

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50091786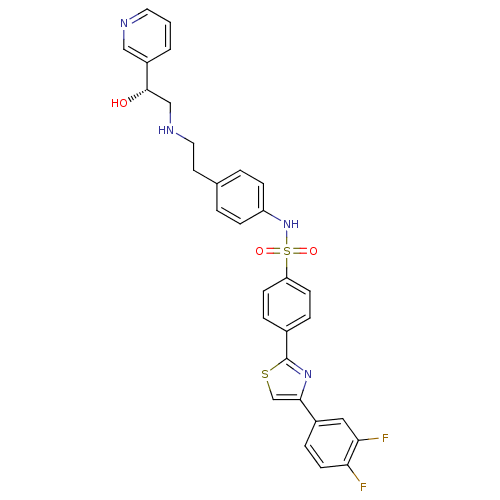 (4-[4-(3,4-Difluoro-phenyl)-thiazol-2-yl]-N-{4-[2-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro functional efficacy as increased cAMP in chinese hamster ovary (CHO) cells expressing human beta-3 receptor | J Med Chem 43: 3832-6 (2000) BindingDB Entry DOI: 10.7270/Q2057F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50092646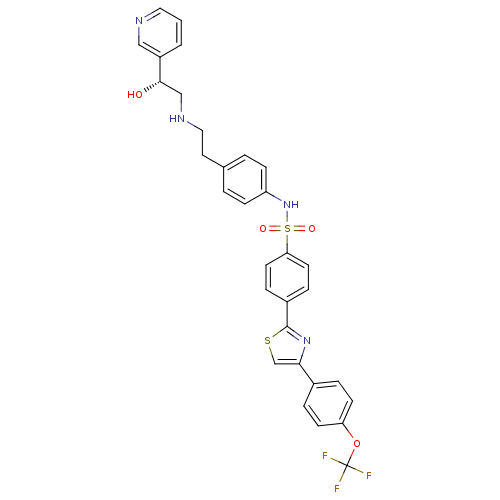 (CHEMBL331744 | N-{4-[2-(2-Hydroxy-2-pyridin-3-yl-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity was evaluated on chinese hamster ovary (CHO) cells expressing the cloned human beta-3 adrenergic receptor in the presence of [125I]-... | J Med Chem 43: 3832-6 (2000) BindingDB Entry DOI: 10.7270/Q2057F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50092645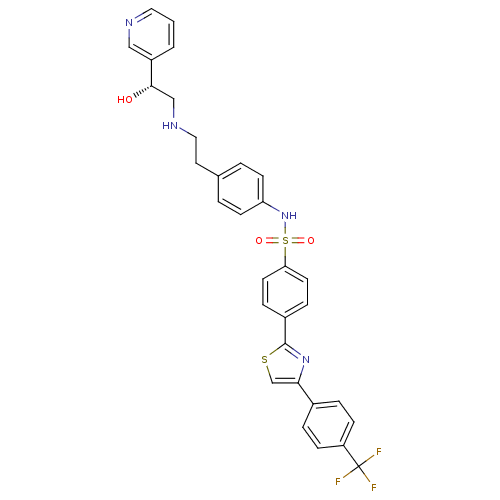 ((R)-N-(4-(2-(2-hydroxy-2-(pyridin-3-yl)ethylamino)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro functional efficacy as increased cAMP in chinese hamster ovary (CHO) cells expressing human beta-3 receptor | J Med Chem 43: 3832-6 (2000) BindingDB Entry DOI: 10.7270/Q2057F5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50076960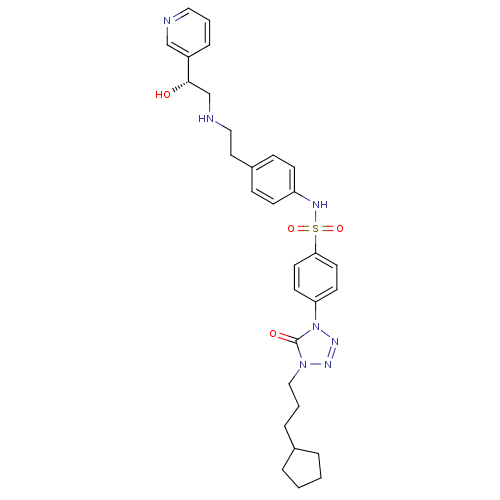 (4-[4-(3-Cyclopentyl-propyl)-5-oxo-4,5-dihydro-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against cloned human Beta-3 adrenergic receptor in the presence of [125I]-iodocyanopindolol expressed in CHO cells by receptor bi... | Bioorg Med Chem Lett 9: 1251-4 (1999) BindingDB Entry DOI: 10.7270/Q2RB73RN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50328307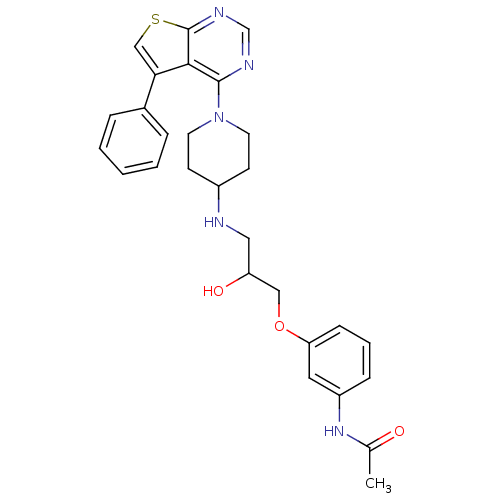 (CHEMBL1258825 | N-(3-(2-hydroxy-3-(1-(5-phenylthie...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as inhibition of isoproterenol-induced cyclic AMP ... | Bioorg Med Chem Lett 20: 6108-15 (2010) Article DOI: 10.1016/j.bmcl.2010.08.039 BindingDB Entry DOI: 10.7270/Q2WM1DNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50328327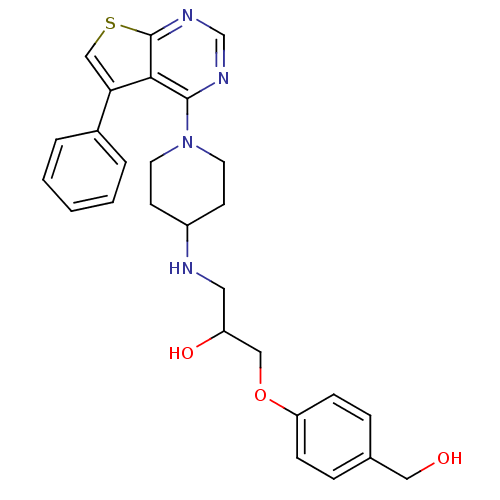 (1-(4-(hydroxymethyl)phenoxy)-3-(1-(5-phenylthieno[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as inhibition of isoproterenol-induced cyclic AMP ... | Bioorg Med Chem Lett 20: 6108-15 (2010) Article DOI: 10.1016/j.bmcl.2010.08.039 BindingDB Entry DOI: 10.7270/Q2WM1DNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50328306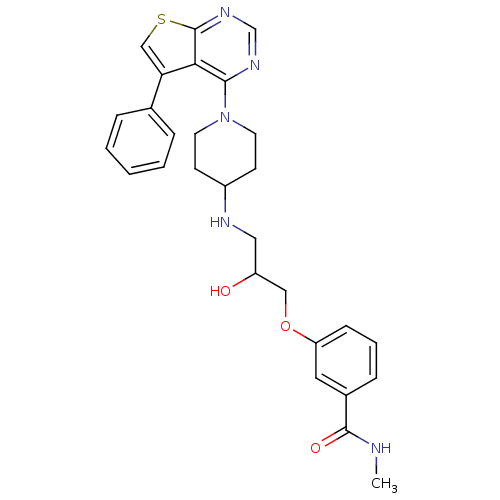 (3-(2-hydroxy-3-(1-(5-phenylthieno[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 402 | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as inhibition of isoproterenol-induced cyclic AMP ... | Bioorg Med Chem Lett 20: 6108-15 (2010) Article DOI: 10.1016/j.bmcl.2010.08.039 BindingDB Entry DOI: 10.7270/Q2WM1DNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50275222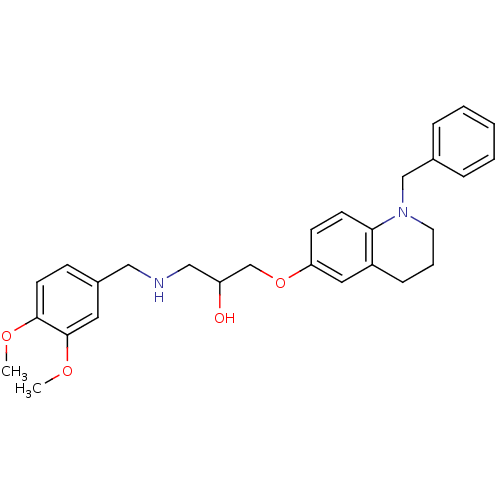 (1-(1-Benzyl-1,2,3,4-tetrahydroquinolin-6-yloxy)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocynopindolol from human adrenergic beta3 receptor | Bioorg Med Chem 17: 830-47 (2009) Article DOI: 10.1016/j.bmc.2008.11.030 BindingDB Entry DOI: 10.7270/Q2RR1Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50274875 (1-(1-Benzyl-1,2,3,4-tetrahydroquinolin-6-yloxy)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocynopindolol from human adrenergic beta3 receptor | Bioorg Med Chem 17: 830-47 (2009) Article DOI: 10.1016/j.bmc.2008.11.030 BindingDB Entry DOI: 10.7270/Q2RR1Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50403975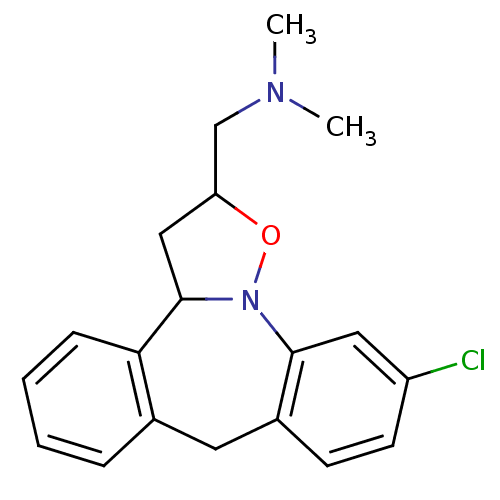 (CHEMBL315772) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 871 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity against Alpha-3A adrenergic receptor | Bioorg Med Chem Lett 12: 3573-7 (2002) BindingDB Entry DOI: 10.7270/Q26M384X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50421590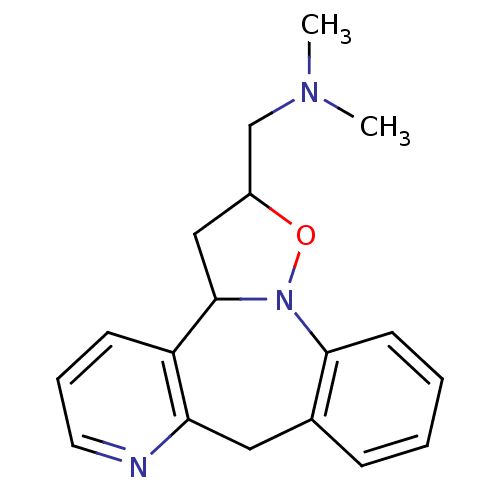 (CHEMBL331188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity against Alpha-3A adrenergic receptor | Bioorg Med Chem Lett 12: 3573-7 (2002) BindingDB Entry DOI: 10.7270/Q26M384X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50421589 (CHEMBL331077) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity against Alpha-3A adrenergic receptor | Bioorg Med Chem Lett 12: 3573-7 (2002) BindingDB Entry DOI: 10.7270/Q26M384X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50275223 (1-(1-Benzyl-1,2,3,4-tetrahydroquinolin-6-yloxy)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocynopindolol from human adrenergic beta3 receptor | Bioorg Med Chem 17: 830-47 (2009) Article DOI: 10.1016/j.bmc.2008.11.030 BindingDB Entry DOI: 10.7270/Q2RR1Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50318991 (2-((1-(2-hydroxy-3-(2-isopropyl-5-methylphenoxy)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG Curated by ChEMBL | Assay Description Antagonist activity at human adrenergic beta3 receptor expressed in CHOK1 cells assessed as inhibition of cAMP accumulation by HTRF assay | Bioorg Med Chem Lett 20: 3399-404 (2010) Article DOI: 10.1016/j.bmcl.2010.04.009 BindingDB Entry DOI: 10.7270/Q2D21XSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50275224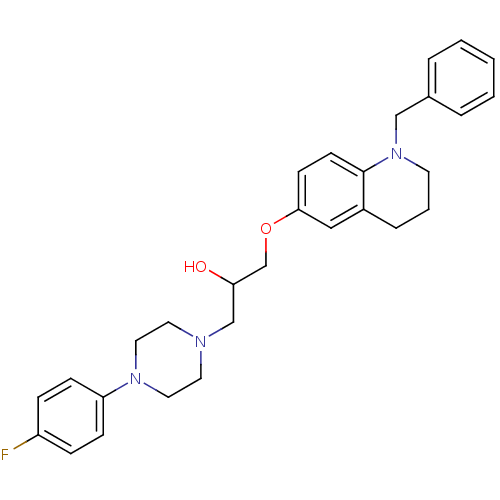 (1-(1-Benzyl-1,2,3,4-tetrahydroquinolin-6-yloxy)-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]iodocynopindolol from human adrenergic beta3 receptor | Bioorg Med Chem 17: 830-47 (2009) Article DOI: 10.1016/j.bmc.2008.11.030 BindingDB Entry DOI: 10.7270/Q2RR1Z3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50146360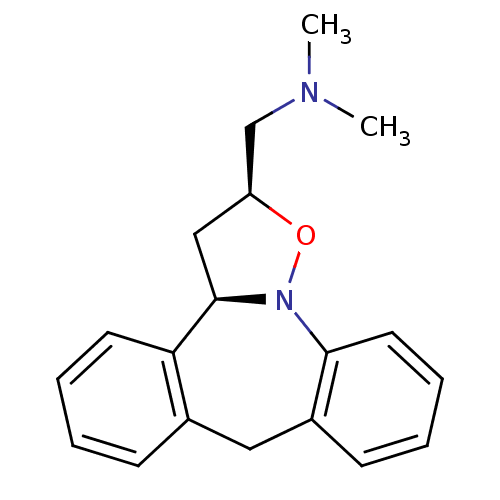 (CHEMBL328573 | Dimethyl-[(2S,3aR)-1-(2,3,3a,8-tetr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity against Alpha-3A adrenergic receptor | Bioorg Med Chem Lett 12: 3573-7 (2002) BindingDB Entry DOI: 10.7270/Q26M384X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50244809 ((2R)-2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of radioligand from adrenergic beta3 receptor | Bioorg Med Chem Lett 18: 4798-801 (2008) Article DOI: 10.1016/j.bmcl.2008.07.103 BindingDB Entry DOI: 10.7270/Q2BC3ZC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50311095 (CHEMBL1079045 | N-(4-(2-amino-2-methylpropyl)pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human adrenergic beta3 receptor | Bioorg Med Chem Lett 19: 6237-40 (2009) Article DOI: 10.1016/j.bmcl.2009.08.076 BindingDB Entry DOI: 10.7270/Q2V124X7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484071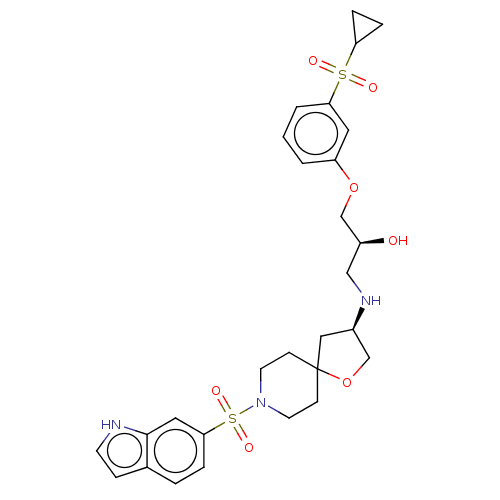 ((S)-1-((R)-8-(1H- pyrrolo[3,2-b]pyridin-6- ylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50328312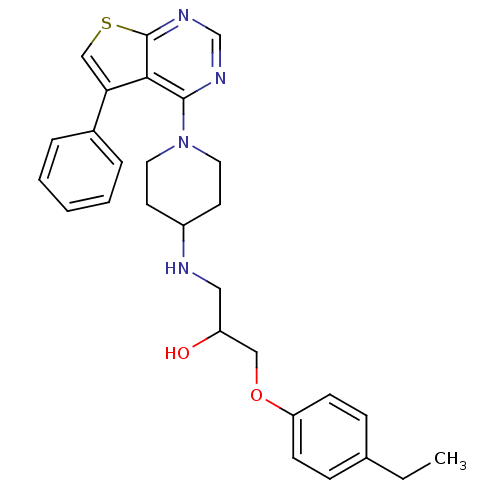 (1-(4-ethylphenoxy)-3-(1-(5-phenylthieno[2,3-d]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
4SC AG Curated by ChEMBL | Assay Description Antagonist activity at human recombinant adrenergic beta3 receptor expressed in CHO cells assessed as inhibition of isoproterenol-induced cyclic AMP ... | Bioorg Med Chem Lett 20: 6108-15 (2010) Article DOI: 10.1016/j.bmcl.2010.08.039 BindingDB Entry DOI: 10.7270/Q2WM1DNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50304075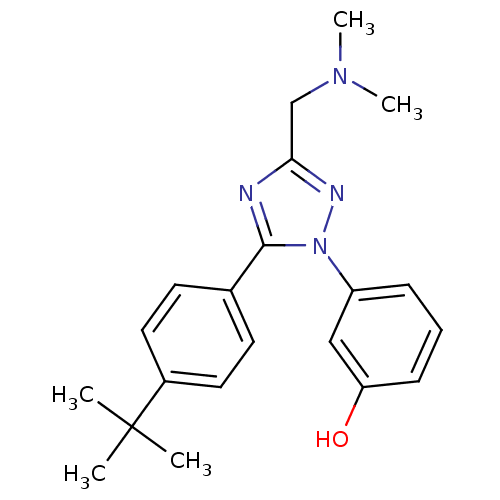 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of beta3 adrenergic receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484061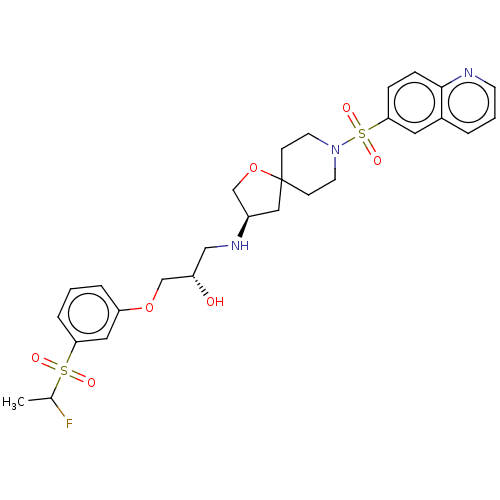 ((2S)-1-(3-(1- fluoroethylsulfonyl) phenoxy)-3-((R)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484073 ((S)-1-((R)-8-(1H- pyrrolo[3,2-b]pyridin-6- ylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484058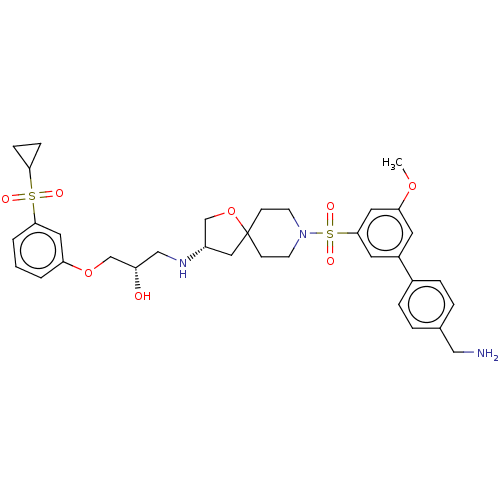 ((S)-1-((S)-8-(4'- (aminomethyl)-5- methoxybiphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484059 ((S)-1-((S)-8-(4'- (aminomethyl)-4- ethoxybiphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484060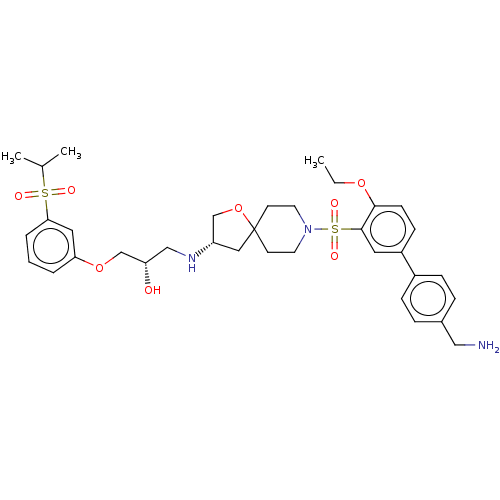 ((S)-1-((S)-8-(4'- (aminomethyl)-4- ethoxybiphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484076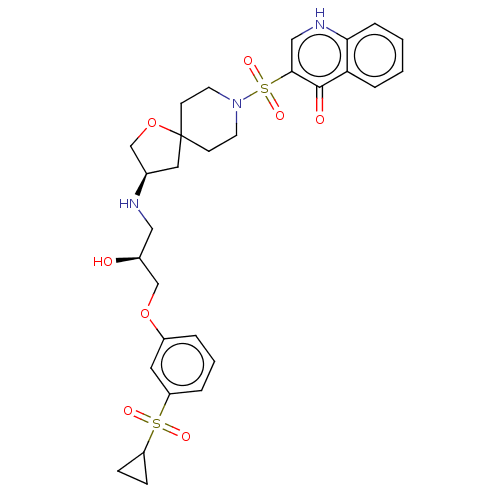 (3-((R)-3-((S)-3-(3- (cyclopropylsulfonyl) phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484050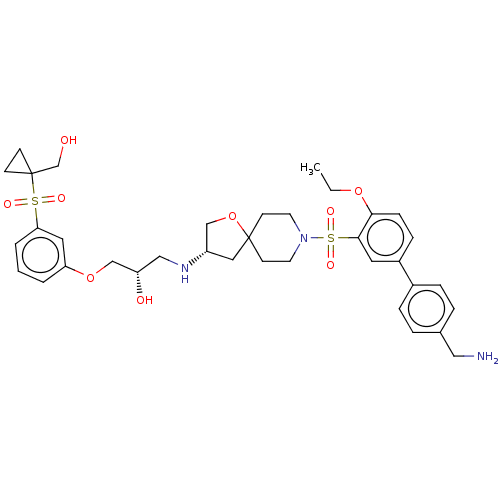 ((S)-1-((S)-8-(4'- (aminomethyl)-4- ethoxybiphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484051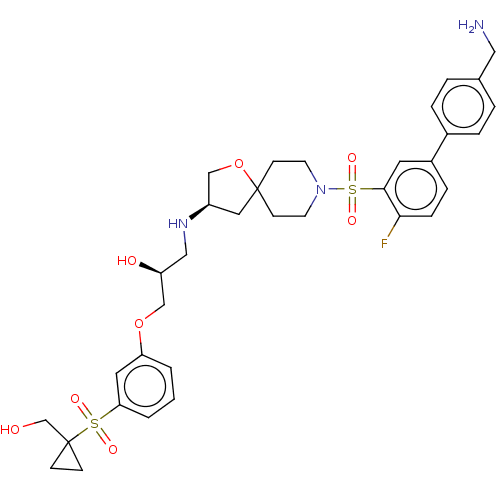 ((S)-1-((R)-8-(4'- (aminomethyl)-4- fluorobiphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484048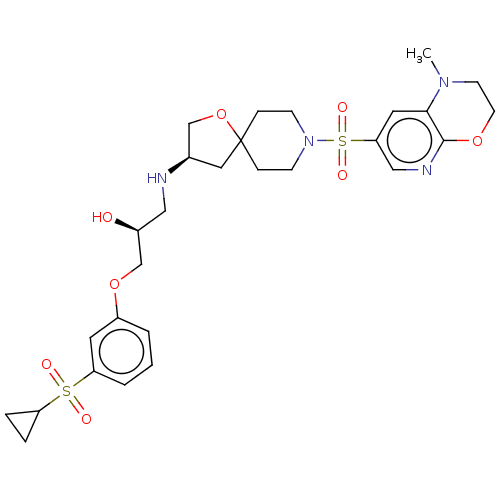 ((S)-1-(3-(cyclopropyl- sulfonyl)phenoxy)- 3-((R)-8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484073 ((S)-1-((R)-8-(1H- pyrrolo[3,2-b]pyridin-6- ylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484057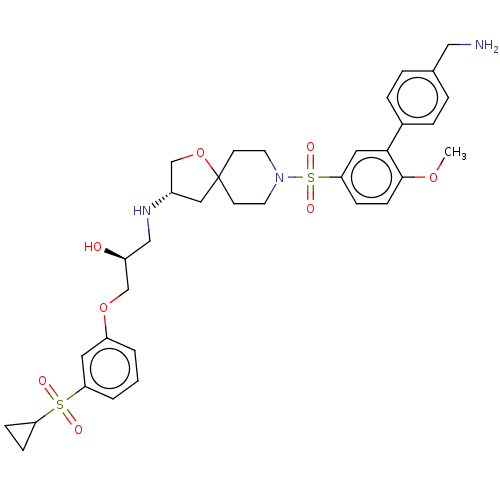 ((S)-1-((S)-8-(4'- (aminomethyl)-6- methoxybiphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484066 ((S)-1-((S)-8-(4'- ((butylamino)methyl) biphenyl-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484047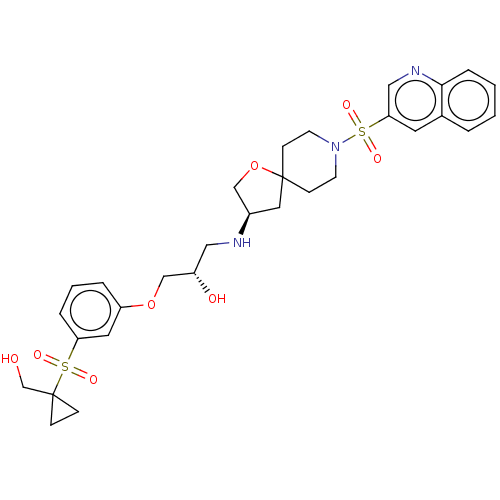 ((S)-1-(3-(1- (hydroxymethyl) cyclopropylsulfonyl) ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484070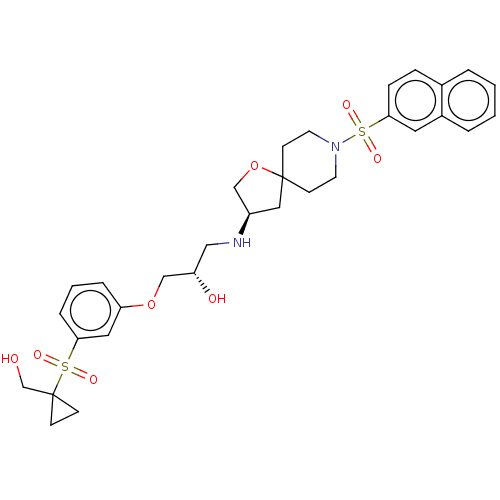 ((S)-1-(3-(1- (hydroxymethyl) cyclopropylsulfonyl) ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484063 ((S)-1-(3-(1- (hydroxymelhyl) cyclopropylsulfonyl) ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484049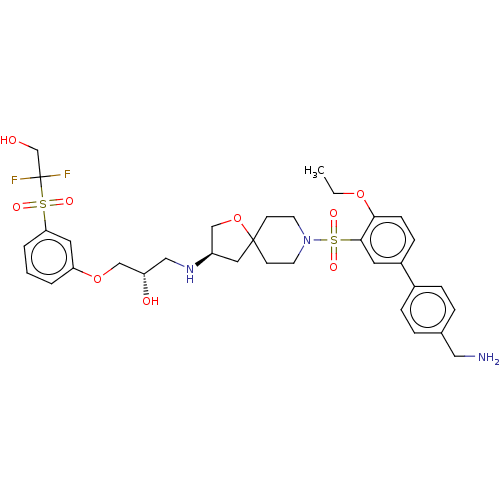 ((S)-1-((R)-8-(4'- (aminomethyl)-4- ethoxybiphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484075 (1-ethyl-8-fluoro-3-((R)- 3-((S)-2-hydroxy-3-(3- (m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484053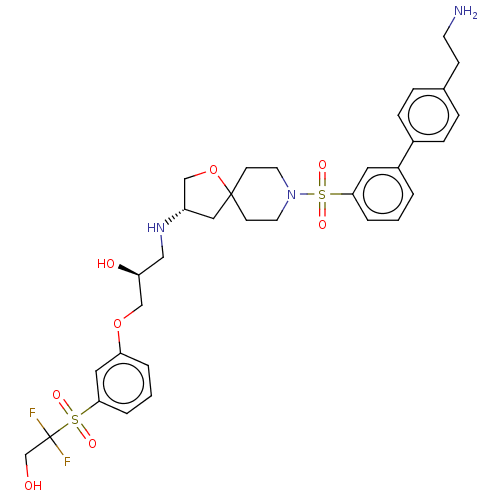 ((S)-1-((S)-8-(4'-(2- aminoethyl)biphenyl-3- ylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484052 ((S)-1-((R)-8-(4'-(1- aminocyclopropyl)-6- methoxyb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484045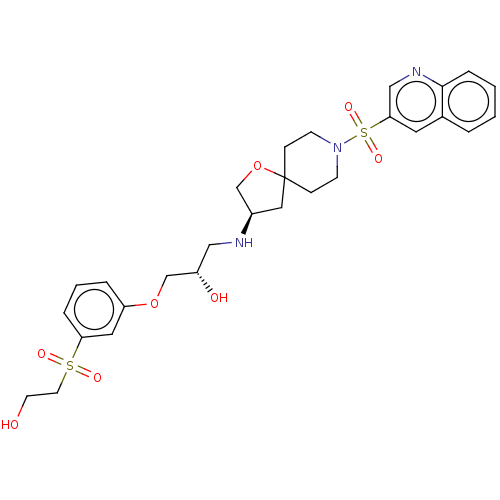 ((S)-1-(3-(2- hydroxyethylsulfonyl) phenoxy)-3-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484054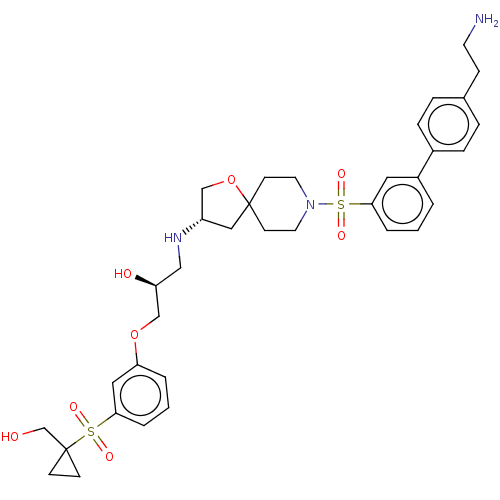 ((S)-1-((S)-8-(4'-(2- aminoethyl)biphenyl-3- ylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484069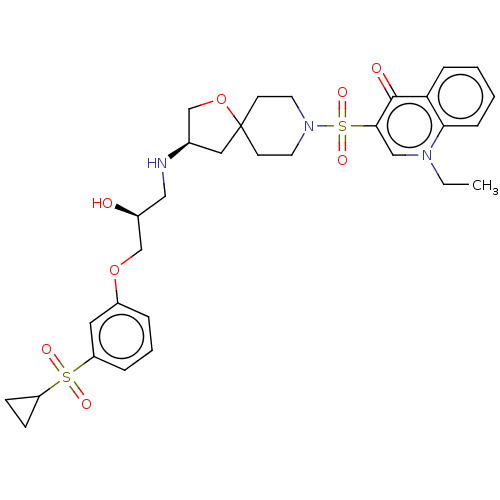 (3-((R)-3-((S)-3-(3- (cyclopropylsulfonyl) phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484065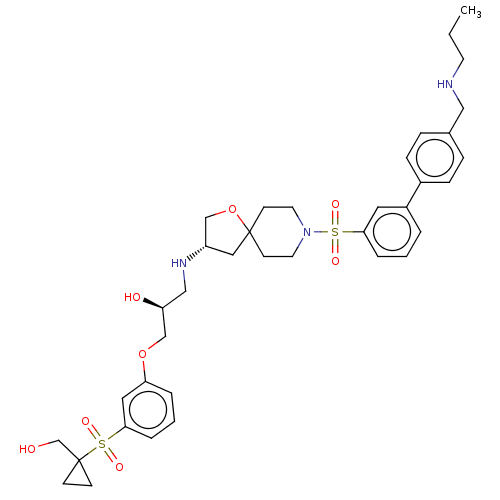 ((S)-l-(3-(l- (hydroxymethyl) cyclopropylsulfonyl) ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484055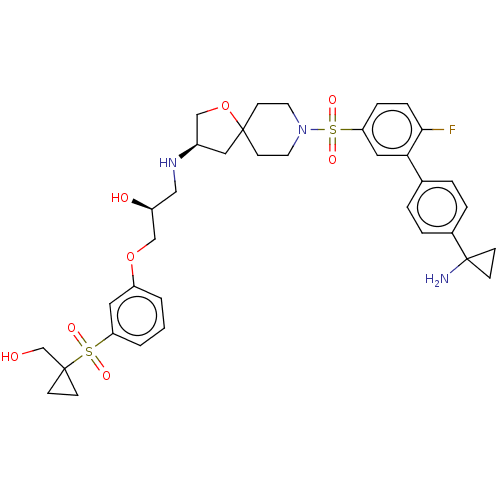 ((S)-1-((R)-8-(4'-(1- aminocyclopropyl)-6- fluorobi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484077 (3-((R)-3-((S)-3-(3- (cyclopropylsulfonyl) phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484078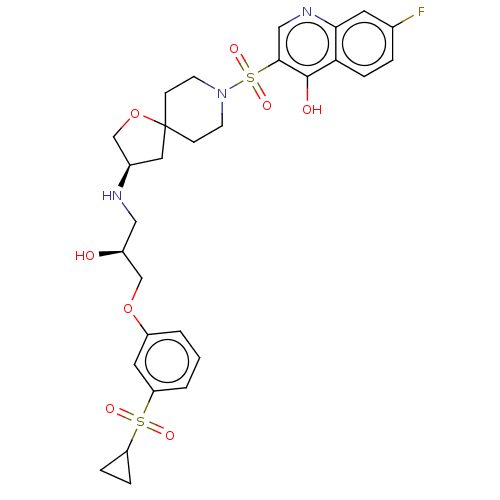 (3-((R)-3-((S)-3-(3- (cyclopropylsulfonyl) phenoxy)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50318666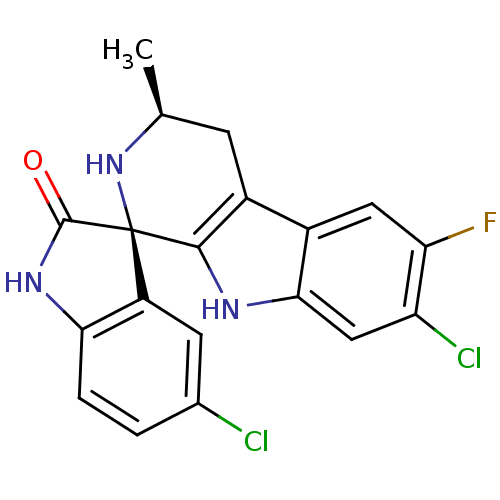 ((1R,3S)-5',7-Dichloro-6-fluoro-3-methyl-2,3,4,9-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Swiss Tropical and Public Health Institute Curated by ChEMBL | Assay Description Binding affinity to human recombinant adrenergic beta3 receptor | Science 329: 1175-80 (2010) Article DOI: 10.1126/science.1193225 BindingDB Entry DOI: 10.7270/Q2SB45ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484079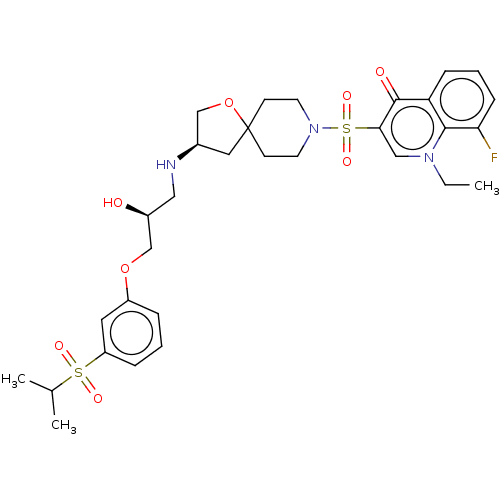 (1-ethyl-8-fluoro-3-((R)- 3-((S)-2-hydroxy-3-(3- (i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM484062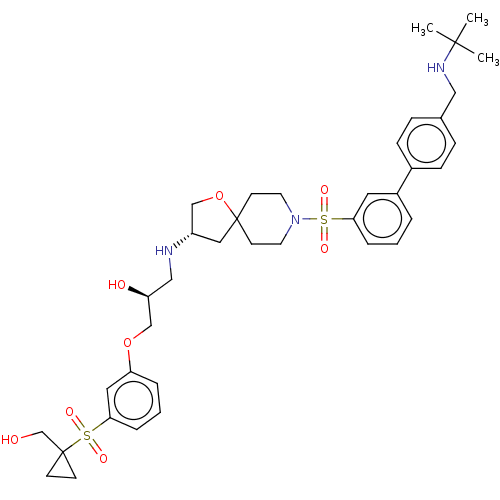 ((S)-1-((S)-8-(4'-((tert- butylamino(methyl) biphen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals, Inc. US Patent | Assay Description Compounds were dissolved and serially diluted (5-fold) in DMSO to generate a 10-point dose response stock. The stock was then diluted 100-fold in ass... | US Patent US10927123 (2021) BindingDB Entry DOI: 10.7270/Q2D221QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 57 total ) | Next | Last >> |