 Found 1385 hits of ic50 data for polymerid = 3676,3678
Found 1385 hits of ic50 data for polymerid = 3676,3678 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348545

(CHEMBL1801384)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C27H26N4O2/c32-27-25-22-8-6-20-16-29-21(15-23(20)26(22)30-24(25)9-10-28-27)7-5-18-1-3-19(4-2-18)17-31-11-13-33-14-12-31/h1-8,15-16,30H,9-14,17H2,(H,28,32)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50297122
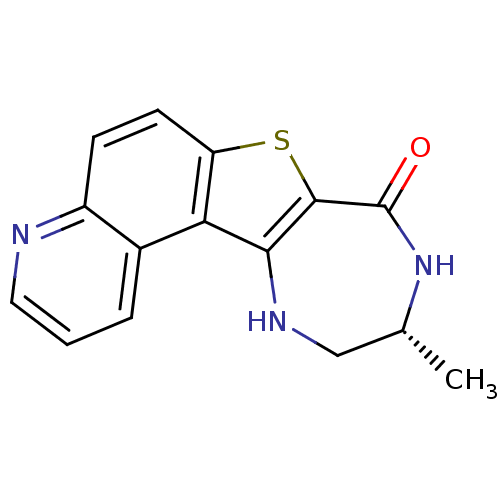
((R)-10-Methyl-9,10,11,12-tetrahydro-7-thia-4,9,12-...)Show InChI InChI=1S/C15H13N3OS/c1-8-7-17-13-12-9-3-2-6-16-10(9)4-5-11(12)20-14(13)15(19)18-8/h2-6,8,17H,7H2,1H3,(H,18,19)/t8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 19: 4882-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.017
BindingDB Entry DOI: 10.7270/Q2J38SM7 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434486

(CHEMBL2385548)Show SMILES O=C1N(Cc2ccccc2-c2cncnc2)c2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C34H28N6O2/c35-18-23-5-7-24(8-6-23)32-17-27-15-26-16-29(39-13-11-36-12-14-39)9-10-31(26)40(34(41)33(27)42-32)21-25-3-1-2-4-30(25)28-19-37-22-38-20-28/h1-10,16-17,19-20,22,36H,11-15,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374681

(CHEMBL257834)Show SMILES CC(C)(C)CN1CCC(CC1)NC(=O)c1csc(NC(=O)c2ccc3cc4C(=O)NCC5(CCC5)n4c3c2)n1 Show InChI InChI=1S/C29H36N6O3S/c1-28(2,3)17-34-11-7-20(8-12-34)31-25(37)21-15-39-27(32-21)33-24(36)19-6-5-18-13-23-26(38)30-16-29(9-4-10-29)35(23)22(18)14-19/h5-6,13-15,20H,4,7-12,16-17H2,1-3H3,(H,30,38)(H,31,37)(H,32,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374682

(CHEMBL403154)Show SMILES CC(C)(C)CN1CCC(CC1)NC(=O)c1csc(NC(=O)c2ccc3cc4C(=O)NCC(C)(C)n4c3c2)n1 Show InChI InChI=1S/C28H36N6O3S/c1-27(2,3)16-33-10-8-19(9-11-33)30-24(36)20-14-38-26(31-20)32-23(35)18-7-6-17-12-22-25(37)29-15-28(4,5)34(22)21(17)13-18/h6-7,12-14,19H,8-11,15-16H2,1-5H3,(H,29,37)(H,30,36)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374683

(CHEMBL258045)Show SMILES CN1CCC(CC1)NC(=O)c1csc(NC(=O)c2ccc3cc4C(=O)NCC5(CCC5)n4c3c2)n1 Show InChI InChI=1S/C25H28N6O3S/c1-30-9-5-17(6-10-30)27-22(33)18-13-35-24(28-18)29-21(32)16-4-3-15-11-20-23(34)26-14-25(7-2-8-25)31(20)19(15)12-16/h3-4,11-13,17H,2,5-10,14H2,1H3,(H,26,34)(H,27,33)(H,28,29,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50297124
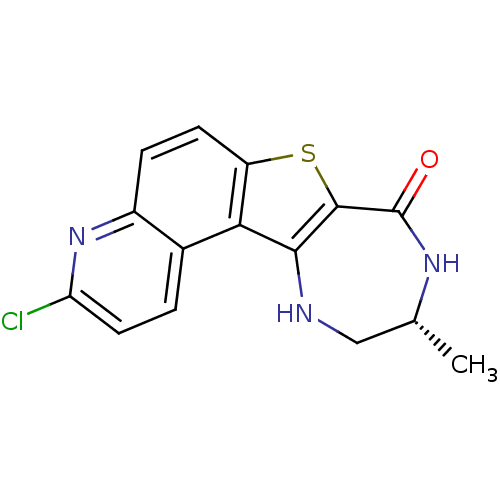
((R)-3-Chloro-10-methyl-9,10,11,12-tetrahydro-7-thi...)Show SMILES C[C@@H]1CNc2c(sc3ccc4nc(Cl)ccc4c23)C(=O)N1 |r| Show InChI InChI=1S/C15H12ClN3OS/c1-7-6-17-13-12-8-2-5-11(16)19-9(8)3-4-10(12)21-14(13)15(20)18-7/h2-5,7,17H,6H2,1H3,(H,18,20)/t7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 19: 4882-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.017
BindingDB Entry DOI: 10.7270/Q2J38SM7 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374681

(CHEMBL257834)Show SMILES CC(C)(C)CN1CCC(CC1)NC(=O)c1csc(NC(=O)c2ccc3cc4C(=O)NCC5(CCC5)n4c3c2)n1 Show InChI InChI=1S/C29H36N6O3S/c1-28(2,3)17-34-11-7-20(8-12-34)31-25(37)21-15-39-27(32-21)33-24(36)19-6-5-18-13-23-26(38)30-16-29(9-4-10-29)35(23)22(18)14-19/h5-6,13-15,20H,4,7-12,16-17H2,1-3H3,(H,30,38)(H,31,37)(H,32,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50395272
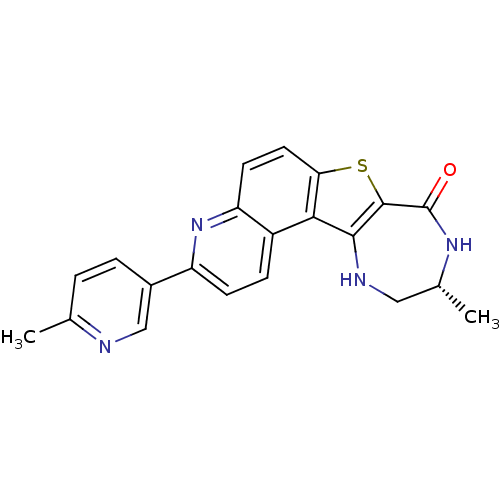
(CHEMBL1231206)Show SMILES C[C@@H]1CNc2c(sc3ccc4nc(ccc4c23)-c2ccc(C)nc2)C(=O)N1 |r| Show InChI InChI=1S/C21H18N4OS/c1-11-3-4-13(10-22-11)15-6-5-14-16(25-15)7-8-17-18(14)19-20(27-17)21(26)24-12(2)9-23-19/h3-8,10,12,23H,9H2,1-2H3,(H,24,26)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01511
BindingDB Entry DOI: 10.7270/Q22Z19M2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50362173

(CHEMBL1938685)Show SMILES N#Cc1ccc(cc1)-c1cc2Cc3cc(ccc3-n3ccnc3-c2o1)N1CCNCC1 Show InChI InChI=1S/C25H21N5O/c26-16-17-1-3-18(4-2-17)23-15-20-13-19-14-21(29-10-7-27-8-11-29)5-6-22(19)30-12-9-28-25(30)24(20)31-23/h1-6,9,12,14-15,27H,7-8,10-11,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using TAMRA-labeled peptide as substrate pre-incubated for 30 mins prior substrate addition measured after 30 mins incubation in da... |
Bioorg Med Chem Lett 22: 1068-72 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.113
BindingDB Entry DOI: 10.7270/Q23J3DDJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374679

(CHEMBL403258)Show SMILES O=C(NC1CCN(Cc2ccccc2)CC1)c1csc(NC(=O)c2ccc3cc4C(=O)NCC5(CCC5)n4c3c2)n1 Show InChI InChI=1S/C31H32N6O3S/c38-27(22-8-7-21-15-26-29(40)32-19-31(11-4-12-31)37(26)25(21)16-22)35-30-34-24(18-41-30)28(39)33-23-9-13-36(14-10-23)17-20-5-2-1-3-6-20/h1-3,5-8,15-16,18,23H,4,9-14,17,19H2,(H,32,40)(H,33,39)(H,34,35,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374687
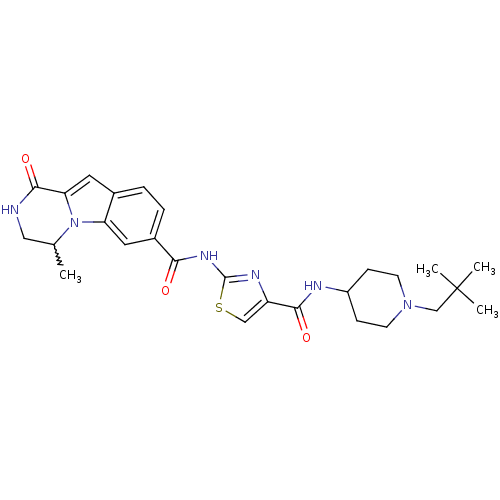
(CHEMBL256194)Show SMILES CC1CNC(=O)c2cc3ccc(cc3n12)C(=O)Nc1nc(cs1)C(=O)NC1CCN(CC(C)(C)C)CC1 |w:1.0| Show InChI InChI=1S/C27H34N6O3S/c1-16-13-28-25(36)22-11-17-5-6-18(12-21(17)33(16)22)23(34)31-26-30-20(14-37-26)24(35)29-19-7-9-32(10-8-19)15-27(2,3)4/h5-6,11-12,14,16,19H,7-10,13,15H2,1-4H3,(H,28,36)(H,29,35)(H,30,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465455
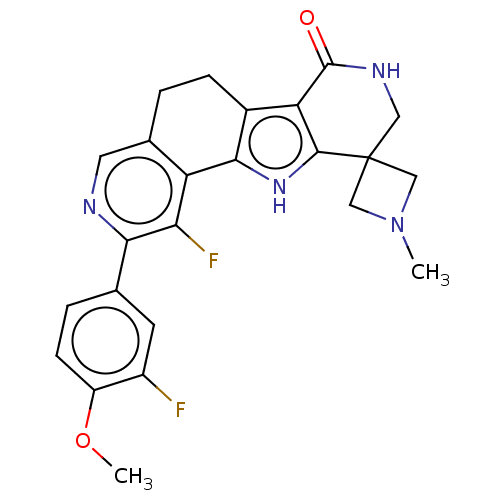
(CHEMBL4282514)Show SMILES COc1ccc(cc1F)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2c1F Show InChI InChI=1S/C24H22F2N4O2/c1-30-10-24(11-30)9-28-23(31)18-14-5-3-13-8-27-20(12-4-6-16(32-2)15(25)7-12)19(26)17(13)21(14)29-22(18)24/h4,6-8,29H,3,5,9-11H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348515
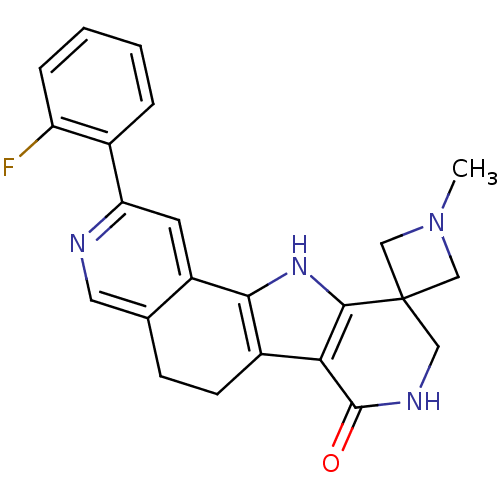
(CHEMBL1233942)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C23H21FN4O/c1-28-11-23(12-28)10-26-22(29)19-15-7-6-13-9-25-18(14-4-2-3-5-17(14)24)8-16(13)20(15)27-21(19)23/h2-5,8-9,27H,6-7,10-12H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MK2 mediated anisomycin-stimulated hsp27 phosphorylation in human THP-1 cells by fluorometric analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465454

(CHEMBL4293121)Show SMILES COc1ccc(cn1)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2c1F Show InChI InChI=1S/C23H22FN5O2/c1-29-10-23(11-29)9-27-22(30)17-14-5-3-12-7-26-19(13-4-6-15(31-2)25-8-13)18(24)16(12)20(14)28-21(17)23/h4,6-8,28H,3,5,9-11H2,1-2H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50465453

(CHEMBL4277929)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(c(F)c4-c3[nH]c21)-c1cccc(F)c1 Show InChI InChI=1S/C23H20F2N4O/c1-29-10-23(11-29)9-27-22(30)17-15-6-5-13-8-26-19(12-3-2-4-14(24)7-12)18(25)16(13)20(15)28-21(17)23/h2-4,7-8,28H,5-6,9-11H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by TR-FRET assay |
ACS Med Chem Lett 9: 392-396 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00098
BindingDB Entry DOI: 10.7270/Q2VX0K5Z |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434488
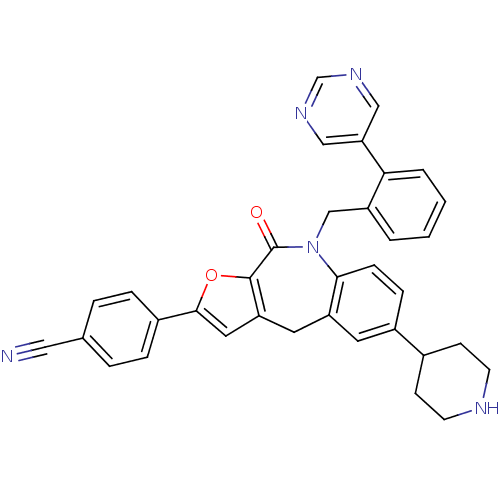
(CHEMBL2385549)Show SMILES O=C1N(Cc2ccccc2-c2cncnc2)c2ccc(cc2Cc2cc(oc12)-c1ccc(cc1)C#N)C1CCNCC1 Show InChI InChI=1S/C35H29N5O2/c36-18-23-5-7-25(8-6-23)33-17-29-16-28-15-26(24-11-13-37-14-12-24)9-10-32(28)40(35(41)34(29)42-33)21-27-3-1-2-4-31(27)30-19-38-22-39-20-30/h1-10,15,17,19-20,22,24,37H,11-14,16,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50362175

(CHEMBL1938687)Show SMILES N#Cc1ccc(cc1)-c1cc2Cc3cc(ccc3-n3cnnc3-c2o1)N1CCNCC1 Show InChI InChI=1S/C24H20N6O/c25-14-16-1-3-17(4-2-16)22-13-19-11-18-12-20(29-9-7-26-8-10-29)5-6-21(18)30-15-27-28-24(30)23(19)31-22/h1-6,12-13,15,26H,7-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using TAMRA-labeled peptide as substrate pre-incubated for 30 mins prior substrate addition measured after 30 mins incubation in da... |
Bioorg Med Chem Lett 22: 1068-72 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.113
BindingDB Entry DOI: 10.7270/Q23J3DDJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374688

(CHEMBL255991)Show SMILES CC(C)CN1CCC(CC1)NC(=O)c1csc(NC(=O)c2ccc3cc4C(=O)NCC(C)n4c3c2)n1 |w:30.31| Show InChI InChI=1S/C26H32N6O3S/c1-15(2)13-31-8-6-19(7-9-31)28-24(34)20-14-36-26(29-20)30-23(33)18-5-4-17-10-22-25(35)27-12-16(3)32(22)21(17)11-18/h4-5,10-11,14-16,19H,6-9,12-13H2,1-3H3,(H,27,35)(H,28,34)(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348492
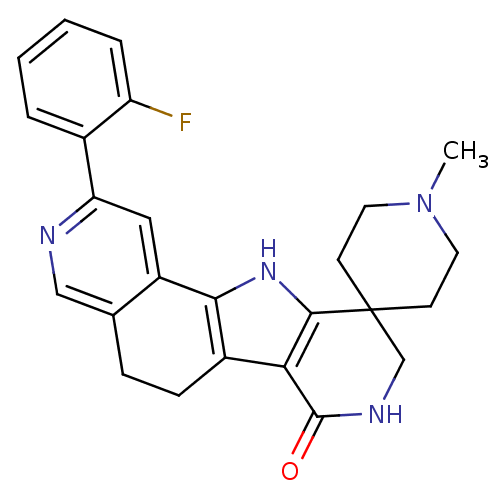
(CHEMBL1801296)Show SMILES CN1CCC2(CC1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C25H25FN4O/c1-30-10-8-25(9-11-30)14-28-24(31)21-17-7-6-15-13-27-20(16-4-2-3-5-19(16)26)12-18(15)22(17)29-23(21)25/h2-5,12-13,29H,6-11,14H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50314776
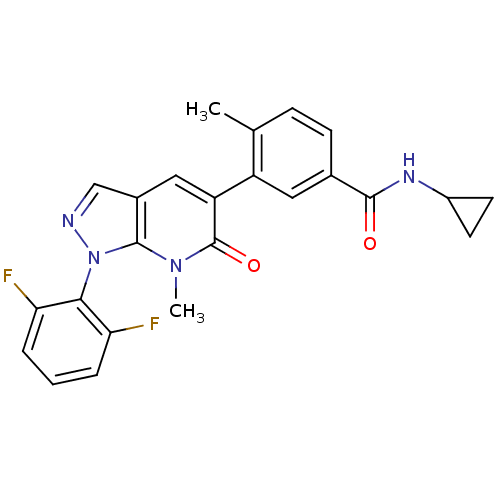
(CHEMBL1089865 | N-Cyclopropyl-3-(1-(2,6-difluoroph...)Show SMILES Cc1ccc(cc1-c1cc2cnn(-c3c(F)cccc3F)c2n(C)c1=O)C(=O)NC1CC1 |(1.2,-4.58,;-.13,-3.81,;-1.47,-4.58,;-2.8,-3.81,;-2.8,-2.27,;-1.47,-1.5,;-.14,-2.27,;1.2,-1.5,;2.53,-2.26,;3.86,-1.49,;5.33,-1.97,;6.23,-.72,;5.3,.54,;6.07,1.87,;7.56,1.95,;8.35,.6,;8.33,3.29,;7.49,4.58,;5.95,4.58,;5.29,3.22,;3.7,3.4,;3.86,.05,;2.53,.82,;2.53,2.36,;1.19,.04,;-.14,.81,;-4.14,-1.5,;-4.14,.04,;-5.47,-2.28,;-6.81,-1.51,;-8.35,-1.51,;-7.58,-.17,)| Show InChI InChI=1S/C24H20F2N4O2/c1-13-6-7-14(22(31)28-16-8-9-16)10-17(13)18-11-15-12-27-30(23(15)29(2)24(18)32)21-19(25)4-3-5-20(21)26/h3-7,10-12,16H,8-9H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced MK2 phosphorylation in human whole blood treated 30 mins before LPS challenge measured after 45 mins by FACS analysis |
J Med Chem 53: 2973-85 (2010)
Article DOI: 10.1021/jm100095x
BindingDB Entry DOI: 10.7270/Q2FX79MD |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434494

(CHEMBL2385542)Show SMILES COc1cncc(c1)-c1ccccc1CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C36H31N5O3/c1-43-31-18-29(21-39-22-31)32-5-3-2-4-26(32)23-41-33-11-10-30(40-14-12-38-13-15-40)17-27(33)16-28-19-34(44-35(28)36(41)42)25-8-6-24(20-37)7-9-25/h2-11,17-19,21-22,38H,12-16,23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374680

(CHEMBL429504)Show SMILES CC1(C)CNC(=O)c2cc3ccc(cc3n12)C(=O)Nc1nc(cs1)C(=O)NC1CCN(Cc2ccccc2)CC1 Show InChI InChI=1S/C30H32N6O3S/c1-30(2)18-31-28(39)25-14-20-8-9-21(15-24(20)36(25)30)26(37)34-29-33-23(17-40-29)27(38)32-22-10-12-35(13-11-22)16-19-6-4-3-5-7-19/h3-9,14-15,17,22H,10-13,16,18H2,1-2H3,(H,31,39)(H,32,38)(H,33,34,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374684

(CHEMBL270148)Show SMILES CN1CCC(CC1)NC(=O)c1csc(NC(=O)c2ccc3cc4C(=O)NCC(C)(C)n4c3c2)n1 Show InChI InChI=1S/C24H28N6O3S/c1-24(2)13-25-22(33)19-10-14-4-5-15(11-18(14)30(19)24)20(31)28-23-27-17(12-34-23)21(32)26-16-6-8-29(3)9-7-16/h4-5,10-12,16H,6-9,13H2,1-3H3,(H,25,33)(H,26,32)(H,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374693

(CHEMBL271309)Show SMILES CC1CNC(=O)c2cc3ccc(cc3n12)C(=O)Nc1nc(cs1)C(=O)NC1CCN(C)CC1 |w:1.0| Show InChI InChI=1S/C23H26N6O3S/c1-13-11-24-22(32)19-9-14-3-4-15(10-18(14)29(13)19)20(30)27-23-26-17(12-33-23)21(31)25-16-5-7-28(2)8-6-16/h3-4,9-10,12-13,16H,5-8,11H2,1-2H3,(H,24,32)(H,25,31)(H,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348542
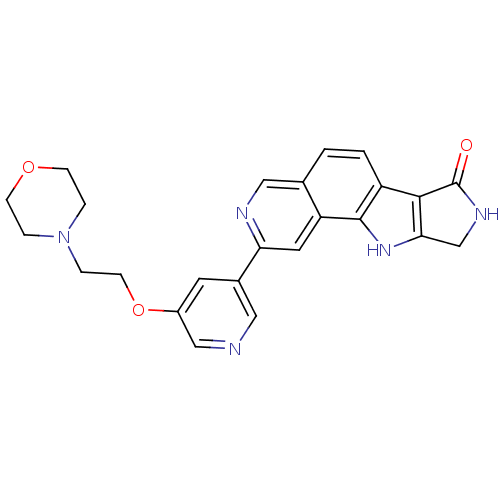
(CHEMBL1801381)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(cc34)-c3cncc(OCCN4CCOCC4)c3)c12 Show InChI InChI=1S/C24H23N5O3/c30-24-22-18-2-1-15-12-26-20(10-19(15)23(18)28-21(22)14-27-24)16-9-17(13-25-11-16)32-8-5-29-3-6-31-7-4-29/h1-2,9-13,28H,3-8,14H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348543

(CHEMBL1801382)Show SMILES COCCOc1cncc(c1)-c1cc2c3[nH]c4CNC(=O)c4c3ccc2cn1 Show InChI InChI=1S/C21H18N4O3/c1-27-4-5-28-14-6-13(8-22-10-14)17-7-16-12(9-23-17)2-3-15-19-18(25-20(15)16)11-24-21(19)26/h2-3,6-10,25H,4-5,11H2,1H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
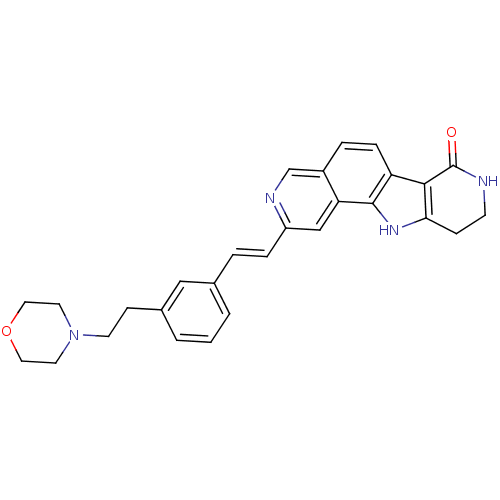
(Homo sapiens (Human)) | BDBM50348546
(CHEMBL1801385)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5cccc(CCN6CCOCC6)c5)cc34)c12 Show InChI InChI=1S/C28H28N4O2/c33-28-26-23-7-5-21-18-30-22(17-24(21)27(23)31-25(26)8-10-29-28)6-4-19-2-1-3-20(16-19)9-11-32-12-14-34-15-13-32/h1-7,16-18,31H,8-15H2,(H,29,33)/b6-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50395272

(CHEMBL1231206)Show SMILES C[C@@H]1CNc2c(sc3ccc4nc(ccc4c23)-c2ccc(C)nc2)C(=O)N1 |r| Show InChI InChI=1S/C21H18N4OS/c1-11-3-4-13(10-22-11)15-6-5-14-16(25-15)7-8-17-18(14)19-20(27-17)21(26)24-12(2)9-23-19/h3-8,10,12,23H,9H2,1-2H3,(H,24,26)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Teijin Pharma Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant MAPKAP-K2 using KKKALSRQLSVAA as substrate after 4 mins by surface plasmon resonance spectroscopic analysis |
J Med Chem 55: 6700-15 (2012)
Article DOI: 10.1021/jm300411k
BindingDB Entry DOI: 10.7270/Q20K29PX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
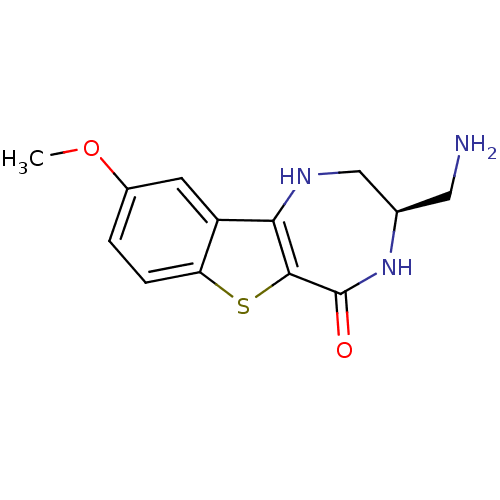
(Homo sapiens (Human)) | BDBM50297151
((3R)-3-(aminomethyl)-9-methoxy-1,2,3,4-tetrahydro-...)Show InChI InChI=1S/C13H15N3O2S/c1-18-8-2-3-10-9(4-8)11-12(19-10)13(17)16-7(5-14)6-15-11/h2-4,7,15H,5-6,14H2,1H3,(H,16,17)/t7-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 19: 4878-81 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.015
BindingDB Entry DOI: 10.7270/Q28K795K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50297119

((R)-10-Methyl-3-(4-methyl-pyridin-3-yl)-9,10,11,12...)Show SMILES C[C@@H]1CNc2c(sc3ccc4nc(ccc4c23)-c2cnccc2C)C(=O)N1 |r| Show InChI InChI=1S/C21H18N4OS/c1-11-7-8-22-10-14(11)16-4-3-13-15(25-16)5-6-17-18(13)19-20(27-17)21(26)24-12(2)9-23-19/h3-8,10,12,23H,9H2,1-2H3,(H,24,26)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 19: 4882-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.017
BindingDB Entry DOI: 10.7270/Q2J38SM7 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50297113
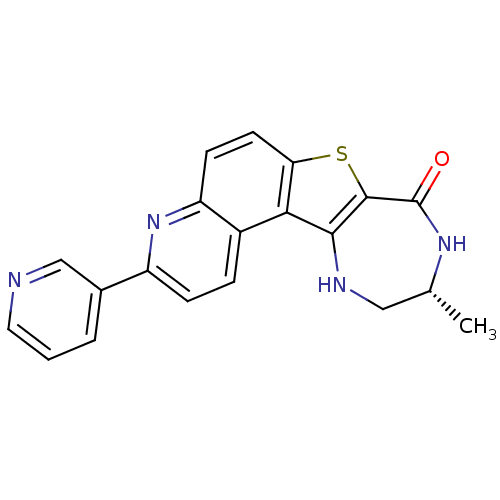
((R)-10-Methyl-3-pyridin-3-yl-9,10,11,12-tetrahydro...)Show SMILES C[C@@H]1CNc2c(sc3ccc4nc(ccc4c23)-c2cccnc2)C(=O)N1 |r| Show InChI InChI=1S/C20H16N4OS/c1-11-9-22-18-17-13-4-5-14(12-3-2-8-21-10-12)24-15(13)6-7-16(17)26-19(18)20(25)23-11/h2-8,10-11,22H,9H2,1H3,(H,23,25)/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 19: 4882-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.017
BindingDB Entry DOI: 10.7270/Q2J38SM7 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50314780
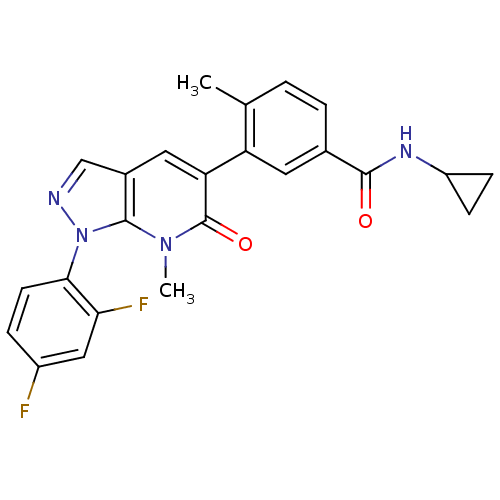
(CHEMBL1091199 | N-Cyclopropyl-3-(1-(2,4-difluoroph...)Show SMILES Cc1ccc(cc1-c1cc2cnn(-c3ccc(F)cc3F)c2n(C)c1=O)C(=O)NC1CC1 Show InChI InChI=1S/C24H20F2N4O2/c1-13-3-4-14(22(31)28-17-6-7-17)9-18(13)19-10-15-12-27-30(23(15)29(2)24(19)32)21-8-5-16(25)11-20(21)26/h3-5,8-12,17H,6-7H2,1-2H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced MK2 phosphorylation in human whole blood treated 30 mins before LPS challenge measured after 45 mins by FACS analysis |
J Med Chem 53: 2973-85 (2010)
Article DOI: 10.1021/jm100095x
BindingDB Entry DOI: 10.7270/Q2FX79MD |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434489
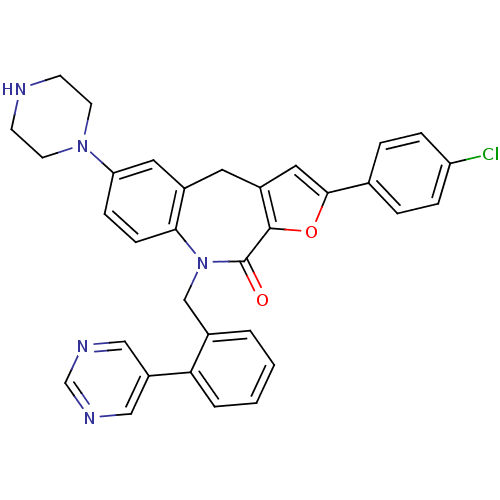
(CHEMBL2385547)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cncnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C33H28ClN5O2/c34-27-7-5-22(6-8-27)31-17-25-15-24-16-28(38-13-11-35-12-14-38)9-10-30(24)39(33(40)32(25)41-31)20-23-3-1-2-4-29(23)26-18-36-21-37-19-26/h1-10,16-19,21,35H,11-15,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50434489

(CHEMBL2385547)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3N(Cc3ccccc3-c3cncnc3)C(=O)c2o1)N1CCNCC1 Show InChI InChI=1S/C33H28ClN5O2/c34-27-7-5-22(6-8-27)31-17-25-15-24-16-28(38-13-11-35-12-14-38)9-10-30(24)39(33(40)32(25)41-31)20-23-3-1-2-4-29(23)26-18-36-21-37-19-26/h1-10,16-19,21,35H,11-15,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) incubated for 30 mins by MK2 IMAP assay |
Bioorg Med Chem Lett 24: 3609-13 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.024
BindingDB Entry DOI: 10.7270/Q2F47QR7 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50384542

(CHEMBL2036616)Show SMILES N#Cc1ccc(cc1)-c1ccc(o1)C1=NOC(N1c1ccc(cc1)N1CCNCC1)c1ccccc1-c1cncnc1 |t:15| Show InChI InChI=1S/C33H27N7O2/c34-19-23-5-7-24(8-6-23)30-13-14-31(41-30)32-38-42-33(29-4-2-1-3-28(29)25-20-36-22-37-21-25)40(32)27-11-9-26(10-12-27)39-17-15-35-16-18-39/h1-14,20-22,33,35H,15-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using 5TAMRA-KKLNRTLSVA-COOH as substrate incubated for 30 mins prior to substrate addition measured after 30 mins by immobilized m... |
ACS Med Chem Lett 3: 100-105 (2012)
Article DOI: 10.1021/ml200238g
BindingDB Entry DOI: 10.7270/Q2K0759N |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348491

(CHEMBL1801305)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(nc4-c3[nH]c21)-c1cccc(F)c1 Show InChI InChI=1S/C22H20FN5O/c1-28-10-22(11-28)9-25-21(29)16-15-6-5-13-8-24-20(12-3-2-4-14(23)7-12)27-17(13)18(15)26-19(16)22/h2-4,7-8,26H,5-6,9-11H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348493
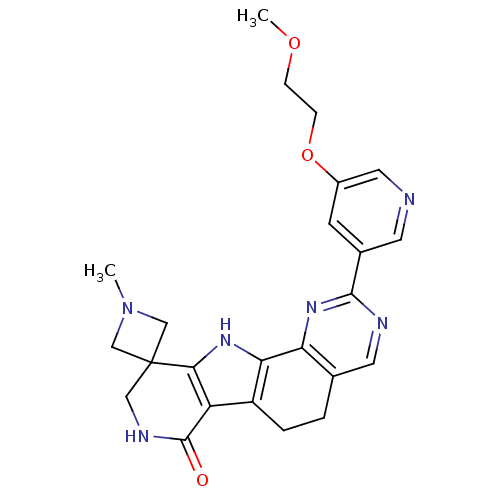
(CHEMBL1801304)Show SMILES COCCOc1cncc(c1)-c1ncc2CCc3c([nH]c4c3C(=O)NCC43CN(C)C3)-c2n1 Show InChI InChI=1S/C24H26N6O3/c1-30-12-24(13-30)11-27-23(31)18-17-4-3-14-9-26-22(29-19(14)20(17)28-21(18)24)15-7-16(10-25-8-15)33-6-5-32-2/h7-10,28H,3-6,11-13H2,1-2H3,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50560259
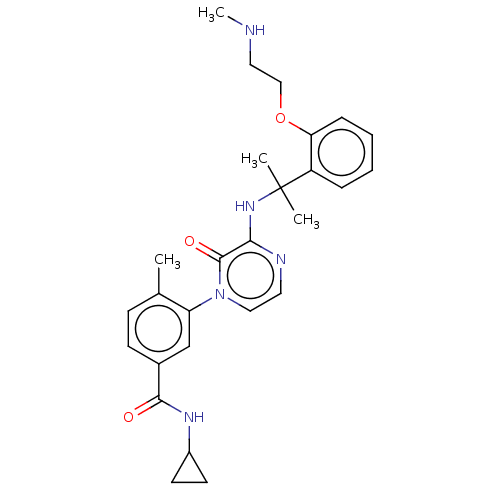
(CHEMBL4792347)Show SMILES CNCCOc1ccccc1C(C)(C)Nc1nccn(-c2cc(ccc2C)C(=O)NC2CC2)c1=O | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human MAPKAPK2 |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127412
BindingDB Entry DOI: 10.7270/Q2V40ZW0 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
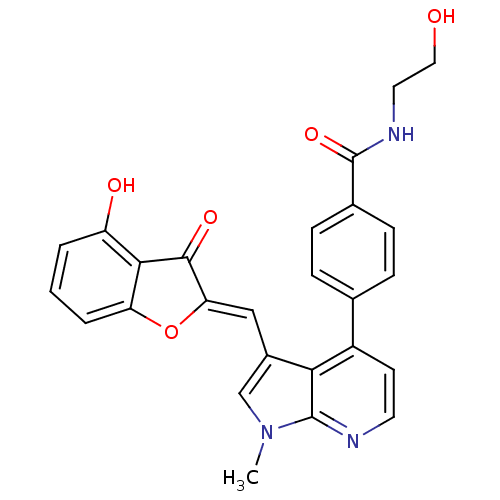
(Homo sapiens (Human)) | BDBM50314344
(4-(3-((4-hydroxy-3-oxobenzofuran-2(3H)-ylidene)met...)Show SMILES Cn1cc(\C=C2/Oc3cccc(O)c3C2=O)c2c(ccnc12)-c1ccc(cc1)C(=O)NCCO Show InChI InChI=1S/C26H21N3O5/c1-29-14-17(13-21-24(32)23-19(31)3-2-4-20(23)34-21)22-18(9-10-27-25(22)29)15-5-7-16(8-6-15)26(33)28-11-12-30/h2-10,13-14,30-31H,11-12H2,1H3,(H,28,33)/b21-13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 20: 2321-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.135
BindingDB Entry DOI: 10.7270/Q2PK0G82 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
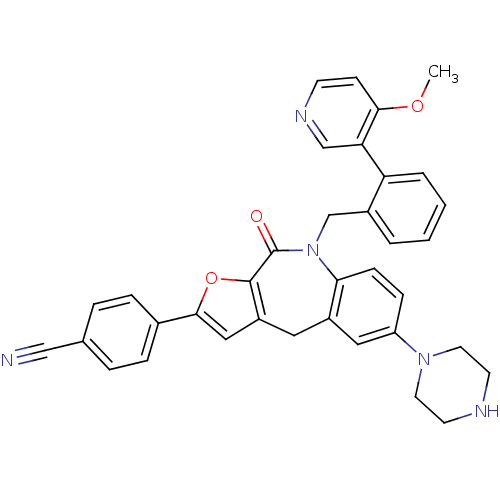
(Homo sapiens (Human)) | BDBM50434490
(CHEMBL2385546)Show SMILES COc1ccncc1-c1ccccc1CN1c2ccc(cc2Cc2cc(oc2C1=O)-c1ccc(cc1)C#N)N1CCNCC1 Show InChI InChI=1S/C36H31N5O3/c1-43-33-12-13-39-22-31(33)30-5-3-2-4-26(30)23-41-32-11-10-29(40-16-14-38-15-17-40)19-27(32)18-28-20-34(44-35(28)36(41)42)25-8-6-24(21-37)7-9-25/h2-13,19-20,22,38H,14-18,23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) phosphorylation using TAMRA labeled peptide as substrate incubated 30 mins before substrate addition measured afte... |
Bioorg Med Chem Lett 23: 3262-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.109
BindingDB Entry DOI: 10.7270/Q2ZS2XX8 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50374689

(CHEMBL256196)Show SMILES CCN1CCC(CC1)NC(=O)c1csc(NC(=O)c2ccc3cc4C(=O)NCC(C)n4c3c2)n1 |w:28.29| Show InChI InChI=1S/C24H28N6O3S/c1-3-29-8-6-17(7-9-29)26-22(32)18-13-34-24(27-18)28-21(31)16-5-4-15-10-20-23(33)25-12-14(2)30(20)19(15)11-16/h4-5,10-11,13-14,17H,3,6-9,12H2,1-2H3,(H,25,33)(H,26,32)(H,27,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of MK2 |
Bioorg Med Chem Lett 18: 938-41 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.037
BindingDB Entry DOI: 10.7270/Q22B8ZWZ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
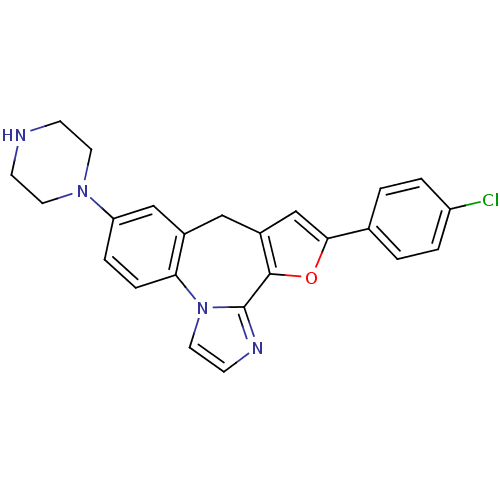
(Homo sapiens (Human)) | BDBM50362172
(CHEMBL1938684)Show SMILES Clc1ccc(cc1)-c1cc2Cc3cc(ccc3-n3ccnc3-c2o1)N1CCNCC1 Show InChI InChI=1S/C24H21ClN4O/c25-19-3-1-16(2-4-19)22-15-18-13-17-14-20(28-10-7-26-8-11-28)5-6-21(17)29-12-9-27-24(29)23(18)30-22/h1-6,9,12,14-15,26H,7-8,10-11,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using TAMRA-labeled peptide as substrate pre-incubated for 30 mins prior substrate addition measured after 30 mins incubation in da... |
Bioorg Med Chem Lett 22: 1068-72 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.113
BindingDB Entry DOI: 10.7270/Q23J3DDJ |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50314784
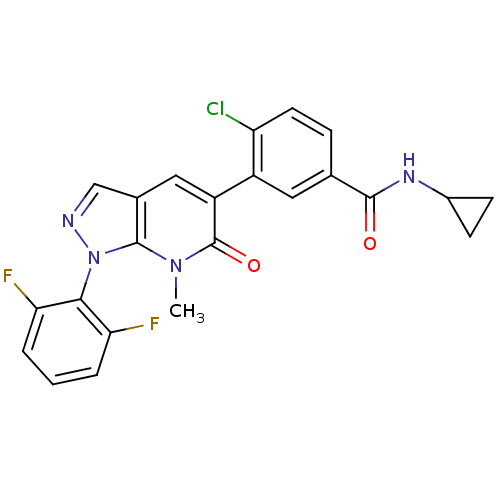
(4-Chloro-N-cyclopropyl-3-(1-(2,6-difluorophenyl)-7...)Show SMILES Cn1c2n(ncc2cc(-c2cc(ccc2Cl)C(=O)NC2CC2)c1=O)-c1c(F)cccc1F |(2.55,2.36,;2.53,.82,;3.86,.04,;5.31,.52,;6.22,-.75,;5.31,-1.98,;3.85,-1.5,;2.51,-2.26,;1.18,-1.48,;-.16,-2.23,;-1.49,-1.45,;-2.83,-2.21,;-2.84,-3.75,;-1.51,-4.53,;-.17,-3.77,;1.16,-4.56,;-4.15,-1.43,;-4.14,.11,;-5.49,-2.19,;-6.82,-1.41,;-8.36,-1.4,;-7.58,-.07,;1.19,.06,;-.13,.85,;6.09,1.85,;7.58,1.92,;8.35,.56,;8.36,3.24,;7.53,4.54,;5.99,4.56,;5.32,3.21,;3.73,3.4,)| Show InChI InChI=1S/C23H17ClF2N4O2/c1-29-22-13(11-27-30(22)20-18(25)3-2-4-19(20)26)10-16(23(29)32)15-9-12(5-8-17(15)24)21(31)28-14-6-7-14/h2-5,8-11,14H,6-7H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of LPS-induced MK2 phosphorylation in human whole blood treated 30 mins before LPS challenge measured after 45 mins by FACS analysis |
J Med Chem 53: 2973-85 (2010)
Article DOI: 10.1021/jm100095x
BindingDB Entry DOI: 10.7270/Q2FX79MD |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348481
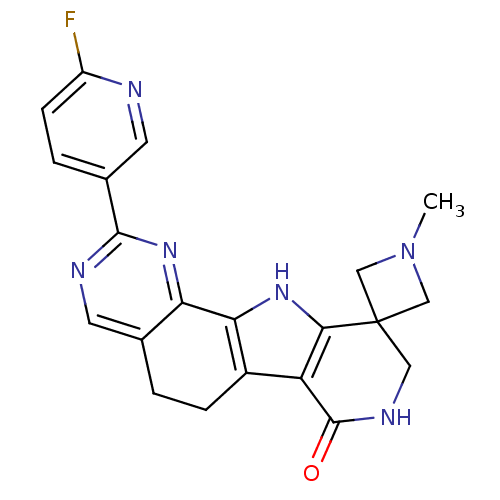
(CHEMBL1801307)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(nc4-c3[nH]c21)-c1ccc(F)nc1 Show InChI InChI=1S/C21H19FN6O/c1-28-9-21(10-28)8-25-20(29)15-13-4-2-11-6-24-19(12-3-5-14(22)23-7-12)27-16(11)17(13)26-18(15)21/h3,5-7,26H,2,4,8-10H2,1H3,(H,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50045412
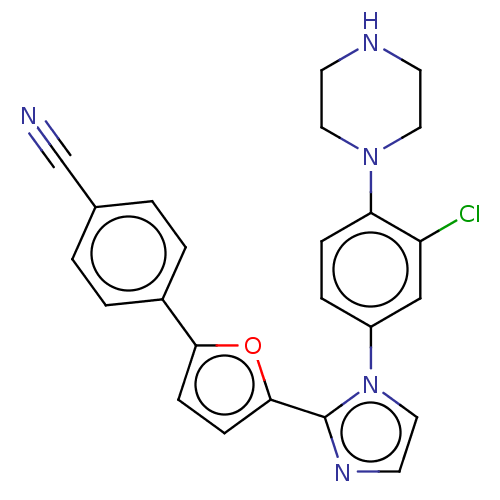
(CHEMBL3314153)Show SMILES Clc1cc(ccc1N1CCNCC1)-n1ccnc1-c1ccc(o1)-c1ccc(cc1)C#N Show InChI InChI=1S/C24H20ClN5O/c25-20-15-19(5-6-21(20)29-12-9-27-10-13-29)30-14-11-28-24(30)23-8-7-22(31-23)18-3-1-17(16-26)2-4-18/h1-8,11,14-15,27H,9-10,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK2 (unknown origin) incubated for 30 mins by MK2 IMAP assay |
Bioorg Med Chem Lett 24: 3609-13 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.024
BindingDB Entry DOI: 10.7270/Q2F47QR7 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50384542

(CHEMBL2036616)Show SMILES N#Cc1ccc(cc1)-c1ccc(o1)C1=NOC(N1c1ccc(cc1)N1CCNCC1)c1ccccc1-c1cncnc1 |t:15| Show InChI InChI=1S/C33H27N7O2/c34-19-23-5-7-24(8-6-23)30-13-14-31(41-30)32-38-42-33(29-4-2-1-3-28(29)25-20-36-22-37-21-25)40(32)27-11-9-26(10-12-27)39-17-15-35-16-18-39/h1-14,20-22,33,35H,15-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using 5TAMRA-KKLNRTLSVA-COOH as substrate incubated for 30 mins prior to substrate addition measured after 30 mins by immobilized m... |
ACS Med Chem Lett 3: 100-105 (2012)
Article DOI: 10.1021/ml200238g
BindingDB Entry DOI: 10.7270/Q2K0759N |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50384542

(CHEMBL2036616)Show SMILES N#Cc1ccc(cc1)-c1ccc(o1)C1=NOC(N1c1ccc(cc1)N1CCNCC1)c1ccccc1-c1cncnc1 |t:15| Show InChI InChI=1S/C33H27N7O2/c34-19-23-5-7-24(8-6-23)30-13-14-31(41-30)32-38-42-33(29-4-2-1-3-28(29)25-20-36-22-37-21-25)40(32)27-11-9-26(10-12-27)39-17-15-35-16-18-39/h1-14,20-22,33,35H,15-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MK2 using 5TAMRA-KKLNRTLSVA-COOH as substrate incubated for 30 mins prior to substrate addition measured after 30 mins by immobilized m... |
ACS Med Chem Lett 3: 100-105 (2012)
Article DOI: 10.1021/ml200238g
BindingDB Entry DOI: 10.7270/Q2K0759N |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348535

(CHEMBL1801374)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C26H24N4O2/c31-26-24-21-8-6-19-14-27-20(13-22(19)25(21)29-23(24)15-28-26)7-5-17-1-3-18(4-2-17)16-30-9-11-32-12-10-30/h1-8,13-14,29H,9-12,15-16H2,(H,28,31)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50362124
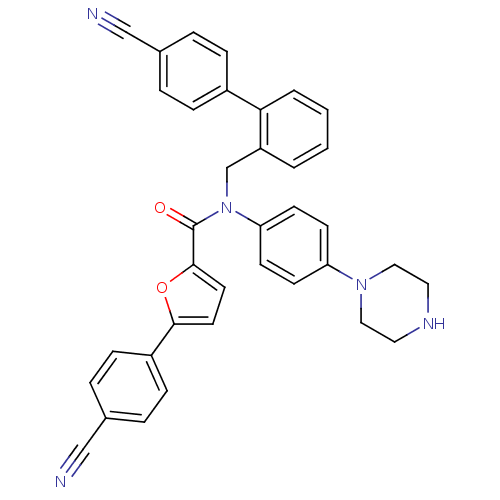
(CHEMBL1940645)Show SMILES O=C(N(Cc1ccccc1-c1ccc(cc1)C#N)c1ccc(cc1)N1CCNCC1)c1ccc(o1)-c1ccc(cc1)C#N Show InChI InChI=1S/C36H29N5O2/c37-23-26-5-9-28(10-6-26)33-4-2-1-3-30(33)25-41(32-15-13-31(14-16-32)40-21-19-39-20-22-40)36(42)35-18-17-34(43-35)29-11-7-27(24-38)8-12-29/h1-18,39H,19-22,25H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 |
Bioorg Med Chem Lett 22: 65-70 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.074
BindingDB Entry DOI: 10.7270/Q2R78FNS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data