 Found 19 hits of ic50 data for polymerid = 3674
Found 19 hits of ic50 data for polymerid = 3674 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MSK2 using GRPRTSSFAEG as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human MSK2 using GRPRTSSFAEG as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50601138
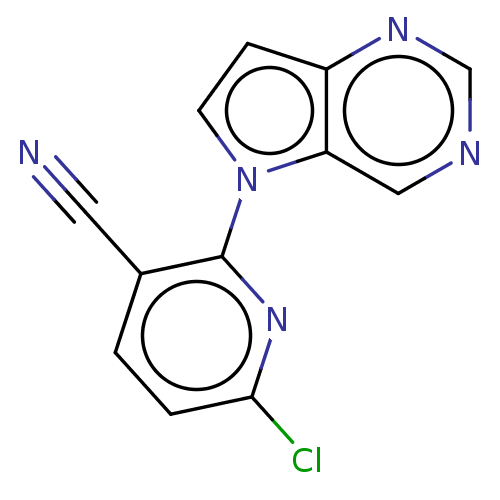
(CHEMBL5184056) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50601139
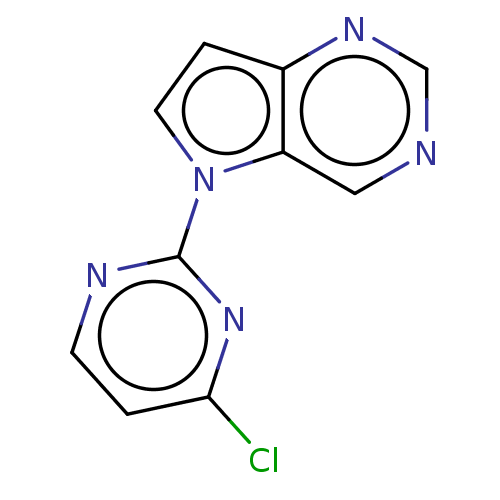
(CHEMBL5190298) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50601160

(CHEMBL5187801) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50462709
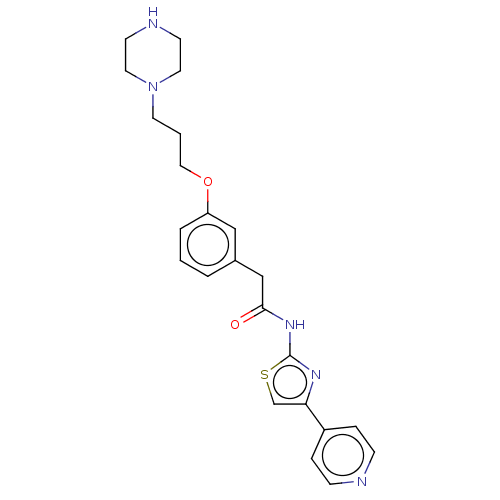
(CHEMBL4245507)Show SMILES O=C(Cc1cccc(OCCCN2CCNCC2)c1)Nc1nc(cs1)-c1ccncc1 Show InChI InChI=1S/C23H27N5O2S/c29-22(27-23-26-21(17-31-23)19-5-7-24-8-6-19)16-18-3-1-4-20(15-18)30-14-2-11-28-12-9-25-10-13-28/h1,3-8,15,17,25H,2,9-14,16H2,(H,26,27,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of MSK2 (unknown origin) |
Bioorg Med Chem Lett 28: 2616-2621 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.043
BindingDB Entry DOI: 10.7270/Q2XP77MH |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM27344
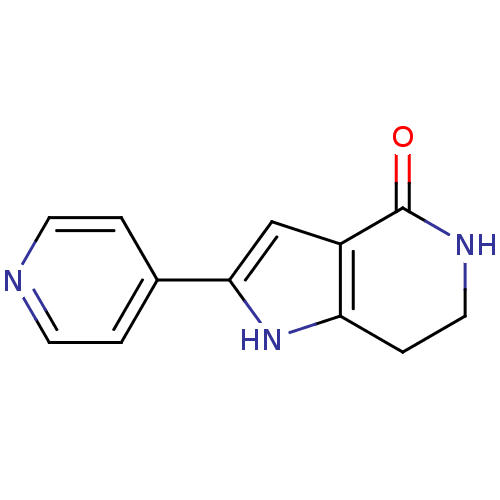
(2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...)Show InChI InChI=1S/C12H11N3O/c16-12-9-7-11(8-1-4-13-5-2-8)15-10(9)3-6-14-12/h1-2,4-5,7,15H,3,6H2,(H,14,16) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50601147
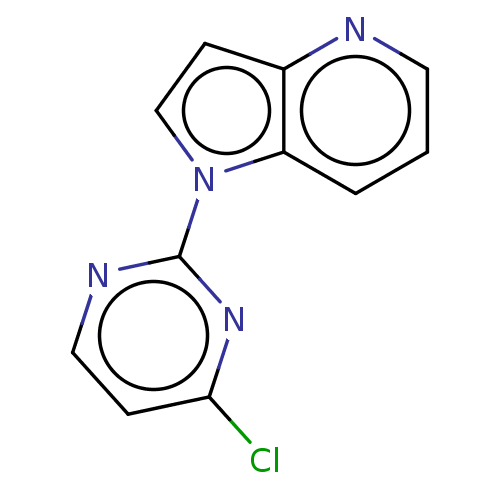
(CHEMBL5183977) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50601137
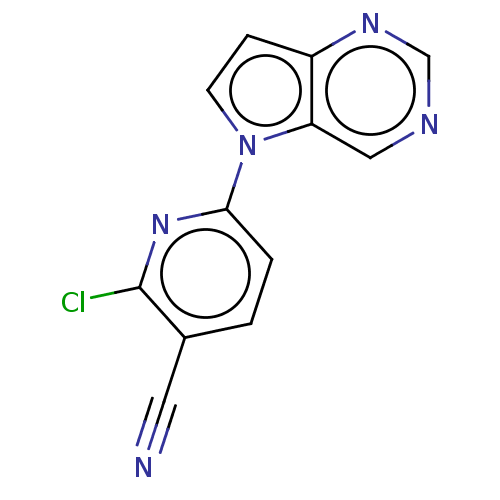
(CHEMBL5198790) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00134
BindingDB Entry DOI: 10.7270/Q2J38XNB |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50436476

(CHEMBL2397316)Show SMILES Cc1nc(NC(=O)C(C)(C)CC(F)(F)F)sc1-c1ccc(N)nc1 Show InChI InChI=1S/C15H17F3N4OS/c1-8-11(9-4-5-10(19)20-6-9)24-13(21-8)22-12(23)14(2,3)7-15(16,17)18/h4-6H,7H2,1-3H3,(H2,19,20)(H,21,22,23) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of MSK2 (unknown origin) |
Bioorg Med Chem Lett 23: 3841-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.077
BindingDB Entry DOI: 10.7270/Q2BG2QCX |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50519662
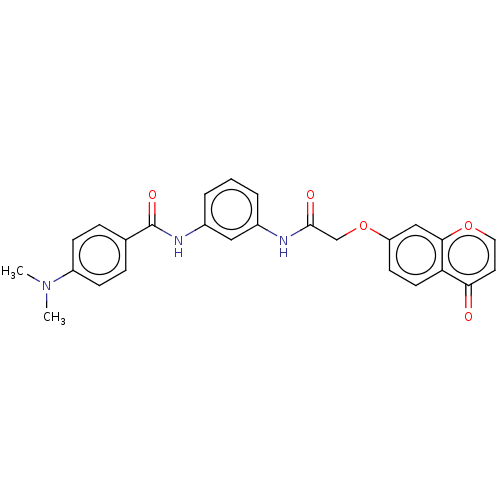
(CHEMBL4438748)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1cccc(NC(=O)COc2ccc3c(c2)occc3=O)c1 Show InChI InChI=1S/C26H23N3O5/c1-29(2)20-8-6-17(7-9-20)26(32)28-19-5-3-4-18(14-19)27-25(31)16-34-21-10-11-22-23(30)12-13-33-24(22)15-21/h3-15H,16H2,1-2H3,(H,27,31)(H,28,32) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human MSK2 (2 to end residues) using GRPRTSSFAEGKK as substrate incubated for 40 mins in presence of [gamma-33ATP by radiom... |
J Med Chem 62: 10691-10710 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01143
BindingDB Entry DOI: 10.7270/Q2MC93FG |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50401152
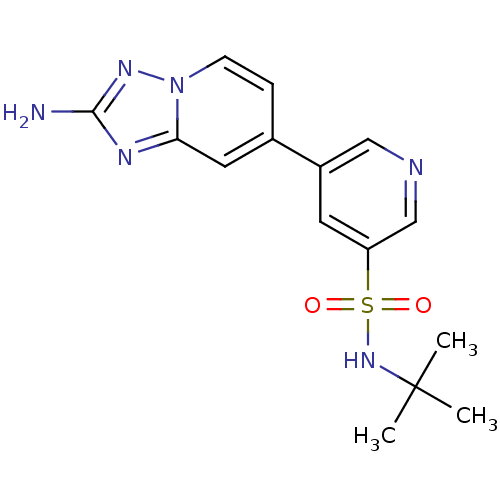
(CHEMBL2205766)Show SMILES CC(C)(C)NS(=O)(=O)c1cncc(c1)-c1ccn2nc(N)nc2c1 Show InChI InChI=1S/C15H18N6O2S/c1-15(2,3)20-24(22,23)12-6-11(8-17-9-12)10-4-5-21-13(7-10)18-14(16)19-21/h4-9,20H,1-3H3,(H2,16,19) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cellzome Ltd
Curated by ChEMBL
| Assay Description
Inhibition of RPS6KA4 |
Bioorg Med Chem Lett 22: 4613-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.090
BindingDB Entry DOI: 10.7270/Q2HQ412B |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50135286

(CHEMBL3745885)Show SMILES Cn1c2nc(Nc3ccc4[nH]ccc4c3)ncc2cc(c1=O)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C22H15F2N5O3S/c1-29-20-13(9-19(21(29)30)33(31,32)18-5-2-14(23)10-16(18)24)11-26-22(28-20)27-15-3-4-17-12(8-15)6-7-25-17/h2-11,25H,1H3,(H,26,27,28) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of human MSK2 using [GRPRTSSFAEG] as substrate |
Bioorg Med Chem 24: 521-44 (2016)
Article DOI: 10.1016/j.bmc.2015.11.045
BindingDB Entry DOI: 10.7270/Q24Q7WT8 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of MSK2 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50359359

(CHEMBL1929238)Show SMILES CN(C)CCN1CCN(CCC1=O)C(=O)c1cc(sc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C25H33Cl2N5O3S/c1-25(2,3)19-15-16(22(36-19)29-24(35)28-18-8-6-7-17(26)21(18)27)23(34)32-10-9-20(33)31(13-14-32)12-11-30(4)5/h6-8,15H,9-14H2,1-5H3,(H2,28,29,35) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of MSK2 |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM30192
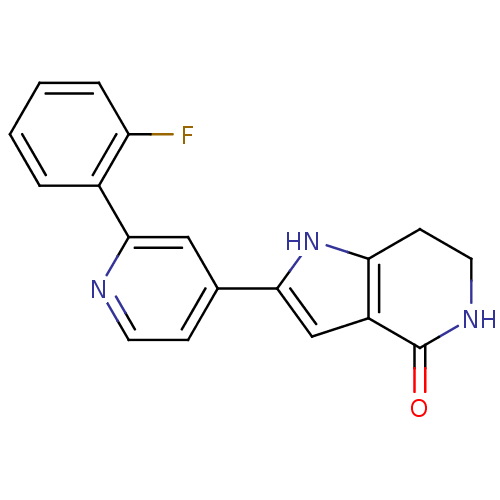
(Pyrrolopyridine, 23)Show InChI InChI=1S/C18H14FN3O/c19-14-4-2-1-3-12(14)17-9-11(5-7-20-17)16-10-13-15(22-16)6-8-21-18(13)23/h1-5,7,9-10,22H,6,8H2,(H,21,23) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM50537742
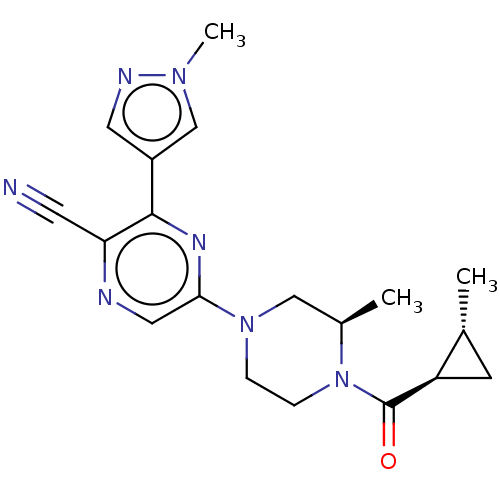
(CHEMBL4634634 | US11179389, Compound 1-14)Show SMILES C[C@@H]1C[C@H]1C(=O)N1CCN(C[C@H]1C)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H23N7O/c1-12-6-15(12)19(27)26-5-4-25(10-13(26)2)17-9-21-16(7-20)18(23-17)14-8-22-24(3)11-14/h8-9,11-13,15H,4-6,10H2,1-3H3/t12-,13-,15-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human GST-tagged RPS6KA4 expressed in baculovirus expression system using ser/Thr01 peptide as substrate incuba... |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126715
BindingDB Entry DOI: 10.7270/Q2HM5CZG |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM30178
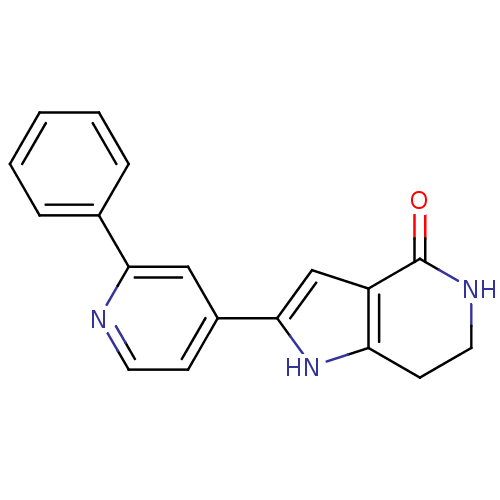
(Pyrrolopyridine, 9)Show InChI InChI=1S/C18H15N3O/c22-18-14-11-17(21-15(14)7-9-20-18)13-6-8-19-16(10-13)12-4-2-1-3-5-12/h1-6,8,10-11,21H,7,9H2,(H,20,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-4
(Homo sapiens (Human)) | BDBM30185
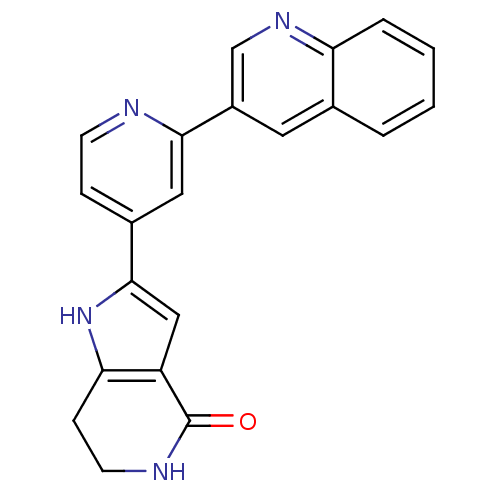
(CHEMBL226403 | Pyrrolopyridine, 16)Show SMILES O=C1NCCc2[nH]c(cc12)-c1ccnc(c1)-c1cnc2ccccc2c1 Show InChI InChI=1S/C21H16N4O/c26-21-16-11-20(25-18(16)6-8-23-21)14-5-7-22-19(10-14)15-9-13-3-1-2-4-17(13)24-12-15/h1-5,7,9-12,25H,6,8H2,(H,23,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer
| Assay Description
Compounds were evaluated as inhibitors of kinase by measuring their effect on kinase induced phosphorylation of substrate. IC50 values were reported ... |
J Med Chem 50: 2647-54 (2007)
Article DOI: 10.1021/jm0611004
BindingDB Entry DOI: 10.7270/Q2794313 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data