

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM26269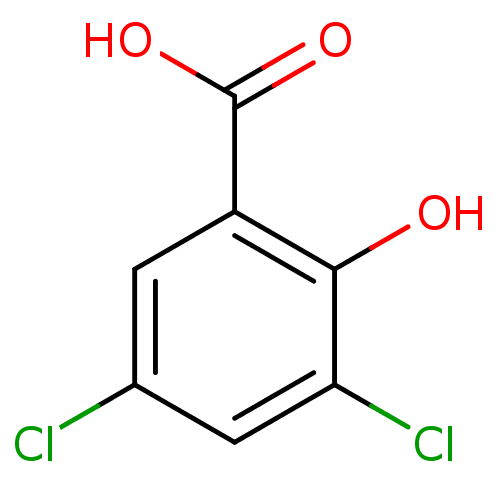 (3,5-dichloro-2-hydroxybenzoic acid | 3,5-dichloros...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of 20-alpha HSD (unknown origin) | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427620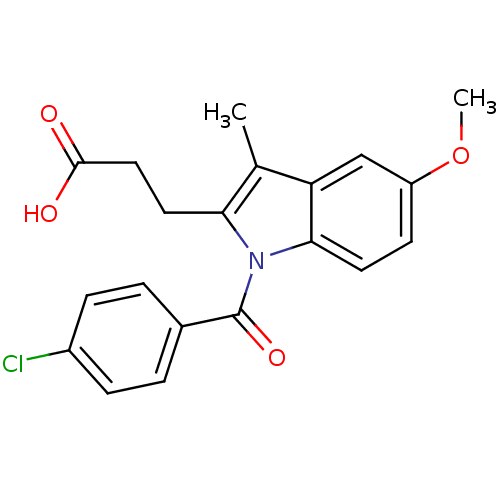 (CHEMBL2323507 | US9346803, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427622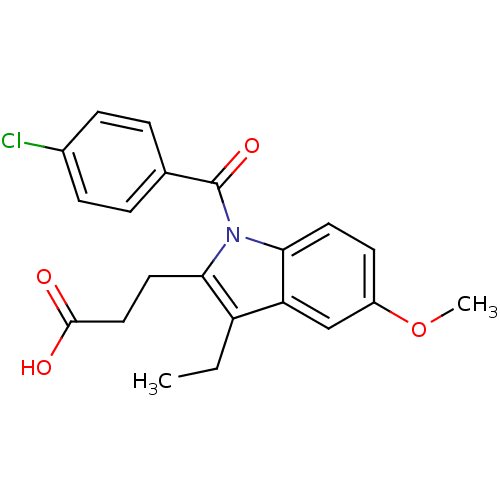 (CHEMBL2323508 | US9346803, Table 2, Compound 10: 3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50249793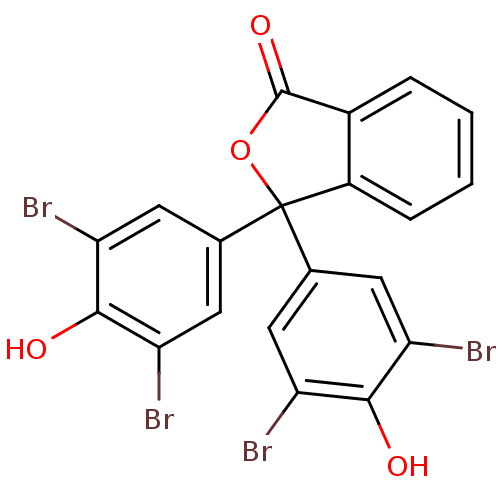 (3',3'',5',5''-tetrabromophenolphthalein | CHEMBL52...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of 20-alpha HSD (unknown origin) | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50158460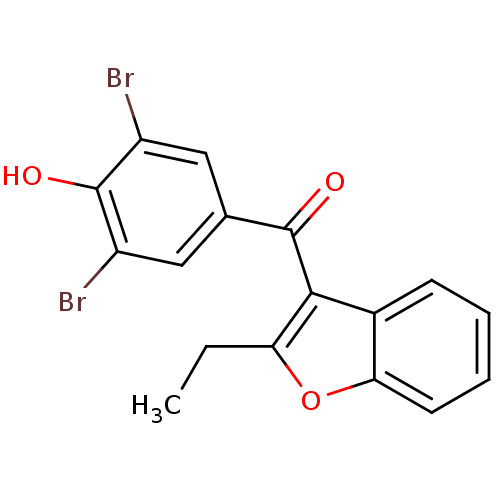 ((3,5-dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of 20-alpha HSD (unknown origin) | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427624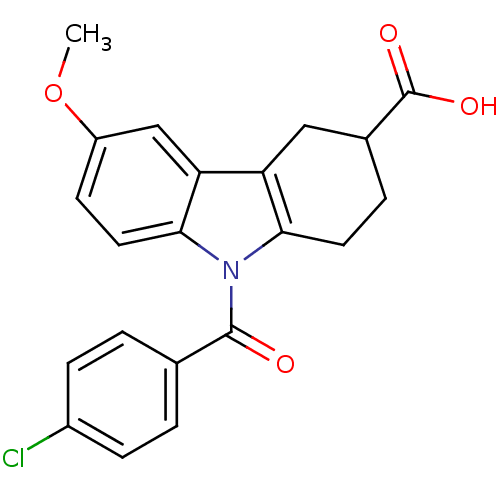 (CHEMBL2323522 | US9346803, Table 2, Compound 11: 9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 76.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427628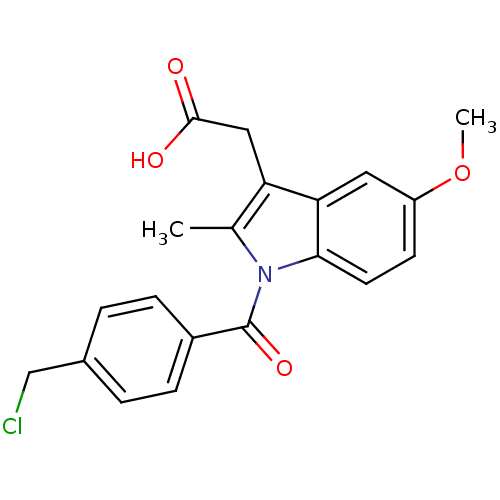 (CHEMBL2323472 | US9346803, Table 2, Compound 8: 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427627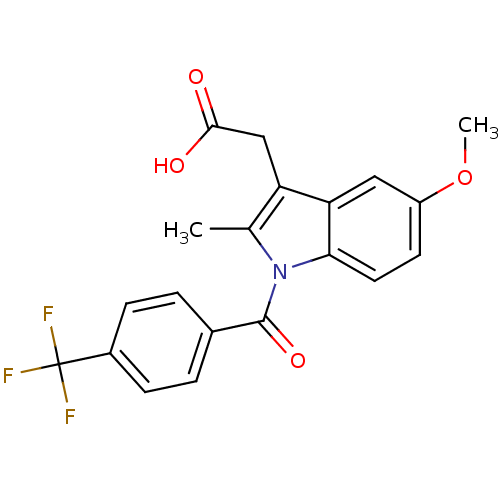 (CHEMBL2323474 | US9346803, Table 2, Compound 9: 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427629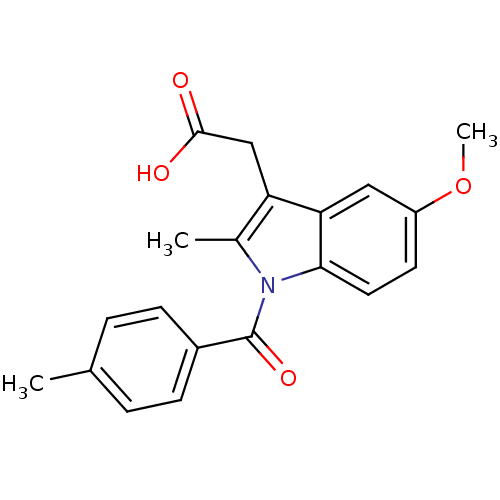 (CHEMBL179587 | US9346803, Table 2, Compound 7: 2-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427625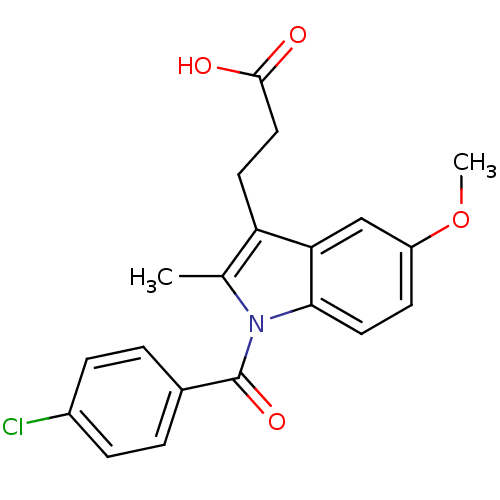 (CHEMBL178687 | US9346803, Table 2, Compound 6: 3-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427626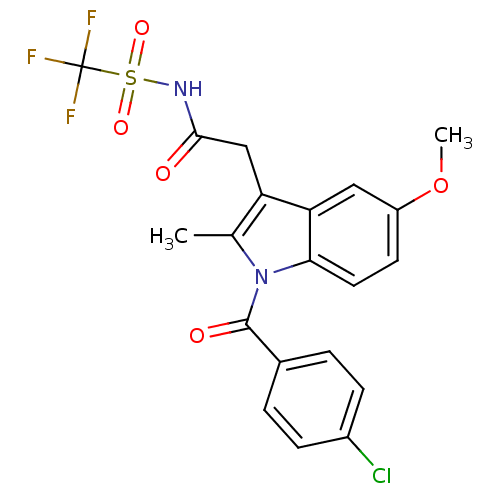 (CHEMBL2323481 | US9346803, Table 2, Compound 5: 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50293598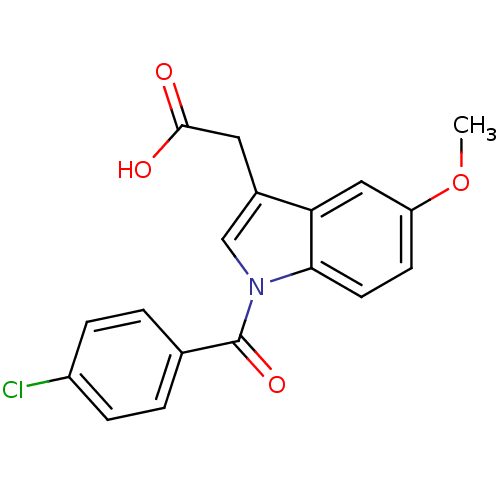 (2'-des-methyl indomethacin | CHEMBL503179 | US9346...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427619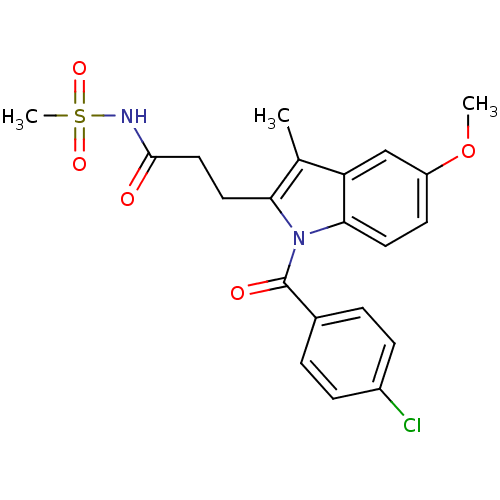 (CHEMBL2323511 | US9346803, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50427621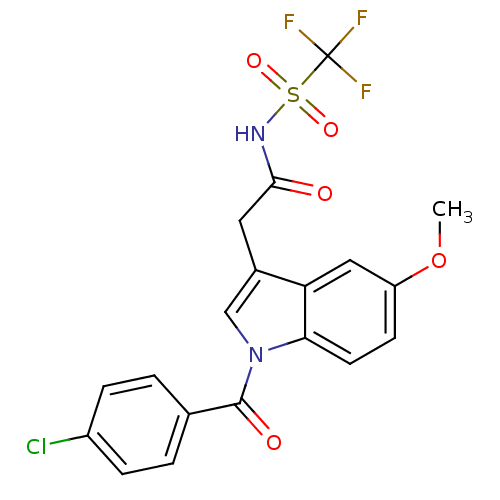 (CHEMBL2323490 | US9346803, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University; The Trustees of the University of Pennsylvania US Patent | Assay Description Inhibitors were initially screened for an ability to block the NADP+ dependent oxidation of the artificial substrate S-tetralol catalyzed by AKR1C3. ... | US Patent US9346803 (2016) BindingDB Entry DOI: 10.7270/Q2154FXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50330427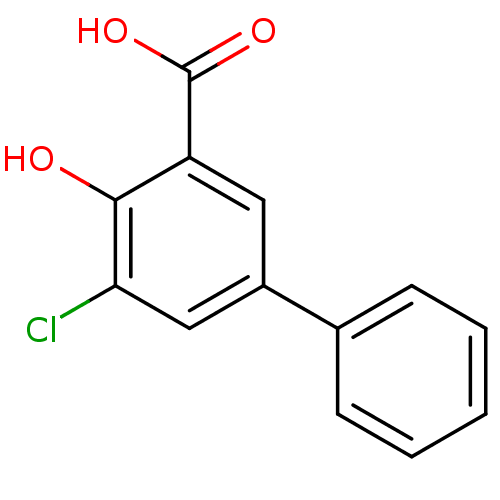 (5-Chloro-4-hydroxybiphenyl-3-carboxylic acid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C1-mediated progesterone metabolism expressed in bovine aortic endothelial cells assessed as formation of 20alpha-hydroxyprog... | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50330426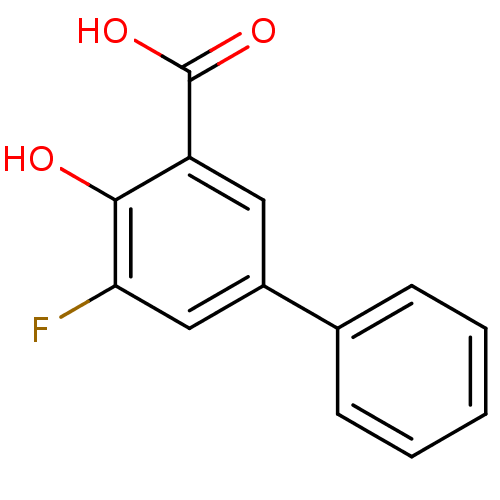 (5-Fluoro-4-hydroxybiphenyl-3-carboxylic acid | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C1-mediated progesterone metabolism expressed in bovine aortic endothelial cells assessed as formation of 20alpha-hydroxyprog... | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466729 (CHEMBL4293881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466730 (CHEMBL4291554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 433 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466716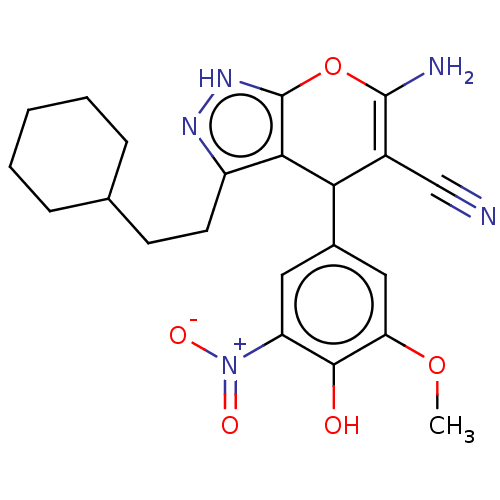 (CHEMBL4287291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 443 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50219490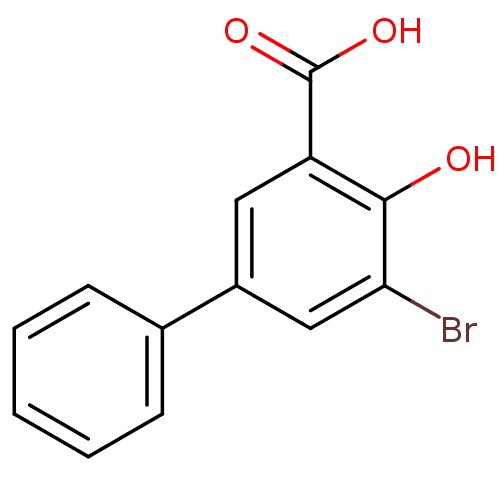 (3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human recombinant 20-alpha HSD expressed in BAEC assessed as inhibition of progesterone metabolism treated 2 hrs before progesterone ch... | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50219490 (3-Bromo-5-phenylsalicylc acid | 5-bromo-4-hydroxyb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of human AKR1C1-mediated progesterone metabolism expressed in bovine aortic endothelial cells assessed as formation of 20alpha-hydroxyprog... | Eur J Med Chem 45: 5309-17 (2010) Article DOI: 10.1016/j.ejmech.2010.08.052 BindingDB Entry DOI: 10.7270/Q2R78FF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466720 (CHEMBL4294478) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 462 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50595596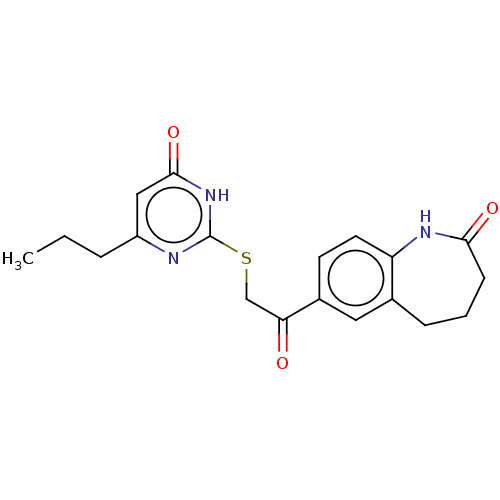 (CHEMBL5203670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00175 BindingDB Entry DOI: 10.7270/Q2DB85WJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466717 (CHEMBL4294539) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 487 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466710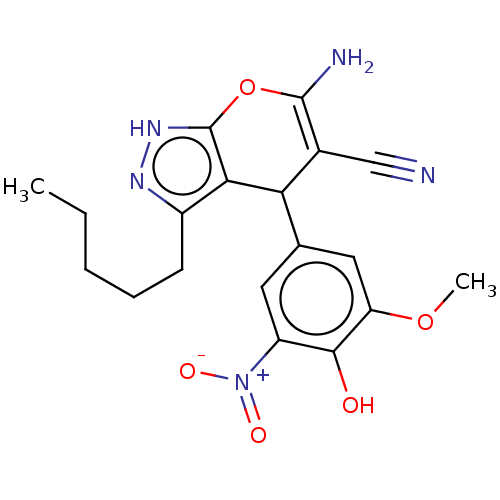 (CHEMBL4290064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 531 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466714 (CHEMBL4282842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 534 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466724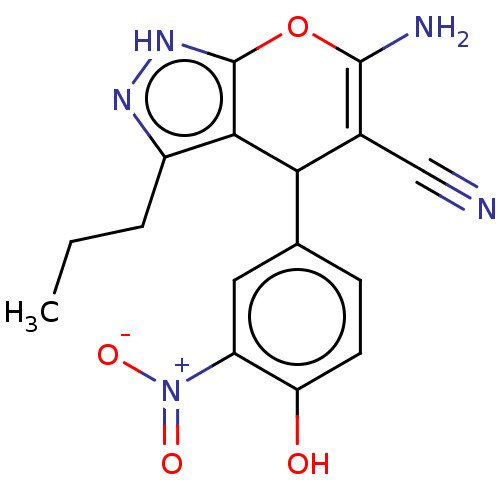 (CHEMBL4287863) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 661 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50396696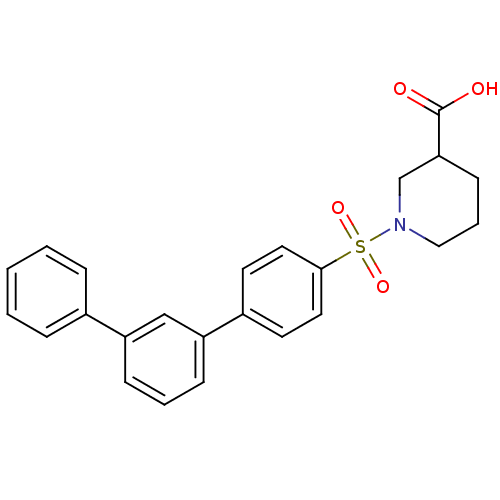 (CHEMBL2172064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C1 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50396716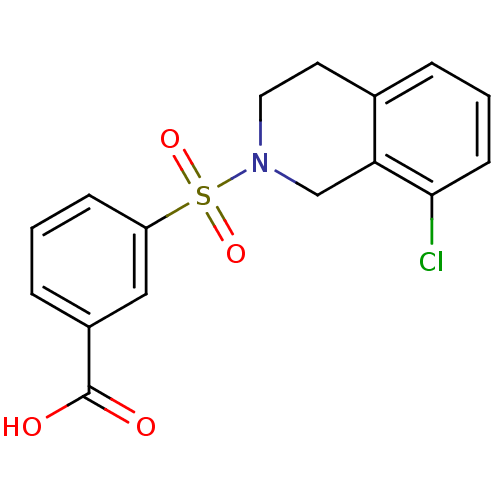 (CHEMBL2172091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C1 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM22971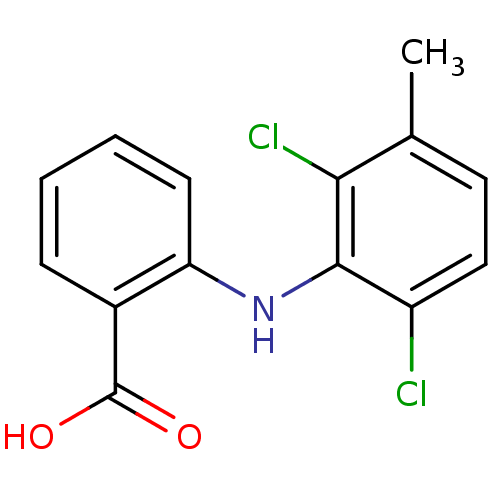 (2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466732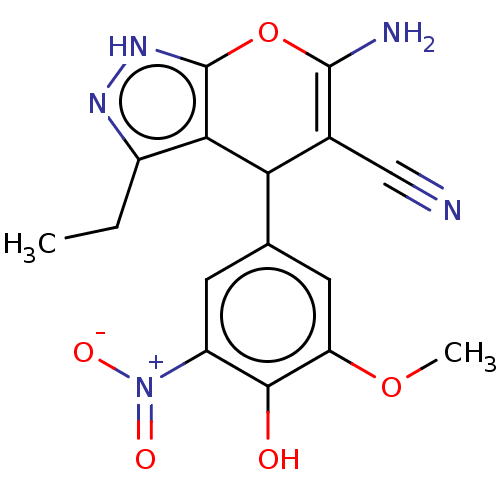 (CHEMBL4286627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466713 (CHEMBL4295234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 801 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466739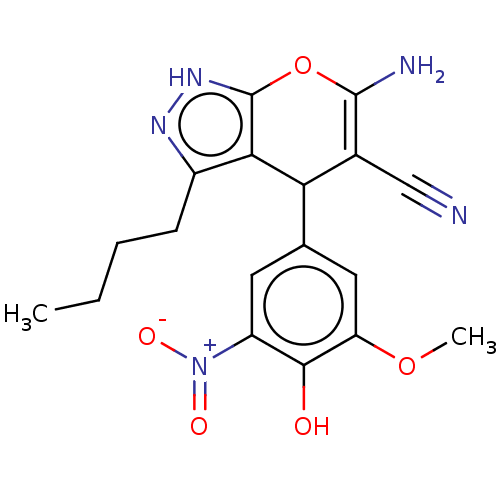 (CHEMBL4279383) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 810 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50396669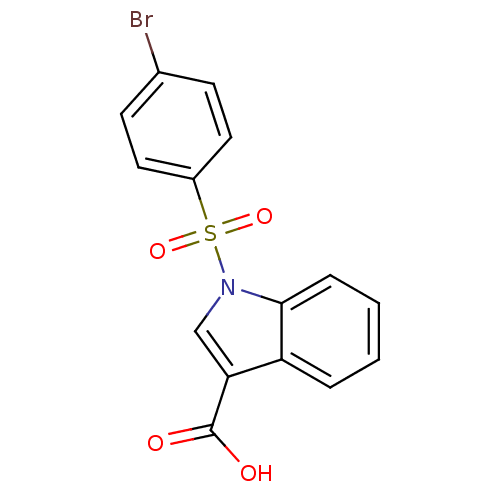 (CHEMBL2172108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C1 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466733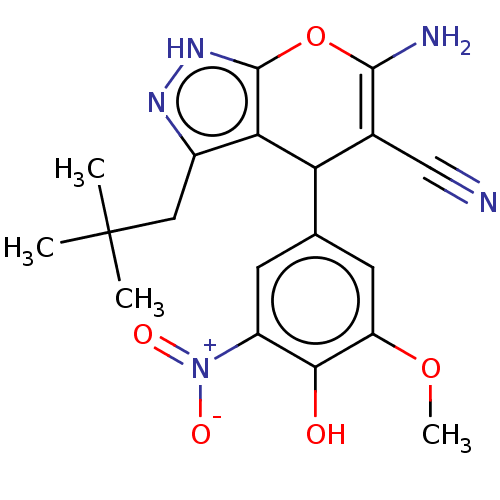 (CHEMBL4280914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 844 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466735 (CHEMBL4286233) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50241817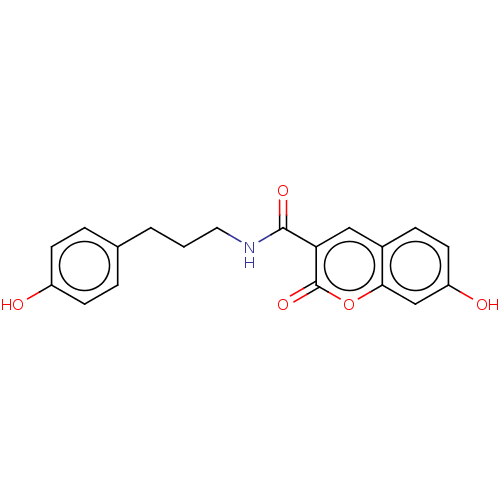 (CHEMBL4081954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 using S-tetralol as substrate | J Med Chem 60: 8441-8455 (2017) Article DOI: 10.1021/acs.jmedchem.7b00830 BindingDB Entry DOI: 10.7270/Q2NZ89S7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM17636 (2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB US Patent | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
The Trustees of the University of Pennsylvania US Patent | Assay Description Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ... | US Patent US9271961 (2016) BindingDB Entry DOI: 10.7270/Q27W6B22 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466711 (CHEMBL4290009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466719 (CHEMBL4279326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466715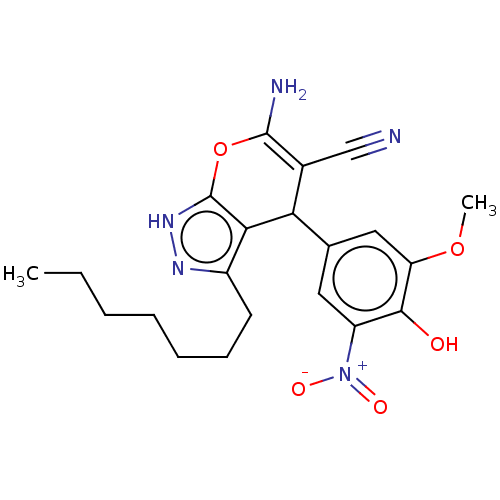 (CHEMBL4293449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466734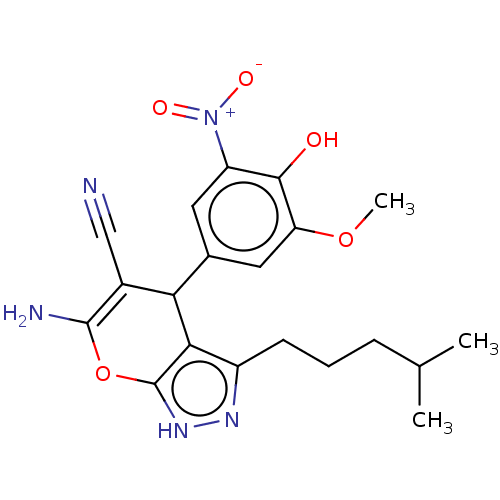 (CHEMBL4278318) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466708 (CHEMBL4277845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50067678 ((6alpha)-17-(Acetyloxy)-6-methylpreg-4-ene-3,20-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli assessed as decrease in oxidation of 1-acenaphthenol substrate by spectrophotome... | Eur J Med Chem 62: 89-97 (2013) Article DOI: 10.1016/j.ejmech.2012.12.045 BindingDB Entry DOI: 10.7270/Q2BR8TH0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50249792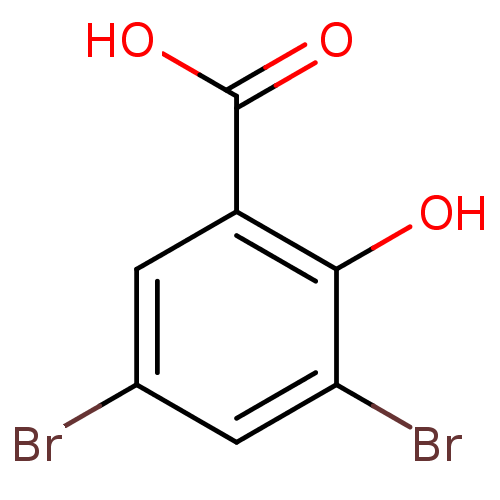 (3,5-Dibromosalicylic acid | CHEMBL447448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Monash University (Parkville Campus) Curated by ChEMBL | Assay Description Inhibition of 20-alpha HSD | J Med Chem 52: 3259-64 (2009) Article DOI: 10.1021/jm9001633 BindingDB Entry DOI: 10.7270/Q2765F6T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466731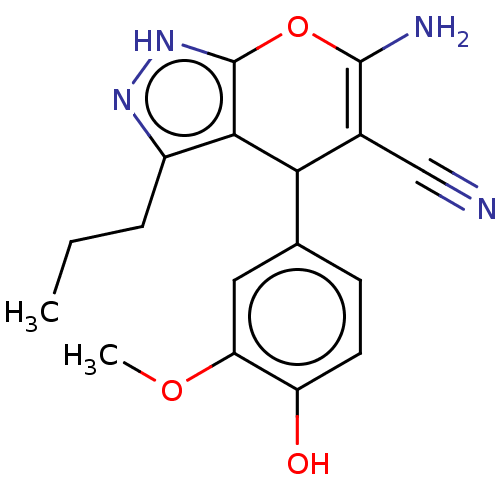 (CHEMBL1510262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50466718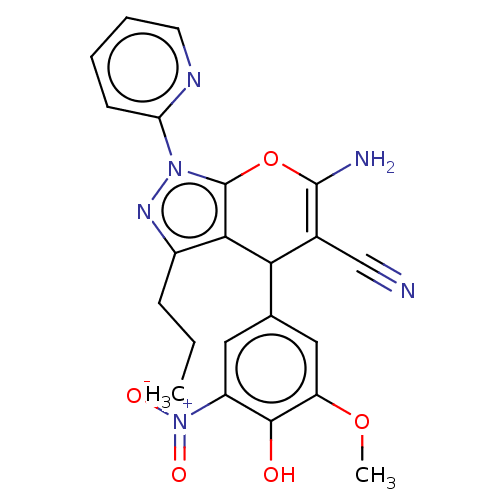 (CHEMBL4288500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou Medical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AKR1C1 expressed in Escherichia coli BL21 cells in presence of 9,10-phenanthrenequinone and NADPH by fluorescence ass... | Bioorg Med Chem 26: 5934-5943 (2018) Article DOI: 10.1016/j.bmc.2018.10.044 BindingDB Entry DOI: 10.7270/Q2NV9MZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM17636 (2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C1 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM17636 (2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C1 (unknown origin) | Eur J Med Chem 62: 738-44 (2013) Article DOI: 10.1016/j.ejmech.2013.01.047 BindingDB Entry DOI: 10.7270/Q2H133CX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 252 total ) | Next | Last >> |