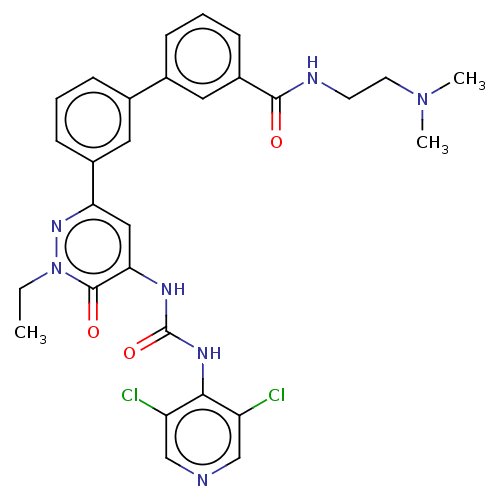 (CHEMBL3980193) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor (unknown origin) | J Med Chem 59: 10479-10497 (2016) Article DOI: 10.1021/acs.jmedchem.6b00829 BindingDB Entry DOI: 10.7270/Q21G0P75 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474232 (5-(5-amino-7-(4-(difluoromethyl)phenyl)-2-((3-f lu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474232 (5-(5-amino-7-(4-(difluoromethyl)phenyl)-2-((3-f lu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474223 (2-(2,6-difluorobenzyl)-8-(2-(dimethylamino) pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM14487 ((2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474223 (2-(2,6-difluorobenzyl)-8-(2-(dimethylamino) pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50055096 (CHEMBL3317576) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Firenze Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A1 receptor expressed in CHO cells assessed as inhibition of CCPA-stimulated cAMP level by scintillation count... | Eur J Med Chem 84: 614-27 (2014) Article DOI: 10.1016/j.ejmech.2014.07.060 BindingDB Entry DOI: 10.7270/Q2BG2QNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50225880 ((1S,2R,4S)-2-[9-((2R,3R,4S,5R)-3,4-dihydroxy-5-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at adenosine A1 receptor expressed in DDT1 MF2 cells assessed as inhibition of (-)-isoproterenol-stimulated cAMP accumulation | Bioorg Med Chem Lett 17: 6779-84 (2007) Article DOI: 10.1016/j.bmcl.2007.10.028 BindingDB Entry DOI: 10.7270/Q24T6K64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50375531 (CHEMBL427737) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at adenosine A1 receptor assessed as inhibition of isoproterenol-stimulated cAMP accumulation in DDT1MF-2 cells | Bioorg Med Chem 16: 1861-73 (2008) Article DOI: 10.1016/j.bmc.2007.11.010 BindingDB Entry DOI: 10.7270/Q29K4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50591168 (CHEMBL5180010) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c01123 BindingDB Entry DOI: 10.7270/Q2KP8643 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at adenosine A1 receptor assessed as inhibition of isoproterenol-stimulated cAMP accumulation in DDT1MF-2 cells | Bioorg Med Chem 16: 1861-73 (2008) Article DOI: 10.1016/j.bmc.2007.11.010 BindingDB Entry DOI: 10.7270/Q29K4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50327337 (5-methoxy-2-(3-methyl-1-phenyl-4-(quinoxalin-6-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Schering Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor by cAMP assay | Bioorg Med Chem Lett 20: 5891-4 (2010) Article DOI: 10.1016/j.bmcl.2010.07.095 BindingDB Entry DOI: 10.7270/Q2FQ9WV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 927 total ) | Next | Last >> |