 Found 4047 hits of ic50 data for polymerid = 2259,50002672
Found 4047 hits of ic50 data for polymerid = 2259,50002672 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182306
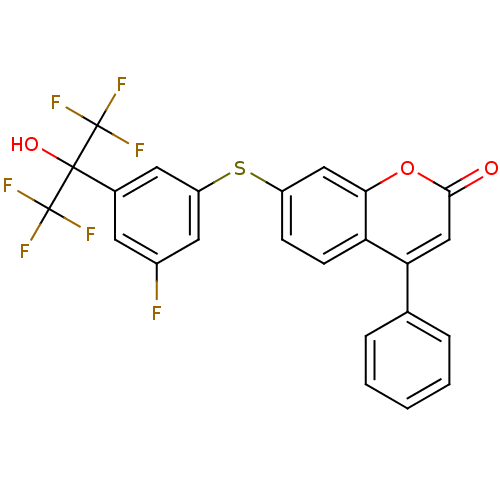
(7-(3-fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypro...)Show SMILES OC(c1cc(F)cc(Sc2ccc3c(cc(=O)oc3c2)-c2ccccc2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H13F7O3S/c25-15-8-14(22(33,23(26,27)28)24(29,30)31)9-17(10-15)35-16-6-7-18-19(13-4-2-1-3-5-13)12-21(32)34-20(18)11-16/h1-12,33H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182303
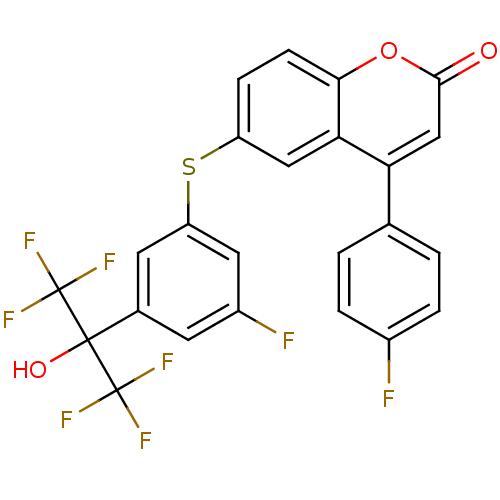
(6-(3-fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypro...)Show SMILES OC(c1cc(F)cc(Sc2ccc3oc(=O)cc(-c4ccc(F)cc4)c3c2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H12F8O3S/c25-14-3-1-12(2-4-14)18-11-21(33)35-20-6-5-16(10-19(18)20)36-17-8-13(7-15(26)9-17)22(34,23(27,28)29)24(30,31)32/h1-11,34H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Mus musculus) | BDBM50043670
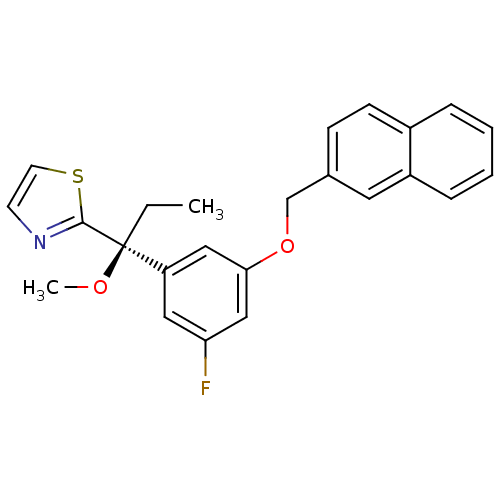
(2-{(S)-1-[3-Fluoro-5-(naphthalen-2-ylmethoxy)-phen...)Show SMILES CC[C@@](OC)(c1nccs1)c1cc(F)cc(OCc2ccc3ccccc3c2)c1 Show InChI InChI=1S/C24H22FNO2S/c1-3-24(27-2,23-26-10-11-29-23)20-13-21(25)15-22(14-20)28-16-17-8-9-18-6-4-5-7-19(18)12-17/h4-15H,3,16H2,1-2H3/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase from mouse macrophage |
J Med Chem 37: 113-24 (1994)
BindingDB Entry DOI: 10.7270/Q2JW8CZP |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | CHEMBL5267320
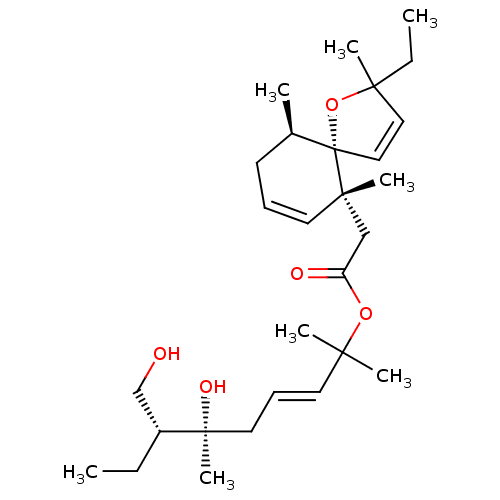
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | CHEMBL5267685

| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182308

(7-(3-fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypro...)Show SMILES OC(c1cc(F)cc(Sc2ccc3c(cc(=O)oc3c2)-c2ccoc2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C22H11F7O4S/c23-13-5-12(20(31,21(24,25)26)22(27,28)29)6-15(7-13)34-14-1-2-16-17(11-3-4-32-10-11)9-19(30)33-18(16)8-14/h1-10,31H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | CHEMBL5270325
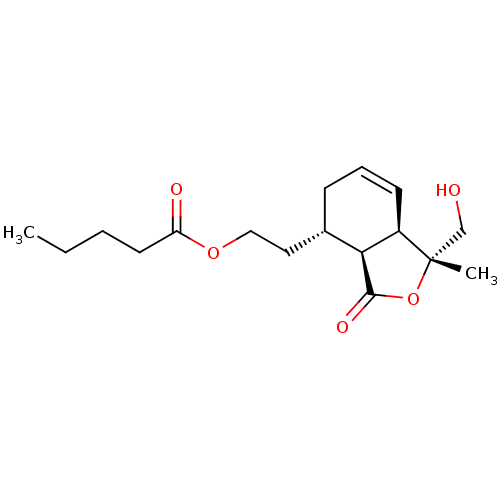
| PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
UniChem
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182304
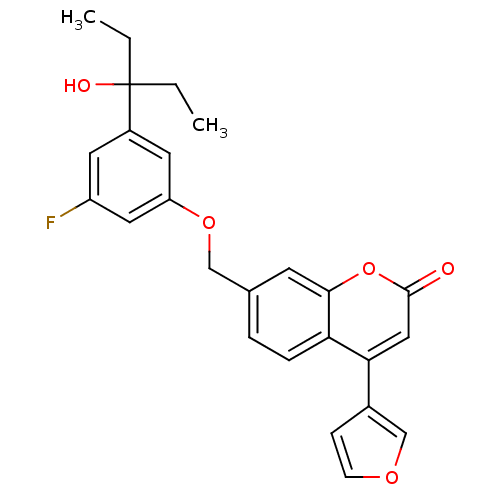
(7-((3-fluoro-5-(3-hydroxypentan-3-yl)phenoxy)methy...)Show SMILES CCC(O)(CC)c1cc(F)cc(OCc2ccc3c(cc(=O)oc3c2)-c2ccoc2)c1 Show InChI InChI=1S/C25H23FO5/c1-3-25(28,4-2)18-10-19(26)12-20(11-18)30-14-16-5-6-21-22(17-7-8-29-15-17)13-24(27)31-23(21)9-16/h5-13,15,28H,3-4,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Mus musculus) | BDBM50043682
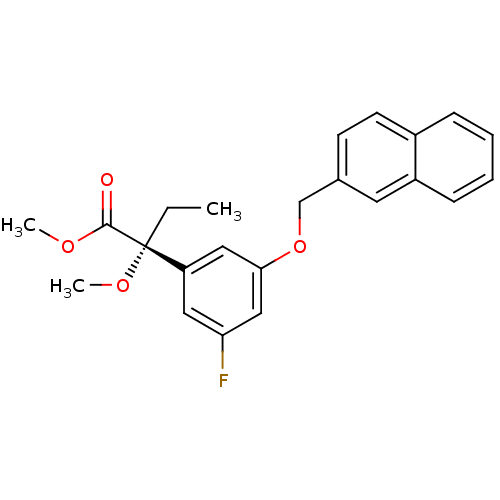
((R)-2-[3-Fluoro-5-(naphthalen-2-ylmethoxy)-phenyl]...)Show SMILES CC[C@](OC)(C(=O)OC)c1cc(F)cc(OCc2ccc3ccccc3c2)c1 Show InChI InChI=1S/C23H23FO4/c1-4-23(27-3,22(25)26-2)19-12-20(24)14-21(13-19)28-15-16-9-10-17-7-5-6-8-18(17)11-16/h5-14H,4,15H2,1-3H3/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase from mouse macrophage |
J Med Chem 37: 113-24 (1994)
BindingDB Entry DOI: 10.7270/Q2JW8CZP |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50029559
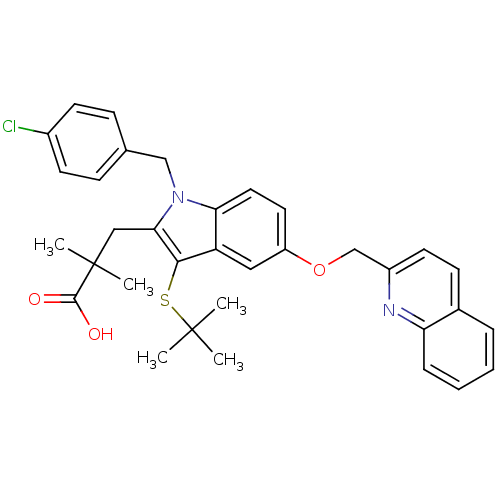
(2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C(O)=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Mus musculus) | BDBM50043669
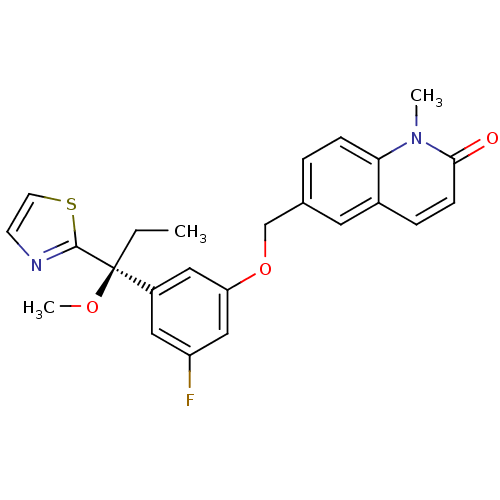
(6-[3-Fluoro-5-((S)-1-methoxy-1-thiazol-2-yl-propyl...)Show SMILES CC[C@@](OC)(c1nccs1)c1cc(F)cc(OCc2ccc3n(C)c(=O)ccc3c2)c1 Show InChI InChI=1S/C24H23FN2O3S/c1-4-24(29-3,23-26-9-10-31-23)18-12-19(25)14-20(13-18)30-15-16-5-7-21-17(11-16)6-8-22(28)27(21)2/h5-14H,4,15H2,1-3H3/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase from mouse macrophage |
J Med Chem 37: 113-24 (1994)
BindingDB Entry DOI: 10.7270/Q2JW8CZP |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182302

(7-((3-fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypr...)Show SMILES OC(c1cc(F)cc(OCc2ccc3c(cc(=O)oc3c2)-c2ccc(F)cc2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C25H14F8O4/c26-16-4-2-14(3-5-16)20-11-22(34)37-21-7-13(1-6-19(20)21)12-36-18-9-15(8-17(27)10-18)23(35,24(28,29)30)25(31,32)33/h1-11,35H,12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182300

(7-((3-fluoro-5-((1S,3S,5R)-3-hydroxy-6,8-dioxa-bic...)Show SMILES O[C@]1(C[C@H]2CO[C@@H](C1)O2)c1cc(F)cc(OCc2ccc3c(cc(nc3c2)C#N)-c2ccoc2)c1 Show InChI InChI=1S/C27H21FN2O5/c28-19-6-18(27(31)10-22-15-34-26(11-27)35-22)7-21(8-19)33-13-16-1-2-23-24(17-3-4-32-14-17)9-20(12-29)30-25(23)5-16/h1-9,14,22,26,31H,10-11,13,15H2/t22?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182305
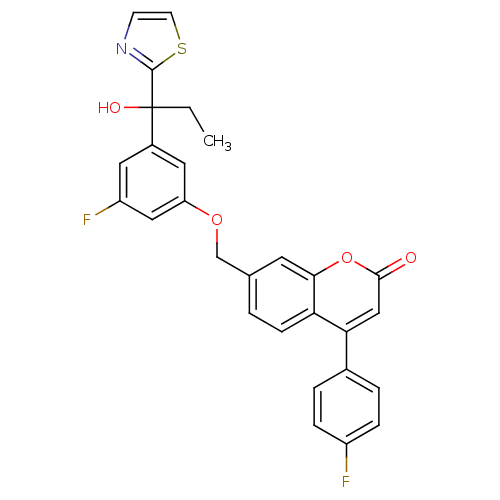
(7-((3-fluoro-5-(1-hydroxy-1-(thiazol-2-yl)propyl)p...)Show SMILES CCC(O)(c1nccs1)c1cc(F)cc(OCc2ccc3c(cc(=O)oc3c2)-c2ccc(F)cc2)c1 Show InChI InChI=1S/C28H21F2NO4S/c1-2-28(33,27-31-9-10-36-27)19-12-21(30)14-22(13-19)34-16-17-3-8-23-24(15-26(32)35-25(23)11-17)18-4-6-20(29)7-5-18/h3-15,33H,2,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50045643

(3-[1-(4-Chloro-benzyl)-4-methyl-6-(quinolin-2-ylme...)Show SMILES CC1Cc2c(OCc3ccc4ccccc4n3)ccc3n(Cc4ccc(Cl)cc4)c(CC(C)(C)C(O)=O)c(S1)c23 Show InChI InChI=1S/C33H31ClN2O3S/c1-20-16-25-29(39-19-24-13-10-22-6-4-5-7-26(22)35-24)15-14-27-30(25)31(40-20)28(17-33(2,3)32(37)38)36(27)18-21-8-11-23(34)12-9-21/h4-15,20H,16-19H2,1-3H3,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of LTB4 biosynthesis in [Ca2+]-ionophore-activated human polymorphonuclear leukocytes |
J Med Chem 36: 2771-87 (1993)
BindingDB Entry DOI: 10.7270/Q28914XB |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50110484

(3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(cc2)S(C)(=O)=O)c1 Show InChI InChI=1S/C29H29FN2O5S/c1-35-29(12-14-36-15-13-29)22-16-23(30)18-26(17-22)37-20-24-19-28(21-6-4-3-5-7-21)32(31-24)25-8-10-27(11-9-25)38(2,33)34/h3-11,16-19H,12-15,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant against arachidonate 5-lipoxygenase |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182307
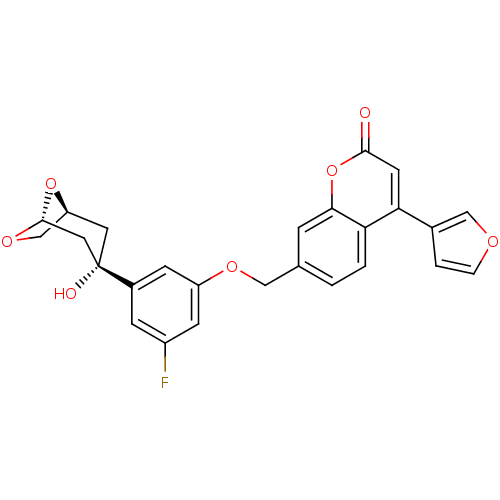
(7-((3-fluoro-5-((1S,3S,5R)-3-hydroxy-6,8-dioxa-bic...)Show SMILES O[C@]1(C[C@H]2CO[C@@H](C1)O2)c1cc(F)cc(OCc2ccc3c(cc(=O)oc3c2)-c2ccoc2)c1 Show InChI InChI=1S/C26H21FO7/c27-18-6-17(26(29)10-20-14-32-25(11-26)33-20)7-19(8-18)31-12-15-1-2-21-22(16-3-4-30-13-16)9-24(28)34-23(21)5-15/h1-9,13,20,25,29H,10-12,14H2/t20?,25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50045673
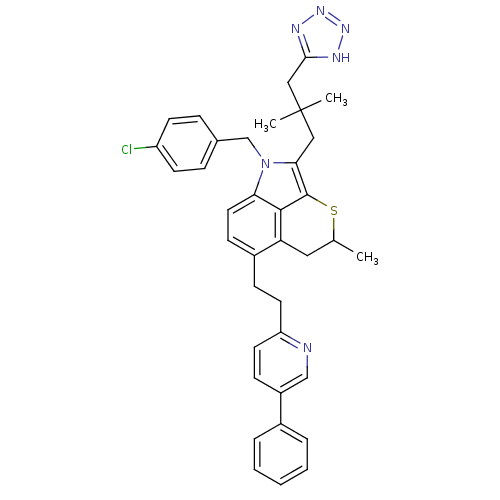
(1-(4-Chloro-benzyl)-2-[2,2-dimethyl-3-(1H-tetrazol...)Show SMILES CC1Cc2c(CCc3ccc(cn3)-c3ccccc3)ccc3n(Cc4ccc(Cl)cc4)c(CC(C)(C)Cc4nnn[nH]4)c(S1)c23 Show InChI InChI=1S/C37H37ClN6S/c1-24-19-31-27(11-16-30-17-12-28(22-39-30)26-7-5-4-6-8-26)13-18-32-35(31)36(45-24)33(20-37(2,3)21-34-40-42-43-41-34)44(32)23-25-9-14-29(38)15-10-25/h4-10,12-15,17-18,22,24H,11,16,19-21,23H2,1-3H3,(H,40,41,42,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of LTB4 biosynthesis in [Ca2+]-ionophore-activated human polymorphonuclear leukocytes |
J Med Chem 36: 2771-87 (1993)
BindingDB Entry DOI: 10.7270/Q28914XB |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50110484

(3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...)Show SMILES COC1(CCOCC1)c1cc(F)cc(OCc2cc(-c3ccccc3)n(n2)-c2ccc(cc2)S(C)(=O)=O)c1 Show InChI InChI=1S/C29H29FN2O5S/c1-35-29(12-14-36-15-13-29)22-16-23(30)18-26(17-22)37-20-24-19-28(21-6-4-3-5-7-21)32(31-24)25-8-10-27(11-9-25)38(2,33)34/h3-11,16-19H,12-15,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Innothera
Curated by ChEMBL
| Assay Description
The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cells |
Bioorg Med Chem Lett 12: 779-82 (2002)
BindingDB Entry DOI: 10.7270/Q20864MC |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182299

(7-((3-fluoro-5-(1-hydroxy-1-(pyridin-2-yl)propyl)p...)Show SMILES CCC(O)(c1cc(F)cc(OCc2ccc3c(cc(=O)oc3c2)-c2ccoc2)c1)c1ccccn1 Show InChI InChI=1S/C28H22FNO5/c1-2-28(32,26-5-3-4-9-30-26)20-12-21(29)14-22(13-20)34-16-18-6-7-23-24(19-8-10-33-17-19)15-27(31)35-25(23)11-18/h3-15,17,32H,2,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182298
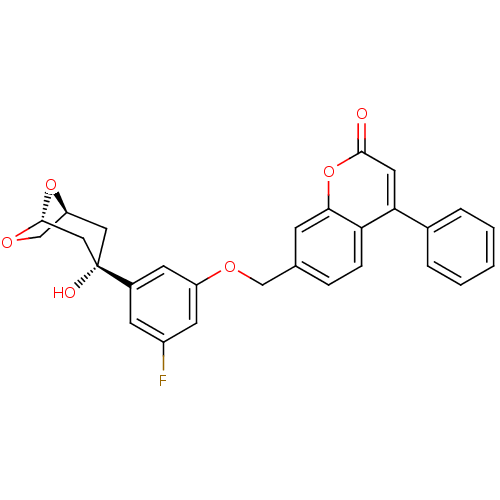
(7-((3-fluoro-5-((1S,3S,5R)-3-hydroxy-6,8-dioxa-bic...)Show SMILES O[C@]1(C[C@H]2CO[C@@H](C1)O2)c1cc(F)cc(OCc2ccc3c(cc(=O)oc3c2)-c2ccccc2)c1 Show InChI InChI=1S/C28H23FO6/c29-20-9-19(28(31)13-22-16-33-27(14-28)34-22)10-21(11-20)32-15-17-6-7-23-24(18-4-2-1-3-5-18)12-26(30)35-25(23)8-17/h1-12,22,27,31H,13-16H2/t22?,27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50004424
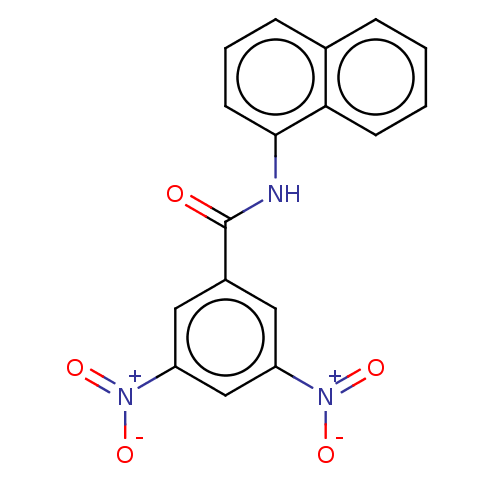
(CHEMBL3238463)Show SMILES [O-][N+](=O)c1cc(cc(c1)[N+]([O-])=O)C(=O)Nc1cccc2ccccc12 Show InChI InChI=1S/C17H11N3O5/c21-17(12-8-13(19(22)23)10-14(9-12)20(24)25)18-16-7-3-5-11-4-1-2-6-15(11)16/h1-10H,(H,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human 5-LOX using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition by H2DCFDA staining-based fluor... |
Bioorg Med Chem 22: 2396-402 (2014)
Article DOI: 10.1016/j.bmc.2014.03.008
BindingDB Entry DOI: 10.7270/Q2X63PF9 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182301
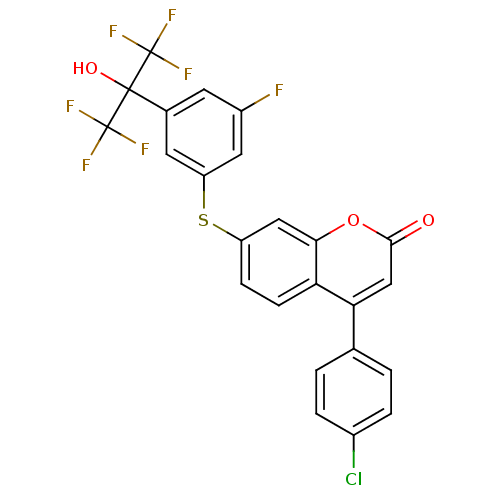
(4-(4-chlorophenyl)-7-(3-fluoro-5-(1,1,1,3,3,3-hexa...)Show SMILES OC(c1cc(F)cc(Sc2ccc3c(cc(=O)oc3c2)-c2ccc(Cl)cc2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H12ClF7O3S/c25-14-3-1-12(2-4-14)19-11-21(33)35-20-10-16(5-6-18(19)20)36-17-8-13(7-15(26)9-17)22(34,23(27,28)29)24(30,31)32/h1-11,34H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of LTB4 production in calcium ionophore A-23187-stimulated human polymorphonuclear leukocyte |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
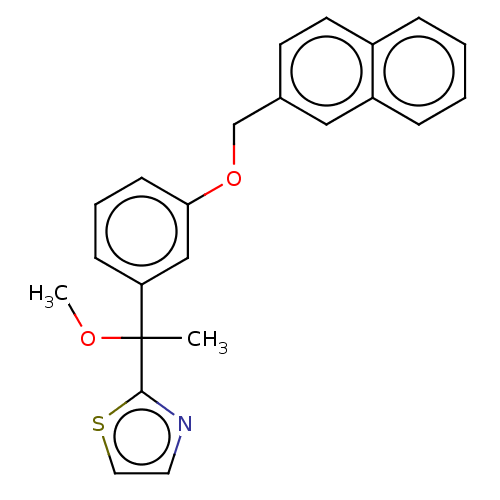
Polyunsaturated fatty acid 5-lipoxygenase
(Mus musculus) | BDBM50000832
(2-{1-Methoxy-1-[3-(naphthalen-2-ylmethoxy)-phenyl]...)Show InChI InChI=1S/C23H21NO2S/c1-23(25-2,22-24-12-13-27-22)20-8-5-9-21(15-20)26-16-17-10-11-18-6-3-4-7-19(18)14-17/h3-15H,16H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in mouse macrophages. |
J Med Chem 34: 2176-86 (1991)
BindingDB Entry DOI: 10.7270/Q2S46QX6 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Mus musculus) | BDBM50043677

((S)-2-[3-Fluoro-5-(naphthalen-2-ylmethoxy)-phenyl]...)Show SMILES CC[C@@](OC)(C(=O)OC)c1cc(F)cc(OCc2ccc3ccccc3c2)c1 Show InChI InChI=1S/C23H23FO4/c1-4-23(27-3,22(25)26-2)19-12-20(24)14-21(13-19)28-15-16-9-10-17-7-5-6-8-18(17)11-16/h5-14H,4,15H2,1-3H3/t23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase from mouse macrophage |
J Med Chem 37: 113-24 (1994)
BindingDB Entry DOI: 10.7270/Q2JW8CZP |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Mus musculus) | BDBM50007052
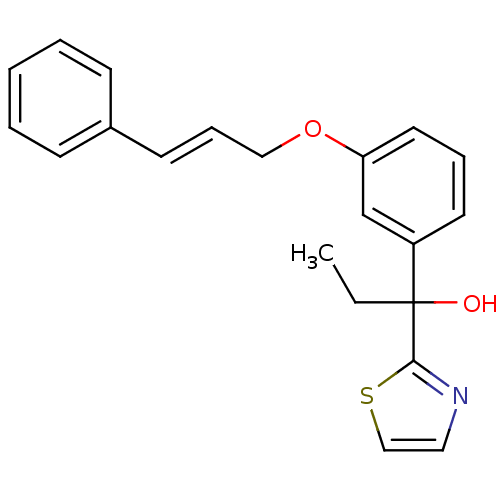
(1-[3-(3-Phenyl-allyloxy)-phenyl]-1-thiazol-2-yl-pr...)Show InChI InChI=1S/C21H21NO2S/c1-2-21(23,20-22-13-15-25-20)18-11-6-12-19(16-18)24-14-7-10-17-8-4-3-5-9-17/h3-13,15-16,23H,2,14H2,1H3/b10-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in mouse macrophages. |
J Med Chem 34: 2176-86 (1991)
BindingDB Entry DOI: 10.7270/Q2S46QX6 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50004425
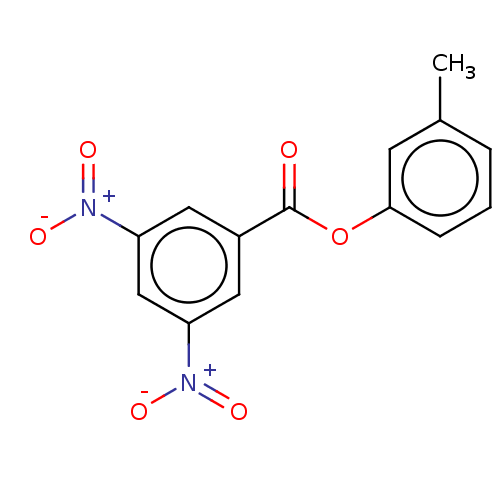
(CHEMBL3238207)Show SMILES Cc1cccc(OC(=O)c2cc(cc(c2)[N+]([O-])=O)[N+]([O-])=O)c1 Show InChI InChI=1S/C14H10N2O6/c1-9-3-2-4-13(5-9)22-14(17)10-6-11(15(18)19)8-12(7-10)16(20)21/h2-8H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human 5-LOX using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition by H2DCFDA staining-based fluor... |
Bioorg Med Chem 22: 2396-402 (2014)
Article DOI: 10.1016/j.bmc.2014.03.008
BindingDB Entry DOI: 10.7270/Q2X63PF9 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50085160

(CHEMBL32842 | L-674636 | {4-(4-Chloro-phenyl)-1-[4...)Show SMILES OC(=O)CSC(CCCc1ccc(Cl)cc1)c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C28H26ClNO3S/c29-23-13-8-20(9-14-23)4-3-7-27(34-19-28(31)32)22-11-16-25(17-12-22)33-18-24-15-10-21-5-1-2-6-26(21)30-24/h1-2,5-6,8-17,27H,3-4,7,18-19H2,(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of calcium ionophore (A-23187)-stimulated LTB4 formation in human neutrophil assay |
J Med Chem 43: 690-705 (2000)
BindingDB Entry DOI: 10.7270/Q2HD7WCR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50016991

(7-Fluoro-2-(4-methoxy-benzyl)-3-methyl-5-propyl-be...)Show InChI InChI=1S/C20H21FO3/c1-4-5-14-11-16(21)20-18(19(14)22)12(2)17(24-20)10-13-6-8-15(23-3)9-7-13/h6-9,11,22H,4-5,10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada Inc.
Curated by ChEMBL
| Assay Description
Concentration required for 50 % inhibition of leukotriene B4 production in human PMN compared with controls in the absence of compound |
J Med Chem 32: 1190-7 (1989)
BindingDB Entry DOI: 10.7270/Q2ST7NVR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50331806
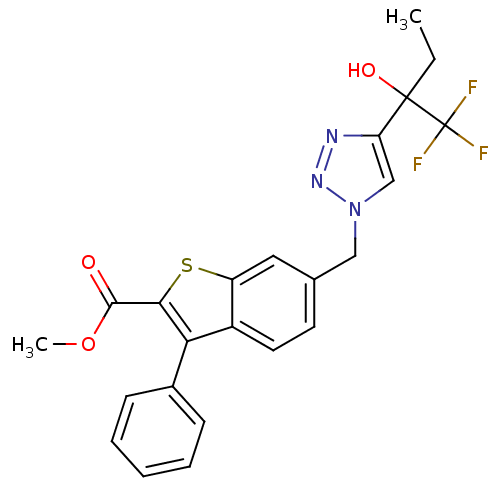
(CHEMBL1290649 | methyl 3-phenyl-6-((4-(1,1,1-trifl...)Show SMILES CCC(O)(c1cn(Cc2ccc3c(c(sc3c2)C(=O)OC)-c2ccccc2)nn1)C(F)(F)F Show InChI InChI=1S/C23H20F3N3O3S/c1-3-22(31,23(24,25)26)18-13-29(28-27-18)12-14-9-10-16-17(11-14)33-20(21(30)32-2)19(16)15-7-5-4-6-8-15/h4-11,13,31H,3,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase assessed as arachidonic acid oxidation |
Bioorg Med Chem Lett 20: 7440-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.024
BindingDB Entry DOI: 10.7270/Q2XG9RC8 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50052018
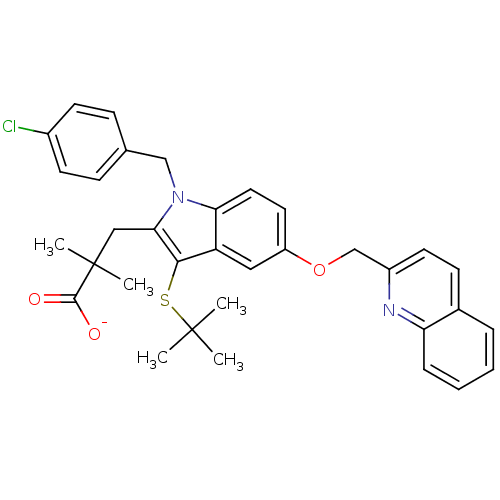
(3-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...)Show SMILES CC(C)(C)Sc1c(CC(C)(C)C([O-])=O)n(Cc2ccc(Cl)cc2)c2ccc(OCc3ccc4ccccc4n3)cc12 Show InChI InChI=1S/C34H35ClN2O3S/c1-33(2,3)41-31-27-18-26(40-21-25-15-12-23-8-6-7-9-28(23)36-25)16-17-29(27)37(20-22-10-13-24(35)14-11-22)30(31)19-34(4,5)32(38)39/h6-18H,19-21H2,1-5H3,(H,38,39)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of calcium ionophore (A-23187)-stimulated LTB4 formation in human neutrophil assay |
J Med Chem 43: 690-705 (2000)
BindingDB Entry DOI: 10.7270/Q2HD7WCR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446686
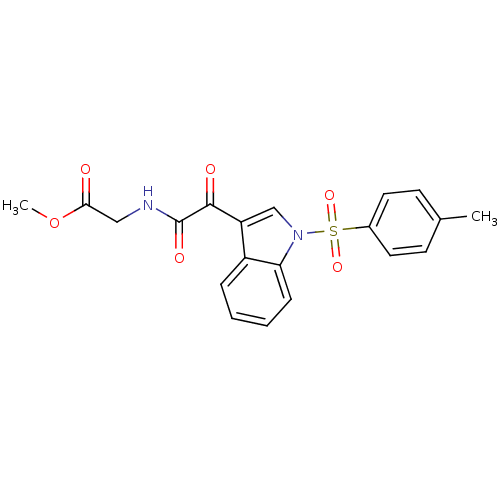
(CHEMBL3113612)Show SMILES COC(=O)CNC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C20H18N2O6S/c1-13-7-9-14(10-8-13)29(26,27)22-12-16(15-5-3-4-6-17(15)22)19(24)20(25)21-11-18(23)28-2/h3-10,12H,11H2,1-2H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA in presence of pig liver esterase |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Mus musculus) | BDBM50000857
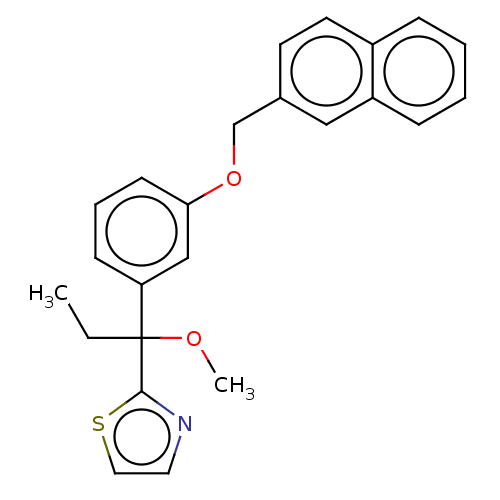
(2-(1-methoxy-1-(3-(naphthalen-2-ylmethoxy)phenyl)p...)Show InChI InChI=1S/C24H23NO2S/c1-3-24(26-2,23-25-13-14-28-23)21-9-6-10-22(16-21)27-17-18-11-12-19-7-4-5-8-20(19)15-18/h4-16H,3,17H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase in mouse macrophages. |
J Med Chem 34: 2176-86 (1991)
BindingDB Entry DOI: 10.7270/Q2S46QX6 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50409117
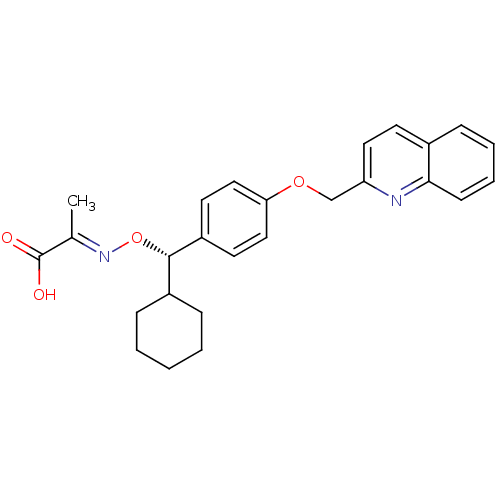
(CHEMBL2093045)Show SMILES C\C(=N/O[C@@H](C1CCCCC1)c1ccc(OCc2ccc3ccccc3n2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H28N2O4/c1-18(26(29)30)28-32-25(20-8-3-2-4-9-20)21-12-15-23(16-13-21)31-17-22-14-11-19-7-5-6-10-24(19)27-22/h5-7,10-16,20,25H,2-4,8-9,17H2,1H3,(H,29,30)/b28-18+/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of calcium ionophore (A-23187)-stimulated LTB4 formation in human neutrophil assay |
J Med Chem 43: 690-705 (2000)
BindingDB Entry DOI: 10.7270/Q2HD7WCR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50041161
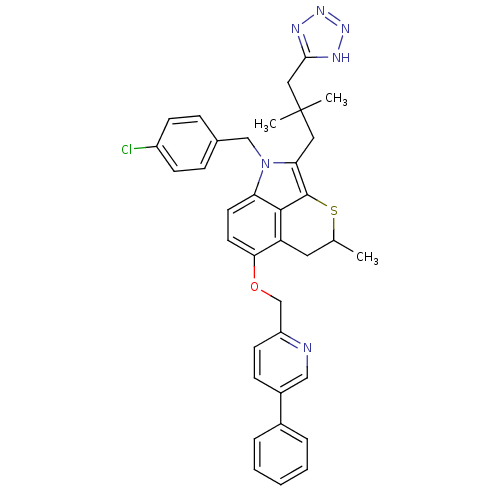
(1-(4-Chloro-benzyl)-2-[2,2-dimethyl-3-(1H-tetrazol...)Show SMILES CC1Cc2c(OCc3ccc(cn3)-c3ccccc3)ccc3n(Cc4ccc(Cl)cc4)c(CC(C)(C)Cc4nnn[nH]4)c(S1)c23 Show InChI InChI=1S/C36H35ClN6OS/c1-23-17-29-32(44-22-28-14-11-26(20-38-28)25-7-5-4-6-8-25)16-15-30-34(29)35(45-23)31(18-36(2,3)19-33-39-41-42-40-33)43(30)21-24-9-12-27(37)13-10-24/h4-16,20,23H,17-19,21-22H2,1-3H3,(H,39,40,41,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of LTB4 biosynthesis in [Ca2+]-ionophore-activated human polymorphonuclear leukocytes |
J Med Chem 36: 2771-87 (1993)
BindingDB Entry DOI: 10.7270/Q28914XB |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446681
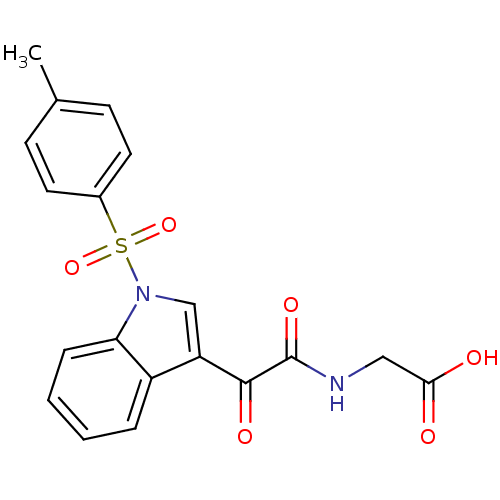
(CHEMBL3113617)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)NCC(O)=O)c2ccccc12 Show InChI InChI=1S/C19H16N2O6S/c1-12-6-8-13(9-7-12)28(26,27)21-11-15(14-4-2-3-5-16(14)21)18(24)19(25)20-10-17(22)23/h2-9,11H,10H2,1H3,(H,20,25)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50085202
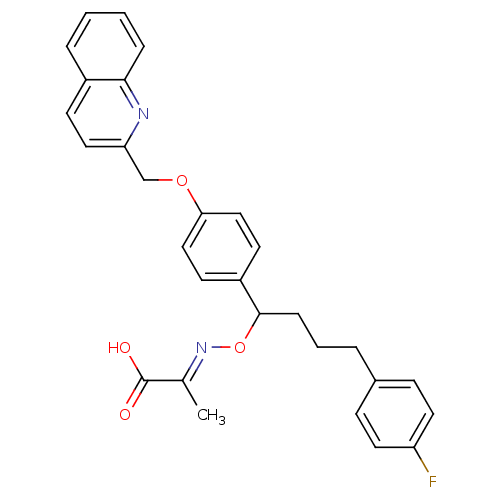
(2-{4-(4-Fluoro-phenyl)-1-[4-(quinolin-2-ylmethoxy)...)Show SMILES C\C(=N/OC(CCCc1ccc(F)cc1)c1ccc(OCc2ccc3ccccc3n2)cc1)C(O)=O Show InChI InChI=1S/C29H27FN2O4/c1-20(29(33)34)32-36-28(8-4-5-21-9-14-24(30)15-10-21)23-12-17-26(18-13-23)35-19-25-16-11-22-6-2-3-7-27(22)31-25/h2-3,6-7,9-18,28H,4-5,8,19H2,1H3,(H,33,34)/b32-20+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of calcium ionophore (A-23187)-stimulated LTB4 formation in human neutrophil assay |
J Med Chem 43: 690-705 (2000)
BindingDB Entry DOI: 10.7270/Q2HD7WCR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50182308

(7-(3-fluoro-5-(1,1,1,3,3,3-hexafluoro-2-hydroxypro...)Show SMILES OC(c1cc(F)cc(Sc2ccc3c(cc(=O)oc3c2)-c2ccoc2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C22H11F7O4S/c23-13-5-12(20(31,21(24,25)26)22(27,28)29)6-15(7-13)34-14-1-2-16-17(11-3-4-32-10-11)9-19(30)33-18(16)8-14/h1-10,31H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5-LO |
Bioorg Med Chem Lett 16: 2528-31 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.085
BindingDB Entry DOI: 10.7270/Q2NV9HVZ |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Mus musculus) | BDBM50043666

((R)-2-[3-Fluoro-5-(1-methyl-2-oxo-1,2-dihydro-quin...)Show SMILES CC[C@](OC)(C(=O)OC)c1cc(F)cc(OCc2ccc3n(C)c(=O)ccc3c2)c1 Show InChI InChI=1S/C23H24FNO5/c1-5-23(29-4,22(27)28-3)17-11-18(24)13-19(12-17)30-14-15-6-8-20-16(10-15)7-9-21(26)25(20)2/h6-13H,5,14H2,1-4H3/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of 5-lipoxygenase from mouse macrophage |
J Med Chem 37: 113-24 (1994)
BindingDB Entry DOI: 10.7270/Q2JW8CZP |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446686

(CHEMBL3113612)Show SMILES COC(=O)CNC(=O)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccc(C)cc1 Show InChI InChI=1S/C20H18N2O6S/c1-13-7-9-14(10-8-13)29(26,27)22-12-16(15-5-3-4-6-17(15)22)19(24)20(25)21-11-18(23)28-2/h3-10,12H,11H2,1-2H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50085229

(CHEMBL159516 | {3-Methyl-3-phenyl-1-[4-(quinolin-2...)Show SMILES CC(C)(CC(O\N=C\C(O)=O)c1ccc(OCc2ccc3ccccc3n2)cc1)c1ccccc1 Show InChI InChI=1S/C29H28N2O4/c1-29(2,23-9-4-3-5-10-23)18-27(35-30-19-28(32)33)22-13-16-25(17-14-22)34-20-24-15-12-21-8-6-7-11-26(21)31-24/h3-17,19,27H,18,20H2,1-2H3,(H,32,33)/b30-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of calcium ionophore (A-23187)-stimulated LTB4 formation in human neutrophil assay |
J Med Chem 43: 690-705 (2000)
BindingDB Entry DOI: 10.7270/Q2HD7WCR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50085231

(CHEMBL159297 | {2-Butyl-1-[4-(quinolin-2-ylmethoxy...)Show SMILES CCCCC(CCCC)C(O\N=C\C(O)=O)c1ccc(OCc2ccc3ccccc3n2)cc1 Show InChI InChI=1S/C28H34N2O4/c1-3-5-9-22(10-6-4-2)28(34-29-19-27(31)32)23-14-17-25(18-15-23)33-20-24-16-13-21-11-7-8-12-26(21)30-24/h7-8,11-19,22,28H,3-6,9-10,20H2,1-2H3,(H,31,32)/b29-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of calcium ionophore (A-23187)-stimulated LTB4 formation in human neutrophil assay |
J Med Chem 43: 690-705 (2000)
BindingDB Entry DOI: 10.7270/Q2HD7WCR |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50446680
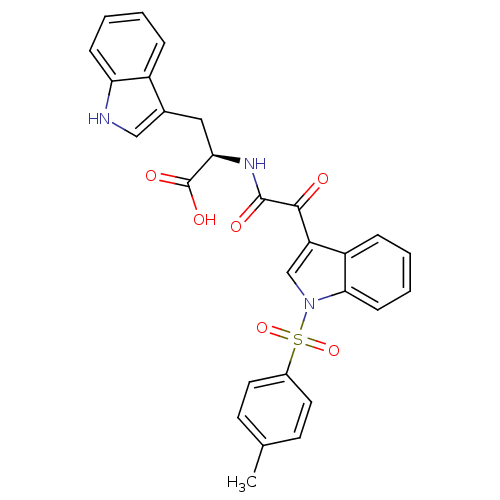
(CHEMBL3113618)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1cc(C(=O)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(O)=O)c2ccccc12 |r| Show InChI InChI=1S/C28H23N3O6S/c1-17-10-12-19(13-11-17)38(36,37)31-16-22(21-7-3-5-9-25(21)31)26(32)27(33)30-24(28(34)35)14-18-15-29-23-8-4-2-6-20(18)23/h2-13,15-16,24,29H,14H2,1H3,(H,30,33)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Guru Nanak Dev University
Curated by ChEMBL
| Assay Description
Inhibition of 5-LOX (unknown origin) using arachidonic acid as substrate after 5 mins by EIA |
Bioorg Med Chem 22: 1642-8 (2014)
Article DOI: 10.1016/j.bmc.2014.01.027
BindingDB Entry DOI: 10.7270/Q2KH0PT3 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50000845

(4-Methoxy-4-[3-(naphthalen-2-ylmethoxy)-phenyl]-te...)Show InChI InChI=1S/C23H24O3/c1-24-23(11-13-25-14-12-23)21-7-4-8-22(16-21)26-17-18-9-10-19-5-2-3-6-20(19)15-18/h2-10,15-16H,11-14,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro potency against human 5-Lipoxygenase |
J Med Chem 37: 512-8 (1994)
BindingDB Entry DOI: 10.7270/Q2J965FV |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50004426
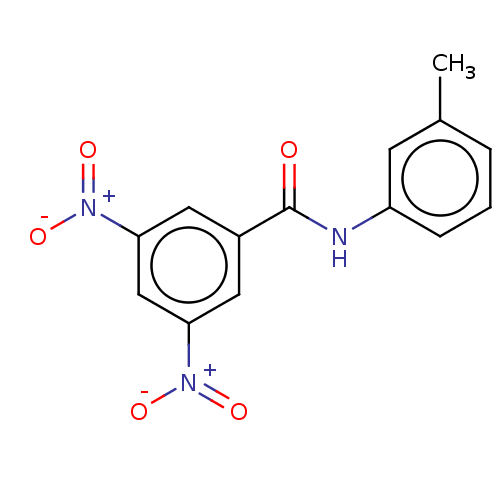
(CHEMBL3238465)Show SMILES Cc1cccc(NC(=O)c2cc(cc(c2)[N+]([O-])=O)[N+]([O-])=O)c1 Show InChI InChI=1S/C14H11N3O5/c1-9-3-2-4-11(5-9)15-14(18)10-6-12(16(19)20)8-13(7-10)17(21)22/h2-8H,1H3,(H,15,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human 5-LOX using arachidonic acid as substrate preincubated for 10 mins followed by substrate addition by H2DCFDA staining-based fluor... |
Bioorg Med Chem 22: 2396-402 (2014)
Article DOI: 10.1016/j.bmc.2014.03.008
BindingDB Entry DOI: 10.7270/Q2X63PF9 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of Human 5-lipoxygenase |
J Med Chem 38: 4538-47 (1995)
BindingDB Entry DOI: 10.7270/Q2C53JW5 |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50031153
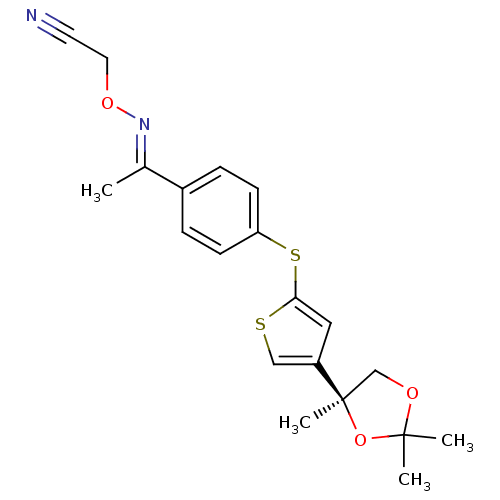
(CHEMBL129970 | [1-{4-[4-((S)-2,2,4-Trimethyl-[1,3]...)Show SMILES C\C(=N/OCC#N)c1ccc(Sc2cc(cs2)[C@@]2(C)COC(C)(C)O2)cc1 Show InChI InChI=1S/C20H22N2O3S2/c1-14(22-24-10-9-21)15-5-7-17(8-6-15)27-18-11-16(12-26-18)20(4)13-23-19(2,3)25-20/h5-8,11-12H,10,13H2,1-4H3/b22-14+/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Zeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of LT biosynthesis in vitro using A-23,187-stimulated human whole blood. |
J Med Chem 38: 3951-6 (1995)
BindingDB Entry DOI: 10.7270/Q22J69WD |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50038460
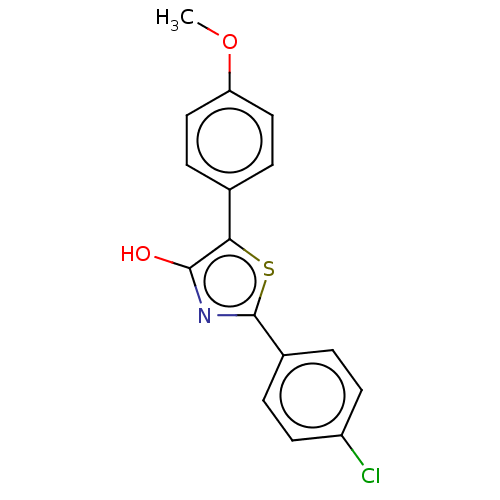
(CHEMBL3353726)Show InChI InChI=1S/C16H12ClNO2S/c1-20-13-8-4-10(5-9-13)14-15(19)18-16(21-14)11-2-6-12(17)7-3-11/h2-9,19H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of 5-LO in human PMNL using arachidonic acid as substrate assessed as product formation incubated for 15 mins prior to substrate addition ... |
Eur J Med Chem 89: 503-23 (2014)
Article DOI: 10.1016/j.ejmech.2014.10.054
BindingDB Entry DOI: 10.7270/Q2959K5T |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50045654

(CHEMBL96253 | N-{4-[1-(4-Chloro-benzyl)-4-methyl-6...)Show SMILES CC1Cc2c(OCc3ccc(cn3)-c3ccccc3)ccc3n(Cc4ccc(Cl)cc4)c(CC(C)(C)CC(=O)NS(C)(=O)=O)c(S1)c23 Show InChI InChI=1S/C37H38ClN3O4S2/c1-24-18-30-33(45-23-29-15-12-27(21-39-29)26-8-6-5-7-9-26)17-16-31-35(30)36(46-24)32(41(31)22-25-10-13-28(38)14-11-25)19-37(2,3)20-34(42)40-47(4,43)44/h5-17,21,24H,18-20,22-23H2,1-4H3,(H,40,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of LTB4 biosynthesis in [Ca2+]-ionophore-activated human polymorphonuclear leukocytes |
J Med Chem 36: 2771-87 (1993)
BindingDB Entry DOI: 10.7270/Q28914XB |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid 5-lipoxygenase
(Homo sapiens (Human)) | BDBM50331797
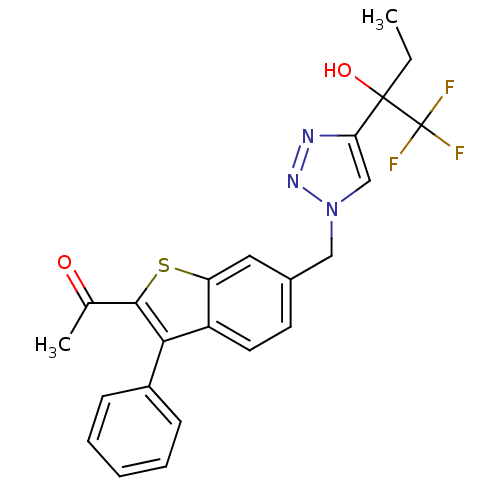
(1-(3-phenyl-6-((4-(1,1,1-trifluoro-2-hydroxybutan-...)Show SMILES CCC(O)(c1cn(Cc2ccc3c(c(sc3c2)C(C)=O)-c2ccccc2)nn1)C(F)(F)F Show InChI InChI=1S/C23H20F3N3O2S/c1-3-22(31,23(24,25)26)19-13-29(28-27-19)12-15-9-10-17-18(11-15)32-21(14(2)30)20(17)16-7-5-4-6-8-16/h4-11,13,31H,3,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 5-lipoxygenase assessed as arachidonic acid oxidation |
Bioorg Med Chem Lett 20: 7440-3 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.024
BindingDB Entry DOI: 10.7270/Q2XG9RC8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data