

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149598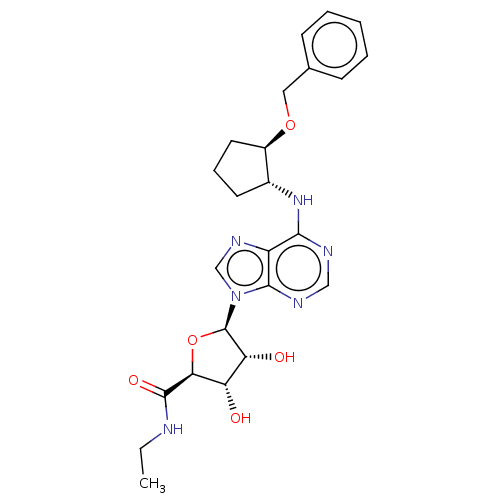 (CHEMBL3770679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0295 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020986 (CHEMBL11002 | N-(2-Dimethylamino-ethyl)-4-(2,6-dio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM82013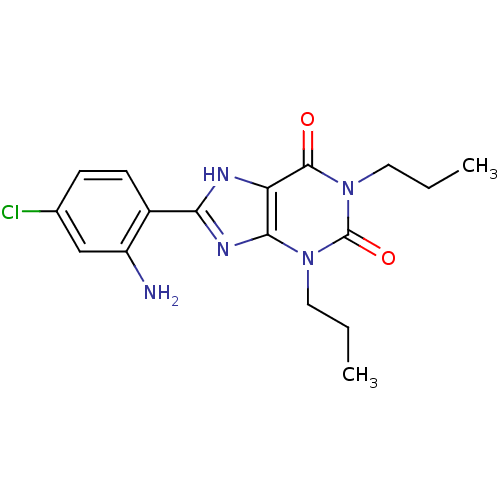 (8-(2-Amino-4-chloro-phenyl)-1,3-dipropyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50550819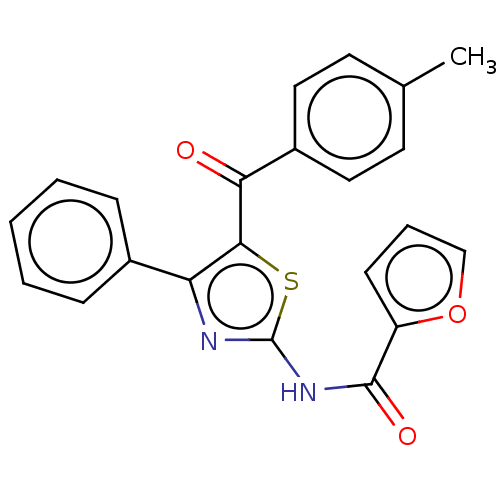 (CHEMBL4796077) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from rat brain cortical membrane A1AR in absence of GTP by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111879 BindingDB Entry DOI: 10.7270/Q24T6P0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50021010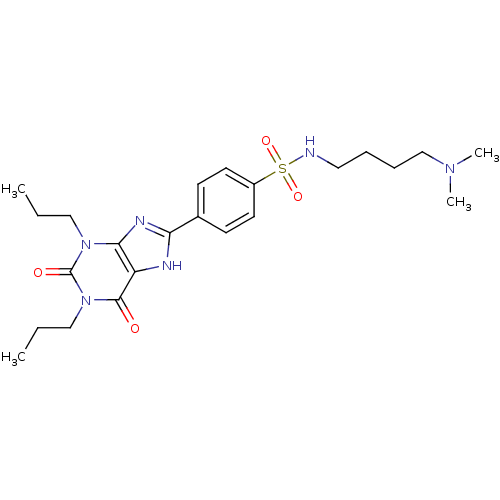 (CHEMBL545362 | N-(4-Dimethylamino-butyl)-4-(2,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50550824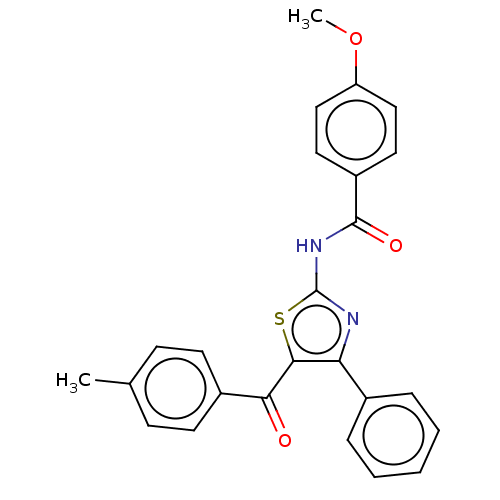 (CHEMBL4797181) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0756 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from rat brain cortical membrane A1AR in absence of GTP by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111879 BindingDB Entry DOI: 10.7270/Q24T6P0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50550824 (CHEMBL4797181) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0763 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from rat brain cortical membrane A1AR in presence of 100 uM of GTP by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111879 BindingDB Entry DOI: 10.7270/Q24T6P0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50021008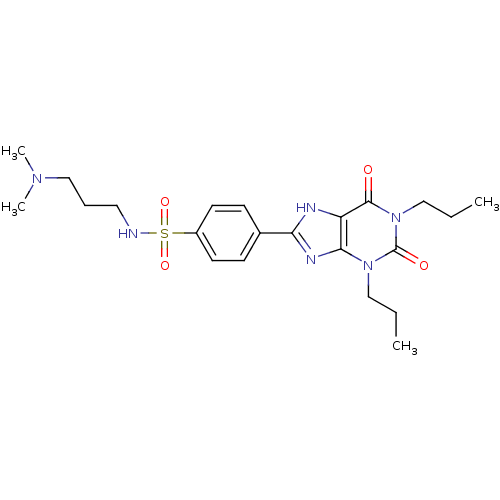 (CHEMBL11036 | N-(3-Dimethylamino-propyl)-4-(2,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50550819 (CHEMBL4796077) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from rat brain cortical membrane A1AR in presence of 100 uM of GTP by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111879 BindingDB Entry DOI: 10.7270/Q24T6P0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50550832 (CHEMBL4764086) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0929 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from rat brain cortical membrane A1AR in absence of GTP by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111879 BindingDB Entry DOI: 10.7270/Q24T6P0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50550832 (CHEMBL4764086) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0981 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]DPCPX from rat brain cortical membrane A1AR in presence of 100 uM of GTP by scintillation counting analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2019.111879 BindingDB Entry DOI: 10.7270/Q24T6P0P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474233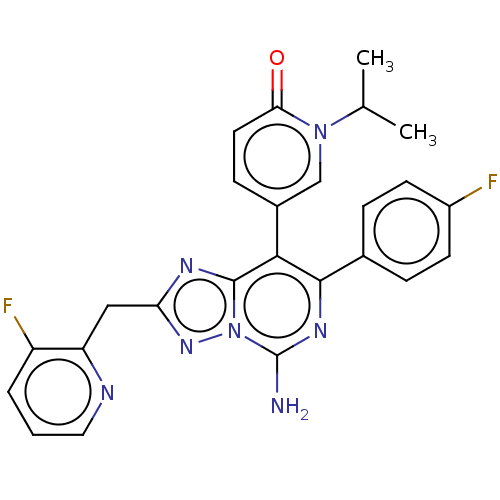 (5-[5-amino-7-(4-fluorophenyl)-2-[(3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474233 (5-[5-amino-7-(4-fluorophenyl)-2-[(3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50375517 (CHEMBL411245) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at adenosine A1 receptor assessed as inhibition of isoproterenol-stimulated cAMP accumulation in DDT1MF-2 cells | Bioorg Med Chem 16: 1861-73 (2008) Article DOI: 10.1016/j.bmc.2007.11.010 BindingDB Entry DOI: 10.7270/Q29K4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50517299 (CHEMBL4533718) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical College of Wisconsin Curated by ChEMBL | Assay Description Agonist activity at human A1A adenosine receptor expressed in HEK cells assessed as inhibition of forskolin-stimulated cAMP production after 60 mins | J Med Chem 62: 1502-1522 (2019) Article DOI: 10.1021/acs.jmedchem.8b01662 BindingDB Entry DOI: 10.7270/Q2TM7FGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50021004 (8-(2,4-Diamino-phenyl)-1,3-dipropyl-3,7-dihydro-pu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474222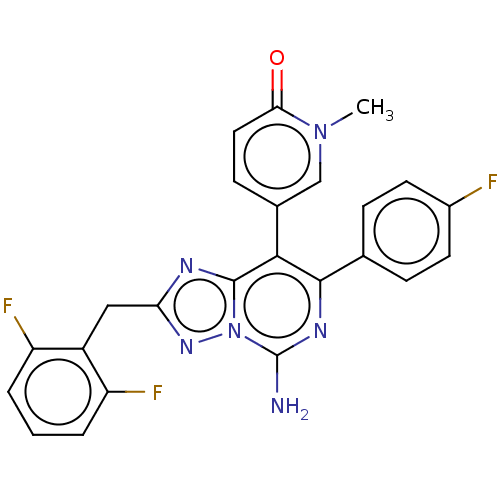 (5-(5-amino-2-(2,6-difluorobenzyl)-7-(4- fluorophen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474222 (5-(5-amino-2-(2,6-difluorobenzyl)-7-(4- fluorophen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.209 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020979 (4-[4-(2,6-Dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50375516 (CHEMBL258759) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Agonist activity at adenosine A1 receptor assessed as inhibition of isoproterenol-stimulated cAMP accumulation in DDT1MF-2 cells | Bioorg Med Chem 16: 1861-73 (2008) Article DOI: 10.1016/j.bmc.2007.11.010 BindingDB Entry DOI: 10.7270/Q29K4C44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Bio-Pharmaceutical Sciences Curated by ChEMBL | Assay Description Evaluated for binding affinity against Adenosine A1 receptor | J Med Chem 35: 629-35 (1992) Checked by Author BindingDB Entry DOI: 10.7270/Q23R0V5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM25400 ((2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia School of Medicine Curated by ChEMBL | Assay Description Binding affinity to adenosine A1 receptor of rat brain membranes without NaCl by inhibition of [125-I]-labeled aminobenzyl adenosine binding | J Med Chem 31: 745-51 (1988) BindingDB Entry DOI: 10.7270/Q2D21Z57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020974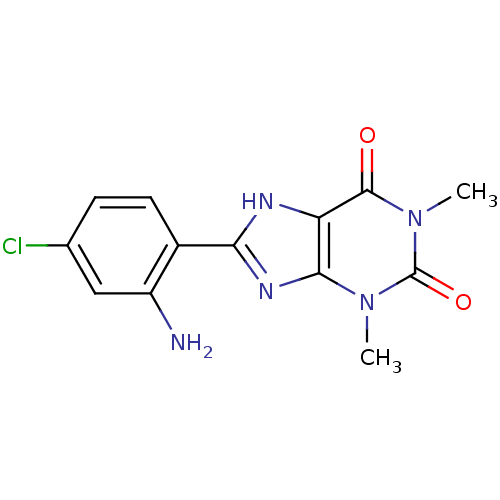 (8-(2-Amino-4-chloro-phenyl)-1,3-dimethyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50385957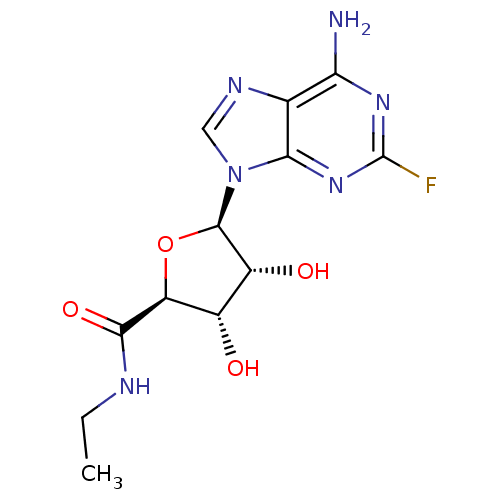 (CHEMBL2042297) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University Curated by ChEMBL | Assay Description Agonist activity at human adenosine A1 receptor expressed in CHO cells assessed as inhibition of forskolin-induced cAMP accumulation | J Med Chem 55: 3521-34 (2012) Article DOI: 10.1021/jm300206u BindingDB Entry DOI: 10.7270/Q2FT8N35 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149597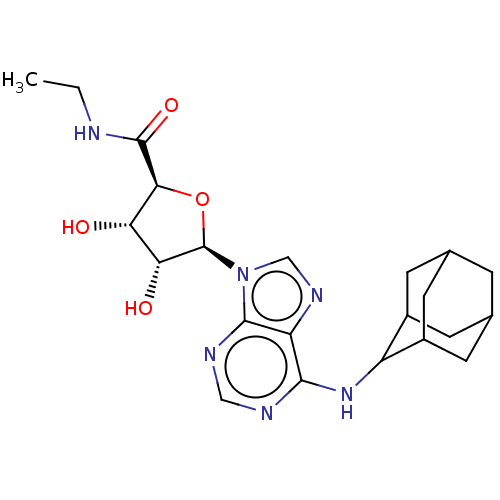 (CHEMBL3771184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50020974 (8-(2-Amino-4-chloro-phenyl)-1,3-dimethyl-3,7-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat brain membrane preparations using N6-[3H]-cyclohexyladenosine as a radioligand | J Med Chem 30: 91-6 (1987) BindingDB Entry DOI: 10.7270/Q2Q23Z84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human recombinant adenosine A1 receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human recombinant adenosine receptor A1 expressed in CHO cells measured after 60 mins by scintillation counting method | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50452317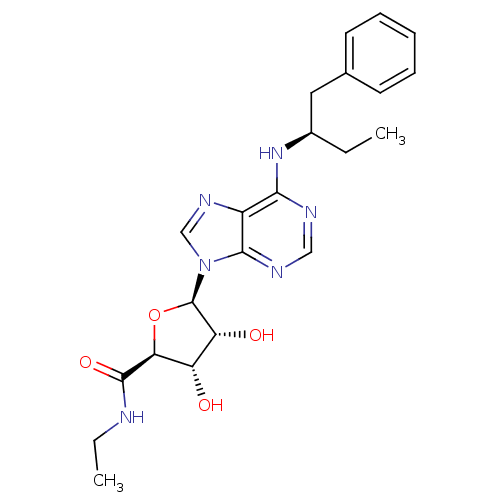 (CHEMBL2094085) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Potency against rat brain adenosine A1 receptor | J Med Chem 29: 1683-9 (1986) BindingDB Entry DOI: 10.7270/Q2Z89D0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50287703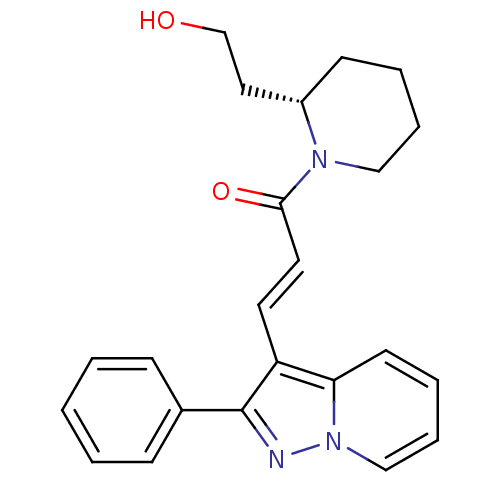 ((E)-1-[(S)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of adenosine induced negative inotropic activity against guinea-pig atria (Adenosine A1 receptor) | Bioorg Med Chem Lett 6: 2059-2062 (1996) Article DOI: 10.1016/0960-894X(96)00368-X BindingDB Entry DOI: 10.7270/Q25Q4W31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50327343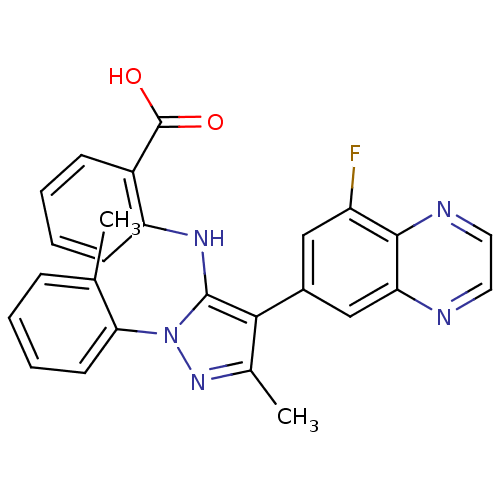 (2-(4-(8-fluoroquinoxalin-6-yl)-3-methyl-1-o-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Schering Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at adenosine A1 receptor by cAMP assay | Bioorg Med Chem Lett 20: 5891-4 (2010) Article DOI: 10.1016/j.bmcl.2010.07.095 BindingDB Entry DOI: 10.7270/Q2FQ9WV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Displacement of [3H]-DPCPX from human recombinant adenosine A1 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50452315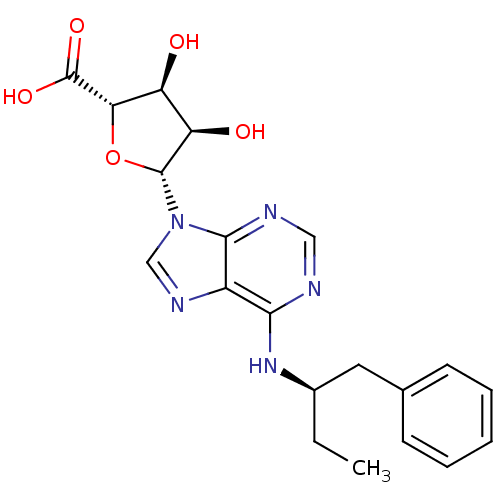 (CHEMBL2094086) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Potency against rat brain adenosine A1 receptor | J Med Chem 29: 1683-9 (1986) BindingDB Entry DOI: 10.7270/Q2Z89D0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50149594 (CHEMBL3771208) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.676 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Warwick Curated by ChEMBL | Assay Description Agonist activity at human Adenosine A1 receptor transfected in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production after 30 m... | J Med Chem 59: 947-64 (2016) Article DOI: 10.1021/acs.jmedchem.5b01402 BindingDB Entry DOI: 10.7270/Q2FX7CBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM82025 (1,3-Dipropyl-8-phenylxanthine | 8-Phenyl-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020976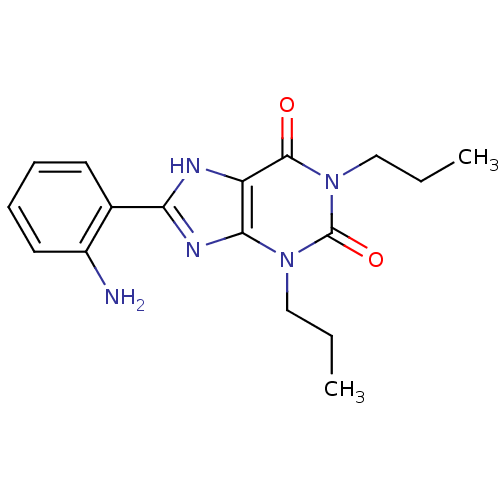 (8-(2-Amino-phenyl)-1,3-dipropyl-3,7-dihydro-purine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020981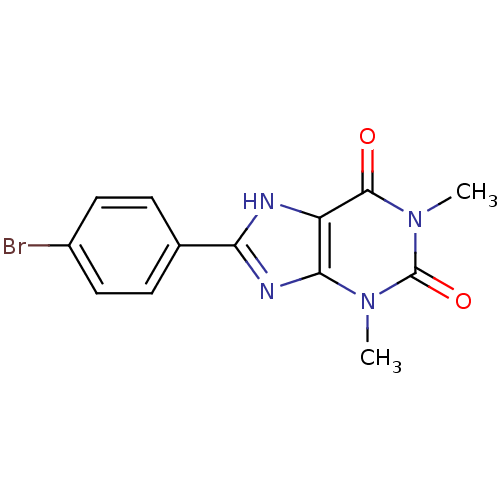 (8-(4-Bromo-phenyl)-1,3-dimethyl-3,7-dihydro-purine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020961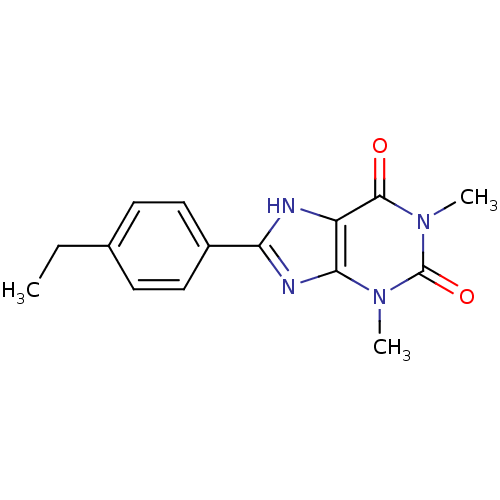 (8-(4-Ethyl-phenyl)-1,3-dimethyl-3,7-dihydro-purine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474230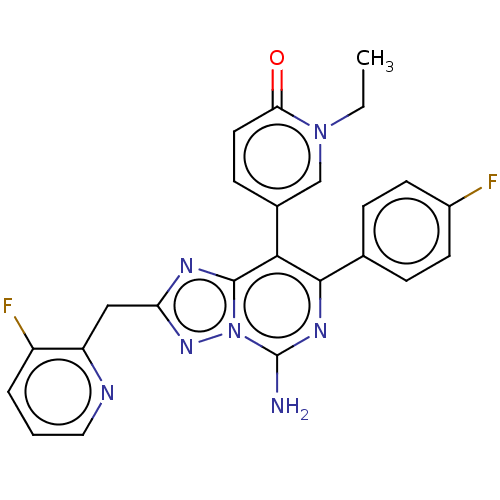 (5-(5-amino-7-(4-fluorophenyl)-2-((3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474237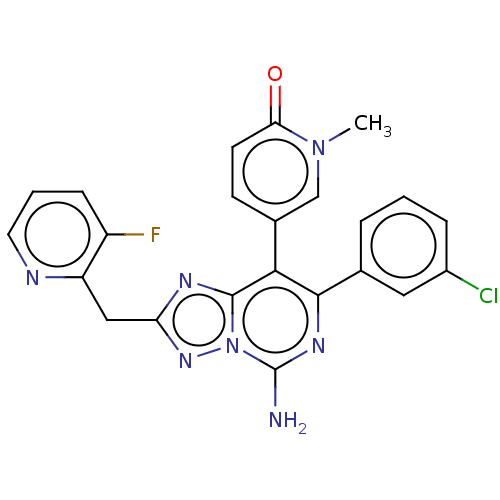 (5-(5-amino-7-(3-chlorophenyl)-2-((3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474237 (5-(5-amino-7-(3-chlorophenyl)-2-((3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA US Patent | Assay Description The compounds at different concentrations were incubate with hA1 membrane (from PerkinElmer) and [3H]-8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) for ... | US Patent US10858365 (2020) BindingDB Entry DOI: 10.7270/Q2Q81H5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020963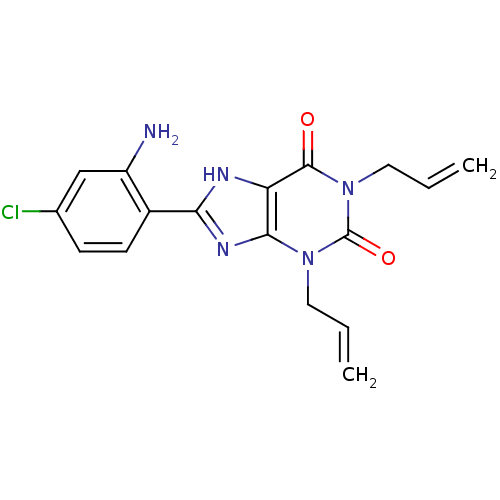 (1,3-Diallyl-8-(2-amino-4-chloro-phenyl)-3,7-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020966 (8-(2-Amino-4-chloro-phenyl)-1,3-diethyl-3,7-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50020988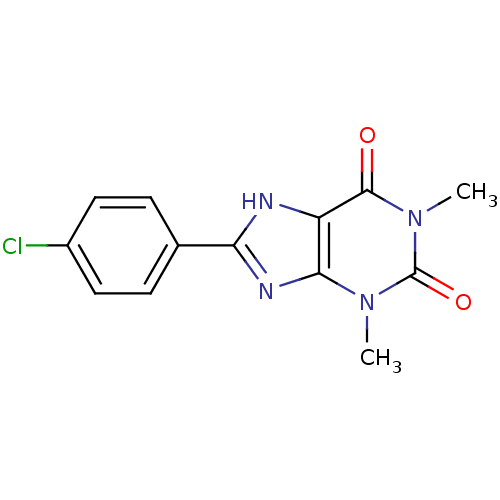 (8-(4-Chloro-phenyl)-1,3-dimethyl-3,7-dihydro-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat brain membrane preparations using N6-[3H]-cyclohexyladenosine as a radioligand | J Med Chem 30: 91-6 (1987) BindingDB Entry DOI: 10.7270/Q2Q23Z84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020988 (8-(4-Chloro-phenyl)-1,3-dimethyl-3,7-dihydro-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM474230 (5-(5-amino-7-(4-fluorophenyl)-2-((3-fluoropyrid in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2W099V1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (BOVINE) | BDBM50020969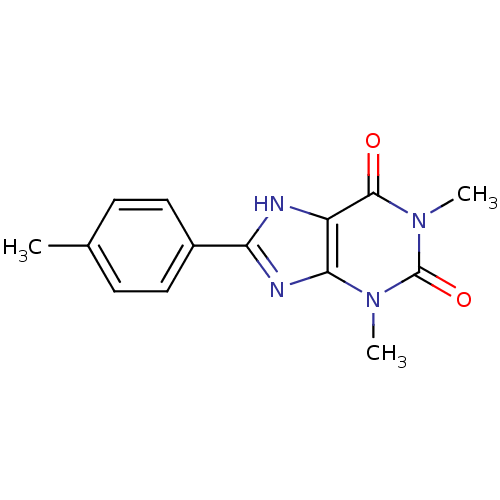 (1,3-Dimethyl-8-p-tolyl-3,7-dihydro-purine-2,6-dion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against bovine brain adenosine A1 receptor by using N6-[3H]- cyclohexyladenosine | J Med Chem 28: 1071-9 (1985) Checked by Author BindingDB Entry DOI: 10.7270/Q2445N2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50020969 (1,3-Dimethyl-8-p-tolyl-3,7-dihydro-purine-2,6-dion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against adenosine A1 receptor in rat brain membrane preparations using N6-[3H]-cyclohexyladenosine as a radioligand | J Med Chem 30: 91-6 (1987) BindingDB Entry DOI: 10.7270/Q2Q23Z84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1480 total ) | Next | Last >> |