 Found 44 hits of ic50 data for polymerid = 2160
Found 44 hits of ic50 data for polymerid = 2160 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489
((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL)
Curated by ChEMBL
| Assay Description
Inhibition of ACE2 (unknown origin) |
J Med Chem 63: 1978-1995 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01780
BindingDB Entry DOI: 10.7270/Q20868NG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ACE2 |
Bioorg Med Chem Lett 18: 1681-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.046
BindingDB Entry DOI: 10.7270/Q29K49Z2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM429241
(med.21724, Compound 13)Show SMILES CC(C)CC(NC(Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
| Assay Description
This is a review article. |
Med Res Rev (2020)
Article DOI: 10.1002/med.21724
BindingDB Entry DOI: 10.7270/Q2JS9ST6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM429137
(med.21724, Compound 10)Show SMILES CCCC(C(CC(C)C)C(=O)NC(CCCCNC(=O)OCc1ccccc1)C(=O)Nc1nccs1)N(O)C=O Show InChI InChI=1S/C28H41N5O6S/c1-4-10-24(33(38)19-34)22(17-20(2)3)25(35)31-23(26(36)32-27-29-15-16-40-27)13-8-9-14-30-28(37)39-18-21-11-6-5-7-12-21/h5-7,11-12,15-16,19-20,22-24,38H,4,8-10,13-14,17-18H2,1-3H3,(H,30,37)(H,31,35)(H,29,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
| Assay Description
This is a review article. |
Med Res Rev (2020)
Article DOI: 10.1002/med.21724
BindingDB Entry DOI: 10.7270/Q2JS9ST6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50606668
(CHEMBL5220314)Show SMILES CC(C)C[C@@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21488
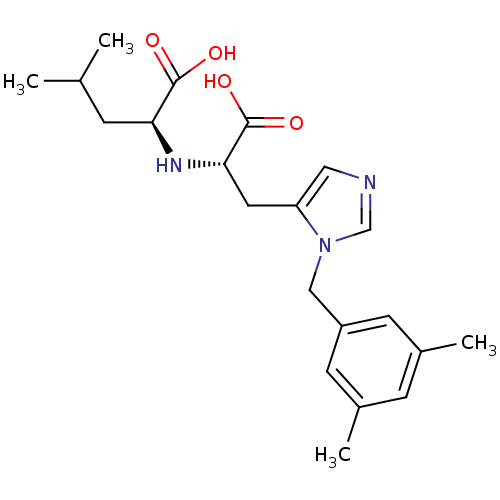
((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dimethylphenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(C)cc(C)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H29N3O4/c1-13(2)5-18(20(25)26)23-19(21(27)28)9-17-10-22-12-24(17)11-16-7-14(3)6-15(4)8-16/h6-8,10,12-13,18-19,23H,5,9,11H2,1-4H3,(H,25,26)(H,27,28)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50606669

(CHEMBL5220968)Show SMILES CC(C)CC(N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21486

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3-methylphenyl)meth...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cccc(C)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H27N3O4/c1-13(2)7-17(19(24)25)22-18(20(26)27)9-16-10-21-12-23(16)11-15-6-4-5-14(3)8-15/h4-6,8,10,12-13,17-18,22H,7,9,11H2,1-3H3,(H,24,25)(H,26,27)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582780
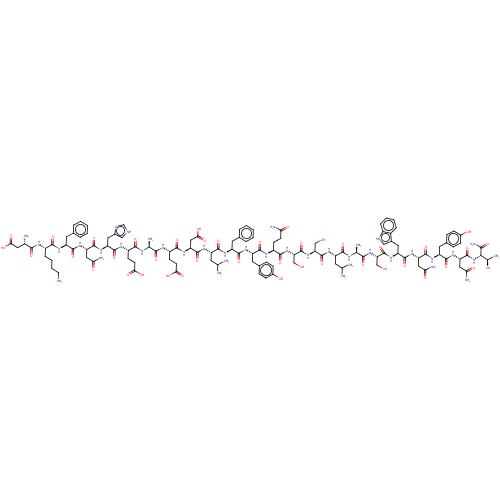
(CHEMBL5091821)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582782
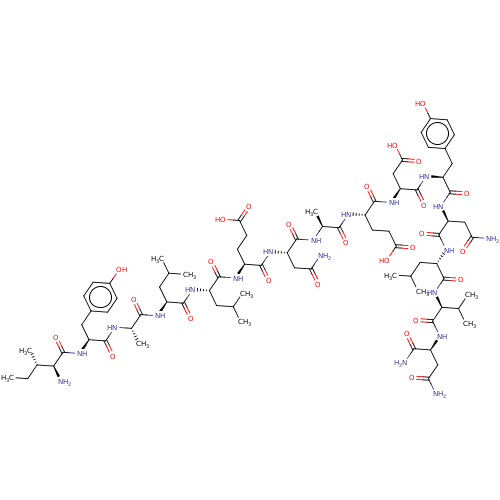
(CHEMBL5088125)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21491
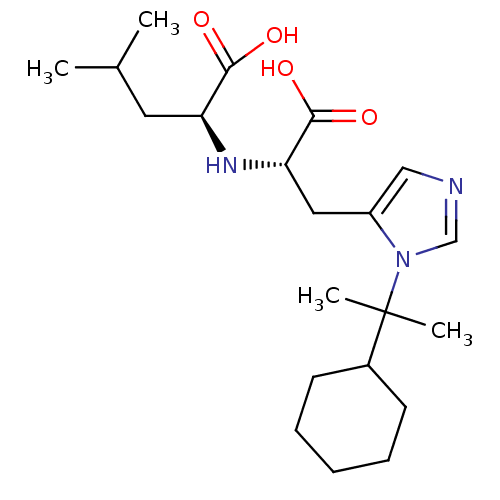
((2S)-2-{[(1S)-1-carboxy-2-[1-(2-cyclohexylpropan-2...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1C(C)(C)C1CCCCC1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H35N3O4/c1-14(2)10-17(19(25)26)23-18(20(27)28)11-16-12-22-13-24(16)21(3,4)15-8-6-5-7-9-15/h12-15,17-18,23H,5-11H2,1-4H3,(H,25,26)(H,27,28)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582783
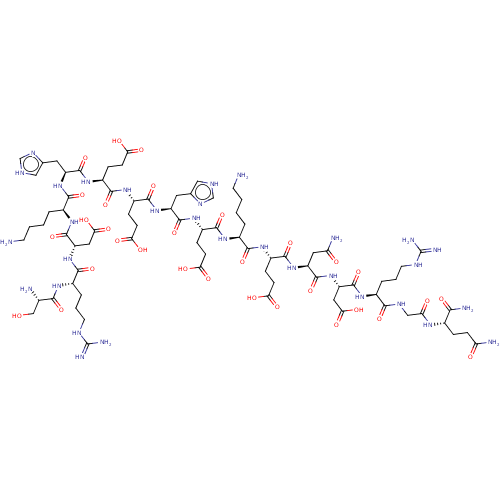
(CHEMBL5093610)Show SMILES NCCCC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50233797

(CHEMBL436639 | DX-600)Show SMILES CC(C)C[C@@H]1NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H]2CCCN2C(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC(=O)[C@@H]2CCCN2C1=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(C)=O)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)CNC(=O)CN)[C@@H](C)O |wU:31.32,8.8,96.103,112.120,128.137,43.49,50.53,64.69,155.166,159.179,193.212,102.113,178.195,wD:19.19,4.3,92.117,140.150,78.85,87.162,212.227,171.182,186.198,122.131,(10.75,-14.8,;10.83,-13.27,;12.19,-12.55,;9.53,-12.43,;9.6,-10.9,;8.3,-10.06,;6.93,-10.77,;6.86,-12.31,;5.63,-9.94,;5.7,-8.4,;7.07,-7.7,;7.14,-6.16,;8.51,-5.44,;8.58,-3.91,;7.28,-3.09,;9.95,-3.2,;4.27,-10.65,;2.97,-9.82,;3.05,-8.28,;1.59,-10.53,;1.54,-12.07,;2.83,-12.9,;4.19,-12.18,;5.47,-13,;5.42,-14.56,;6.72,-15.38,;3.9,-15.18,;2.76,-14.44,;.3,-9.7,;-1.06,-10.41,;-1.14,-11.95,;-2.36,-9.57,;-2.29,-8.04,;-.92,-7.33,;.38,-8.17,;1.75,-7.45,;1.81,-5.91,;3.17,-5.21,;.51,-5.09,;-.86,-5.81,;-3.72,-10.29,;-3.8,-11.83,;-2.5,-12.66,;-5.16,-12.54,;-6.55,-11.85,;-7.62,-12.95,;-6.92,-14.3,;-5.4,-14.05,;-4.1,-14.87,;-2.74,-14.15,;-4.16,-16.41,;-5.53,-17.13,;-5.57,-18.67,;-4.27,-19.49,;-4.9,-21.07,;-6.44,-21.01,;-7.49,-22.11,;-8.97,-21.75,;-9.42,-20.27,;-8.34,-19.16,;-6.88,-19.54,;-2.86,-17.23,;-2.92,-18.77,;-3.7,-20.09,;-1.61,-19.6,;-1.66,-21.14,;-2.45,-22.46,;-3.98,-22.61,;-4.33,-24.1,;-3,-24.88,;-2.71,-26.39,;-1.24,-26.88,;-.09,-25.85,;-.4,-24.36,;-1.85,-23.88,;-.25,-18.88,;1.06,-19.7,;.99,-21.23,;2.42,-18.97,;2.48,-17.44,;3.84,-16.72,;5.14,-17.54,;6.5,-16.82,;7.81,-17.63,;3.72,-19.8,;3.66,-21.33,;2.3,-22.06,;4.97,-22.15,;6.34,-21.44,;7.63,-22.26,;21.81,-15.99,;20.43,-15.3,;20.34,-13.77,;18.96,-13.08,;17.68,-13.92,;17.77,-15.46,;16.31,-13.23,;16.21,-11.69,;17.51,-10.85,;15.02,-14.07,;13.65,-13.38,;12.35,-14.22,;13.55,-11.84,;14.74,-10.87,;14.18,-9.43,;12.66,-9.54,;12.27,-11,;10.97,-10.19,;11.04,-8.66,;21.63,-12.92,;21.54,-11.39,;23.01,-13.62,;24.29,-12.77,;24.2,-11.24,;25.5,-10.39,;25.56,-8.85,;27.07,-8.44,;27.9,-9.73,;26.94,-10.94,;25.67,-13.46,;25.76,-15,;26.96,-12.63,;28.34,-13.3,;28.42,-14.84,;27.15,-15.69,;29.64,-12.47,;29.54,-10.92,;31.01,-13.16,;32.29,-12.31,;32.2,-10.77,;33.49,-9.93,;34.86,-10.63,;36.15,-9.78,;36.06,-8.25,;37.35,-7.4,;34.68,-7.55,;33.4,-8.4,;33.67,-13,;33.76,-14.55,;34.94,-12.16,;36.33,-12.85,;36.41,-14.38,;37.79,-15.08,;39.08,-14.24,;37.88,-16.62,;37.61,-12.01,;37.53,-10.46,;39,-12.7,;40.28,-11.85,;41.65,-12.54,;41.75,-14.09,;42.94,-11.71,;4.91,-23.7,;3.55,-24.42,;2.25,-23.59,;3.49,-25.96,;4.78,-26.77,;6.15,-26.05,;6.21,-24.51,;7.45,-26.87,;7.39,-28.41,;6.03,-29.13,;4.74,-28.31,;3.38,-29.03,;3.31,-30.57,;1.96,-31.29,;4.63,-31.39,;5.98,-30.66,;8.82,-26.16,;10.12,-26.97,;10.07,-28.51,;11.48,-26.26,;11.7,-24.73,;13.21,-24.46,;13.93,-25.82,;12.86,-26.93,;13.13,-28.44,;11.94,-29.44,;14.57,-28.99,;14.84,-30.5,;13.66,-31.49,;12.21,-30.95,;13.91,-33,;15.75,-27.99,;17.2,-28.52,;17.46,-30.03,;18.38,-27.53,;18.26,-25.99,;19.7,-25.4,;20.69,-26.59,;19.86,-27.9,;20.44,-29.32,;19.49,-30.55,;21.96,-29.54,;22.54,-30.97,;21.59,-32.19,;22.16,-33.61,;23.7,-33.83,;21.21,-34.82,;22.92,-28.32,;24.43,-28.55,;25.01,-29.97,;25.38,-27.33,;26.91,-27.55,;27.85,-26.33,;27.28,-24.9,;29.38,-26.55,;30.33,-25.34,;31.86,-25.56,;32.43,-26.98,;32.81,-24.34,;34.33,-24.56,;2.13,-26.67,;.81,-25.85,;2.07,-28.21,)| Show InChI InChI=1S/C140H184N34O39S2/c1-72(2)50-100-137(211)172-47-15-25-110(172)134(208)167-104(68-176)128(202)168-105(130(204)159-98(57-81-64-145-71-152-81)125(199)166-103(67-175)127(201)157-94(52-76-28-36-83(180)37-29-76)122(196)160-99(58-115(188)189)118(192)151-61-73(3)177)69-214-215-70-106(169-135(209)117(74(4)178)170-126(200)96(54-78-32-40-85(182)41-33-78)162-132(206)108-23-14-49-174(108)139(213)102(59-116(190)191)165-133(207)109-24-12-46-171(109)136(210)92(42-43-114(186)187)153-113(185)66-150-112(184)65-149-111(183)60-142)129(203)155-90(20-9-10-44-141)119(193)158-97(55-79-62-147-88-18-7-5-16-86(79)88)124(198)164-101(56-80-63-148-89-19-8-6-17-87(80)89)138(212)173-48-13-22-107(173)131(205)161-95(53-77-30-38-84(181)39-31-77)123(197)156-93(51-75-26-34-82(179)35-27-75)121(195)154-91(120(194)163-100)21-11-45-146-140(143)144/h5-8,16-19,26-41,62-64,71-72,74,90-110,117,147-148,175-176,178-182H,9-15,20-25,42-61,65-70,141-142H2,1-4H3,(H,145,152)(H,149,183)(H,150,184)(H,151,192)(H,153,185)(H,154,195)(H,155,203)(H,156,197)(H,157,201)(H,158,193)(H,159,204)(H,160,196)(H,161,205)(H,162,206)(H,163,194)(H,164,198)(H,165,207)(H,166,199)(H,167,208)(H,168,202)(H,169,209)(H,170,200)(H,186,187)(H,188,189)(H,190,191)(H4,143,144,146)/t74-,90+,91+,92+,93+,94+,95+,96+,97+,98+,99+,100+,101+,102+,103+,104+,105+,106+,107+,108+,109+,110+,117+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ACE2 |
Bioorg Med Chem Lett 18: 1681-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.046
BindingDB Entry DOI: 10.7270/Q29K49Z2 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21487

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,4-dimethylphenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1ccc(C)c(C)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H29N3O4/c1-13(2)7-18(20(25)26)23-19(21(27)28)9-17-10-22-12-24(17)11-16-6-5-14(3)15(4)8-16/h5-6,8,10,12-13,18-19,23H,7,9,11H2,1-4H3,(H,25,26)(H,27,28)/t18-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582778

(CHEMBL5081268)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582779
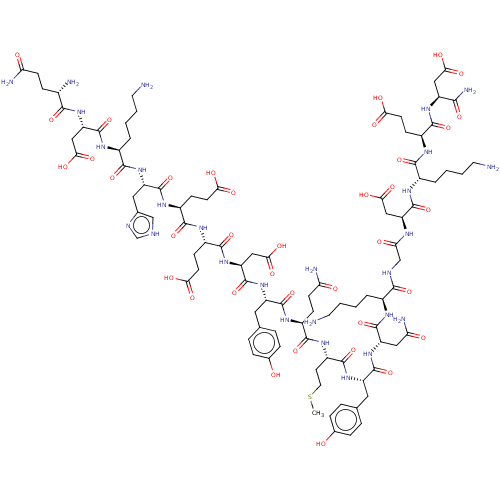
(CHEMBL5081573)Show SMILES CSCC[C@H](NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CCC(N)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21482
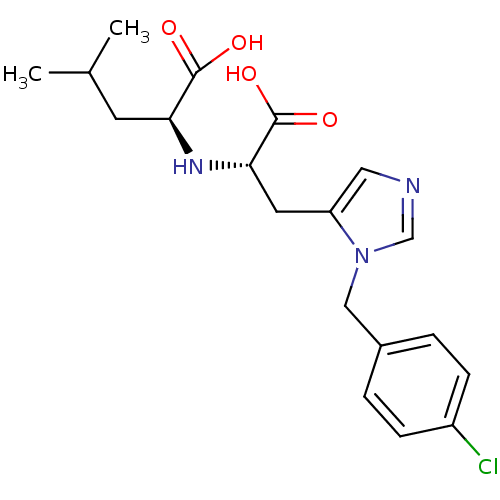
((2S)-2-{[(1S)-1-carboxy-2-{1-[(4-chlorophenyl)meth...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1ccc(Cl)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24ClN3O4/c1-12(2)7-16(18(24)25)22-17(19(26)27)8-15-9-21-11-23(15)10-13-3-5-14(20)6-4-13/h3-6,9,11-12,16-17,22H,7-8,10H2,1-2H3,(H,24,25)(H,26,27)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21484

((2S)-2-{[(1S)-1-carboxy-2-{1-[(4-methylphenyl)meth...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1ccc(C)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H27N3O4/c1-13(2)8-17(19(24)25)22-18(20(26)27)9-16-10-21-12-23(16)11-15-6-4-14(3)5-7-15/h4-7,10,12-13,17-18,22H,8-9,11H2,1-3H3,(H,24,25)(H,26,27)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582781
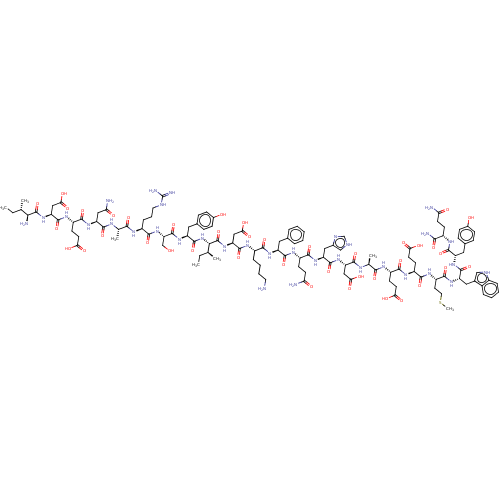
(CHEMBL5078837)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 expressed in HEK293T cells using FMZ as substrate by NanoLuc luciferase assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21483
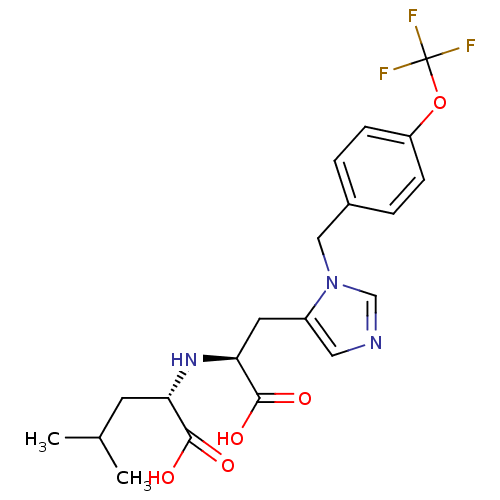
((2S)-2-{[(1S)-1-carboxy-2-(1-{[4-(trifluoromethoxy...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1ccc(OC(F)(F)F)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H24F3N3O5/c1-12(2)7-16(18(27)28)25-17(19(29)30)8-14-9-24-11-26(14)10-13-3-5-15(6-4-13)31-20(21,22)23/h3-6,9,11-12,16-17,25H,7-8,10H2,1-2H3,(H,27,28)(H,29,30)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21481

((2S)-2-{[(1S)-1-carboxy-2-{1-[(4-nitrophenyl)methy...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1ccc(cc1)[N+]([O-])=O)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24N4O6/c1-12(2)7-16(18(24)25)21-17(19(26)27)8-15-9-20-11-22(15)10-13-3-5-14(6-4-13)23(28)29/h3-6,9,11-12,16-17,21H,7-8,10H2,1-2H3,(H,24,25)(H,26,27)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582783

(CHEMBL5093610)Show SMILES NCCCC[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 by ITC assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50559137

(CHEMBL4785828)Show SMILES CC(C)C[C@H](NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O)[C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 by ITC assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50582782

(CHEMBL5088125)Show SMILES CC[C@H](C)[C@H](N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(N)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of SARS-COV2 S-RBD binding to human ACE2 by ITC assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00477
BindingDB Entry DOI: 10.7270/Q23N279S |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM429226
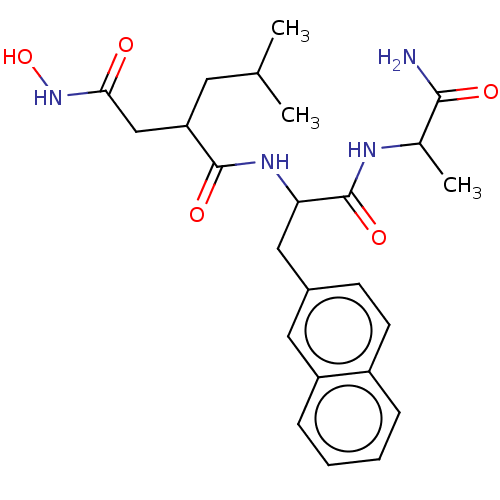
(med.21724, Compound 11)Show SMILES CC(C)CC(CC(=O)NO)C(=O)NC(Cc1ccc2ccccc2c1)C(=O)NC(C)C(N)=O Show InChI InChI=1S/C24H32N4O5/c1-14(2)10-19(13-21(29)28-33)23(31)27-20(24(32)26-15(3)22(25)30)12-16-8-9-17-6-4-5-7-18(17)11-16/h4-9,11,14-15,19-20,33H,10,12-13H2,1-3H3,(H2,25,30)(H,26,32)(H,27,31)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
| Assay Description
This is a review article. |
Med Res Rev (2020)
Article DOI: 10.1002/med.21724
BindingDB Entry DOI: 10.7270/Q2JS9ST6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM429240
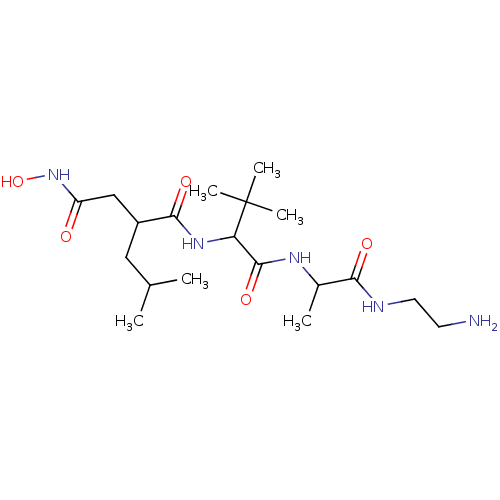
(med.21724, Compound 12)Show SMILES CC(C)CC(CC(=O)NO)C(=O)NC(C(=O)NC(C)C(=O)NCCN)C(C)(C)C Show InChI InChI=1S/C19H37N5O5/c1-11(2)9-13(10-14(25)24-29)17(27)23-15(19(4,5)6)18(28)22-12(3)16(26)21-8-7-20/h11-13,15,29H,7-10,20H2,1-6H3,(H,21,26)(H,22,28)(H,23,27)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
| Assay Description
This is a review article. |
Med Res Rev (2020)
Article DOI: 10.1002/med.21724
BindingDB Entry DOI: 10.7270/Q2JS9ST6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21480

((2S)-2-{[(1S)-1-carboxy-2-[1-(cyclohexylmethyl)-1H...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1CC1CCCCC1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H31N3O4/c1-13(2)8-16(18(23)24)21-17(19(25)26)9-15-10-20-12-22(15)11-14-6-4-3-5-7-14/h10,12-14,16-17,21H,3-9,11H2,1-2H3,(H,23,24)(H,25,26)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21485

((2S)-2-{[(1S)-1-carboxy-2-{1-[(2-methylphenyl)meth...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1ccccc1C)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H27N3O4/c1-13(2)8-17(19(24)25)22-18(20(26)27)9-16-10-21-12-23(16)11-15-7-5-4-6-14(15)3/h4-7,10,12-13,17-18,22H,8-9,11H2,1-3H3,(H,24,25)(H,26,27)/t17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21478
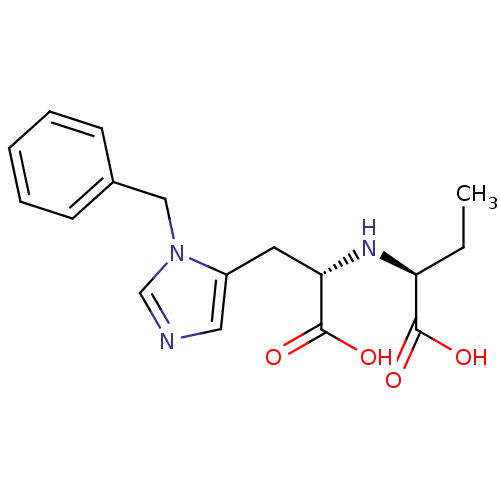
((2S)-2-{[(1S)-2-(1-benzyl-1H-imidazol-5-yl)-1-carb...)Show SMILES CC[C@H](N[C@@H](Cc1cncn1Cc1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H21N3O4/c1-2-14(16(21)22)19-15(17(23)24)8-13-9-18-11-20(13)10-12-6-4-3-5-7-12/h3-7,9,11,14-15,19H,2,8,10H2,1H3,(H,21,22)(H,23,24)/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21479
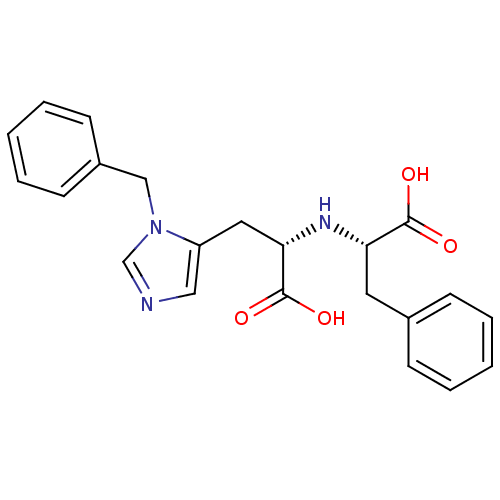
((2S)-2-{[(1S)-2-(1-benzyl-1H-imidazol-5-yl)-1-carb...)Show SMILES OC(=O)[C@H](Cc1cncn1Cc1ccccc1)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C22H23N3O4/c26-21(27)19(11-16-7-3-1-4-8-16)24-20(22(28)29)12-18-13-23-15-25(18)14-17-9-5-2-6-10-17/h1-10,13,15,19-20,24H,11-12,14H2,(H,26,27)(H,28,29)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | 6.5 | 22 |
Millennium Pharmaceuticals
| Assay Description
Enzyme assay was conducted in 384-well microplates format. Activity was monitored by measuring increase in fluorescence (excitation @320 nm, emission... |
J Am Chem Soc 124: 11852-3 (2002)
Article DOI: 10.1021/ja0277226
BindingDB Entry DOI: 10.7270/Q2KP80FN |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM21489

((2S)-2-{[(1S)-1-carboxy-2-{1-[(3,5-dichlorophenyl)...)Show SMILES CC(C)C[C@H](N[C@@H](Cc1cncn1Cc1cc(Cl)cc(Cl)c1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H23Cl2N3O4/c1-11(2)3-16(18(25)26)23-17(19(27)28)7-15-8-22-10-24(15)9-12-4-13(20)6-14(21)5-12/h4-6,8,10-11,16-17,23H,3,7,9H2,1-2H3,(H,25,26)(H,27,28)/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM22985
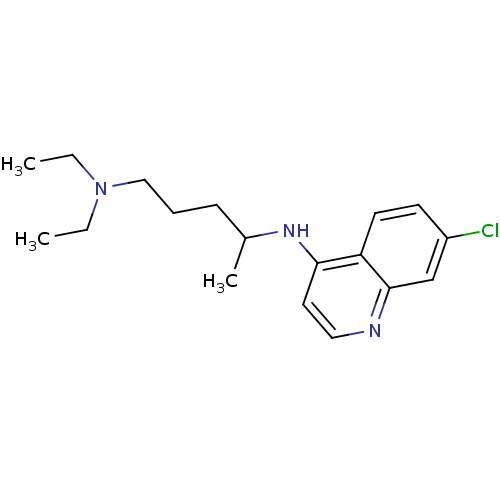
(Aralen | CHEMBL76 | CHLOROQUINE PHOSPHATE | Chloro...)Show InChI InChI=1S/C18H26ClN3/c1-4-22(5-2)12-6-7-14(3)21-17-10-11-20-18-13-15(19)8-9-16(17)18/h8-11,13-14H,4-7,12H2,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
| Assay Description
This is a review article. |
Med Res Rev (2020)
Article DOI: 10.1002/med.21724
BindingDB Entry DOI: 10.7270/Q2JS9ST6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM15236

(3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...)Show SMILES Oc1cc(O)c2c(c1)oc(-c1cc(O)c(O)c(O)c1)c(O)c2=O Show InChI InChI=1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SARS-COV2 His-tagged S-RBD binding to recombinant human ACE2 expressed in HEK293T cells incubated for 0.5 hrs by alpha scre... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01547
BindingDB Entry DOI: 10.7270/Q25H7M47 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM152157
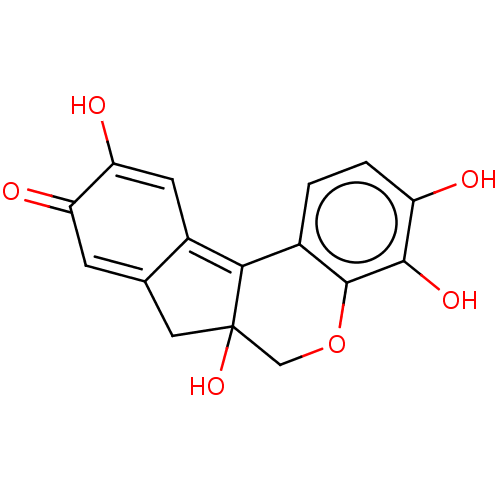
(US8987474, Hematin)Show SMILES OC1=CC2=C3c4ccc(O)c(O)c4OCC3(O)CC2=CC1=O |c:3,21,t:1| Show InChI InChI=1S/C16H12O6/c17-10-2-1-8-13-9-4-12(19)11(18)3-7(9)5-16(13,21)6-22-15(8)14(10)20/h1-4,17,19-21H,5-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant SARS-COV2 His-tagged S-RBD binding to recombinant human ACE2 expressed in HEK293T cells incubated for 0.5 hrs by alpha scre... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01547
BindingDB Entry DOI: 10.7270/Q25H7M47 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50416875
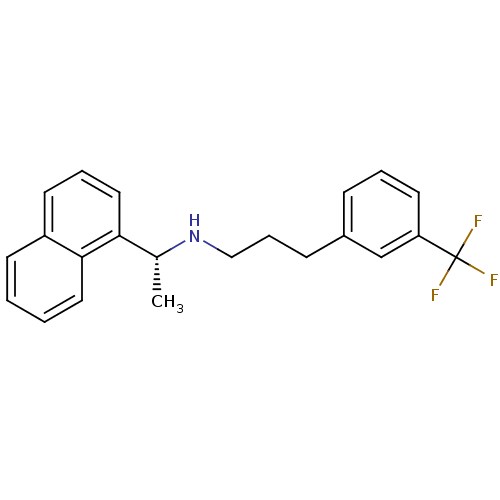
(AMG-073 | AMG073 HCL | CINACALCET | CINACALCET HYD...)Show SMILES C[C@@H](NCCCc1cccc(c1)C(F)(F)F)c1cccc2ccccc12 |r| Show InChI InChI=1S/C22H22F3N/c1-16(20-13-5-10-18-9-2-3-12-21(18)20)26-14-6-8-17-7-4-11-19(15-17)22(23,24)25/h2-5,7,9-13,15-16,26H,6,8,14H2,1H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human ACE2 using MCA-Tyr-Val-Ala-Asp-Ala-Pro-Lys(DNP)-OH as substrate preincubated for 15 mins followed by substrate additi... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01547
BindingDB Entry DOI: 10.7270/Q25H7M47 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50233798
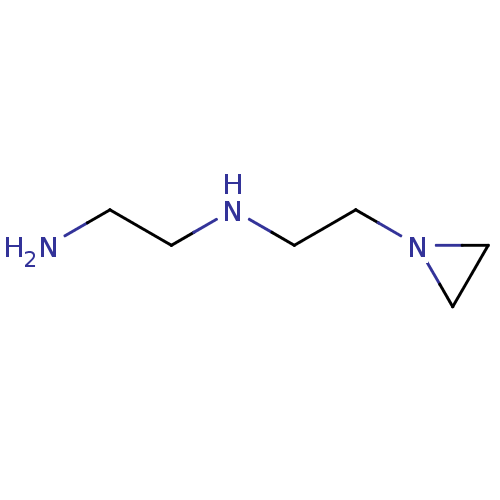
(CHEMBL398940 | N-(2-aminoethyl)-1-aziridine-ethana...)Show InChI InChI=1S/C6H15N3/c7-1-2-8-3-4-9-5-6-9/h8H,1-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Functional H2 receptor antagonistic activity in vitro assay on the isolated spontaneously beating guinea-pig right atrium |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50233798

(CHEMBL398940 | N-(2-aminoethyl)-1-aziridine-ethana...)Show InChI InChI=1S/C6H15N3/c7-1-2-8-3-4-9-5-6-9/h8H,1-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ACE2 |
Bioorg Med Chem Lett 18: 1681-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.046
BindingDB Entry DOI: 10.7270/Q29K49Z2 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50606670

(CHEMBL3219078)Show SMILES CNC(=O)CCCC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)-c1ccc(Cl)cc1Cl |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116389
BindingDB Entry DOI: 10.7270/Q2GF0ZM3 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50233798

(CHEMBL398940 | N-(2-aminoethyl)-1-aziridine-ethana...)Show InChI InChI=1S/C6H15N3/c7-1-2-8-3-4-9-5-6-9/h8H,1-7H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
| Assay Description
This is a review article. |
Med Res Rev (2020)
Article DOI: 10.1002/med.21724
BindingDB Entry DOI: 10.7270/Q2JS9ST6 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
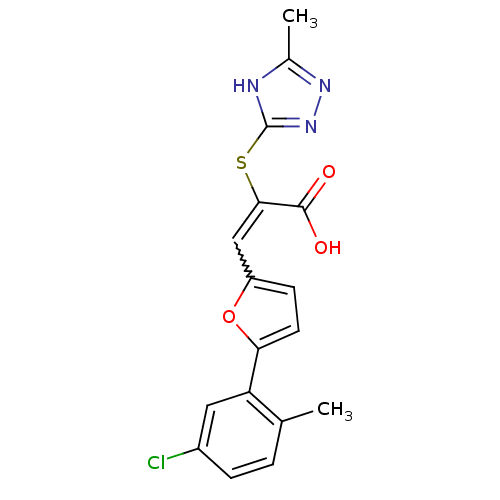
(Homo sapiens (Human)) | BDBM52871
((E)-3-[5-(5-Chloro-2-methyl-phenyl)-furan-2-yl]-2-...)Show SMILES Cc1nnc(SC(=Cc2ccc(o2)-c2cc(Cl)ccc2C)C(O)=O)[nH]1 |w:7.7| Show InChI InChI=1S/C17H14ClN3O3S/c1-9-3-4-11(18)7-13(9)14-6-5-12(24-14)8-15(16(22)23)25-17-19-10(2)20-21-17/h3-8H,1-2H3,(H,22,23)(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ACE2 |
Bioorg Med Chem Lett 18: 1681-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.046
BindingDB Entry DOI: 10.7270/Q29K49Z2 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50005641

(CHEMBL3235416)Show SMILES CNC(=O)[C@H](Cc1c[nH]cn1)NC(=O)CN(CCCc1ccccc1)CC(O)=O |r| Show InChI InChI=1S/C20H27N5O4/c1-21-20(29)17(10-16-11-22-14-23-16)24-18(26)12-25(13-19(27)28)9-5-8-15-6-3-2-4-7-15/h2-4,6-7,11,14,17H,5,8-10,12-13H2,1H3,(H,21,29)(H,22,23)(H,24,26)(H,27,28)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant ACE2-mediated amyloid beta hydrolysis using Mca-Tyr-Val-Ala-Asp-Pro-Ala-Lys-(DNP)-OH as substrate after 20 mins by fl... |
Eur J Med Chem 79: 184-93 (2014)
Article DOI: 10.1016/j.ejmech.2014.04.009
BindingDB Entry DOI: 10.7270/Q27H1M34 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
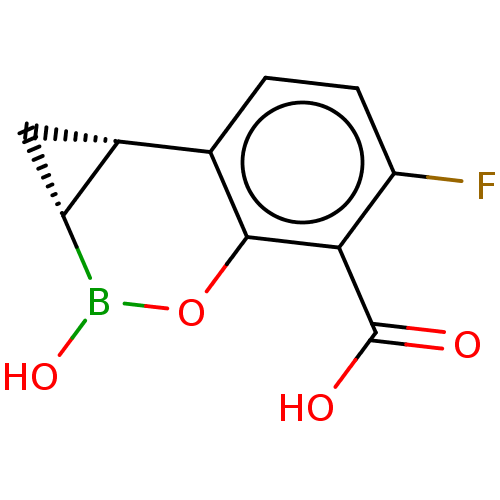
(Homo sapiens (Human)) | BDBM50541448
(CHEMBL4633785)Show SMILES [H][C@@]12C[C@]1([H])c1ccc(F)c(C(O)=O)c1OB2O |r| Show InChI InChI=1S/C10H8BFO4/c12-7-2-1-4-5-3-6(5)11(15)16-9(4)8(7)10(13)14/h1-2,5-6,15H,3H2,(H,13,14)/t5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Qpex Biopharma, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mammalian ACE-2 (unknown origin) using Mca-APK(Dnp) as substrate preincubated for 10 mins followed by substrate addition measured after... |
J Med Chem 63: 7491-7507 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01976
BindingDB Entry DOI: 10.7270/Q2377D7Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data