

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50240412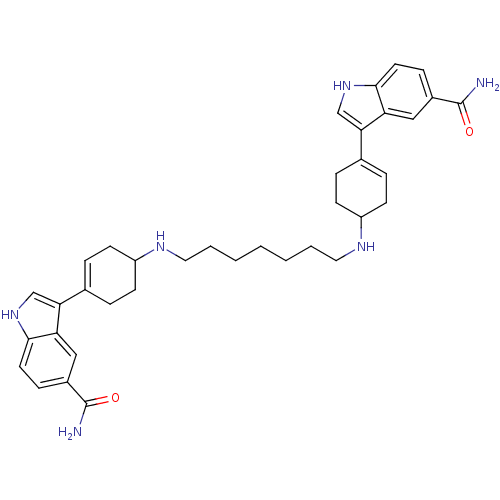 (3-(4-{7-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance, Inc. Curated by ChEMBL | Assay Description Binding affinity to 5HT1E receptor | J Med Chem 51: 3609-16 (2008) Article DOI: 10.1021/jm7011722 BindingDB Entry DOI: 10.7270/Q26T0MDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50240412 (3-(4-{7-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1E receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50285314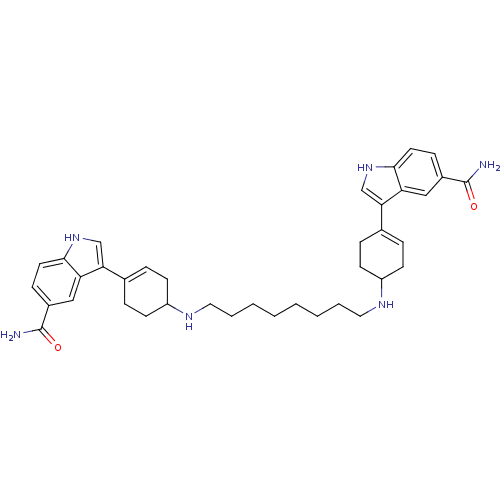 (3-(4-{8-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1E receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31044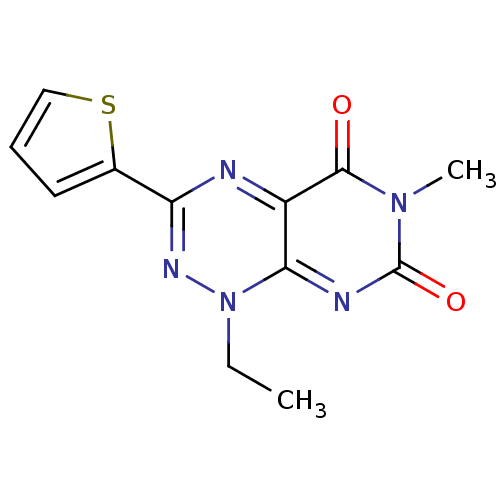 (1-Ethyl-6-methyl-3-thiophen-2-yl-1H-pyrimido[5,4-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 629 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31041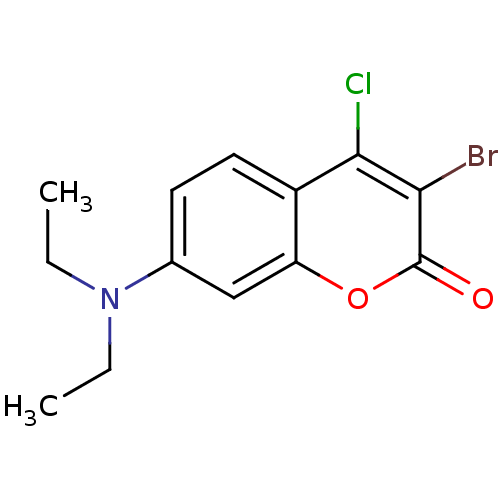 (3-Bromo-4-chloro-7-diethylamino-chromen-2-one | 3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 999 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50285315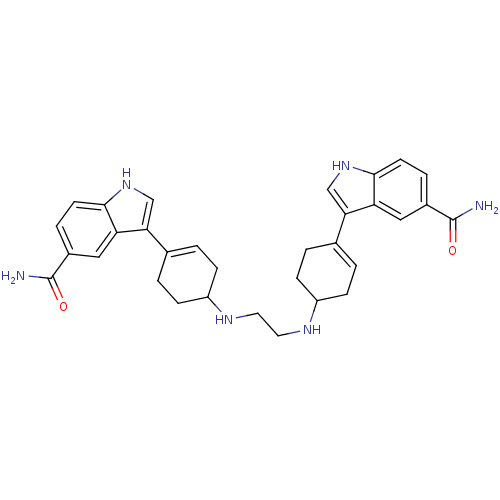 (3-(4-{2-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1E receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50285317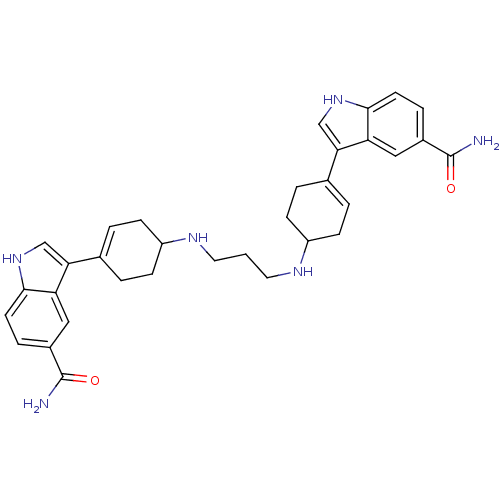 (3-(4-{3-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1E receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50285313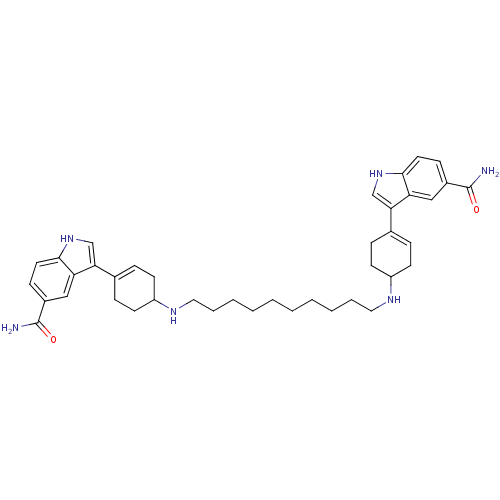 (3-(4-{10-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1E receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50285316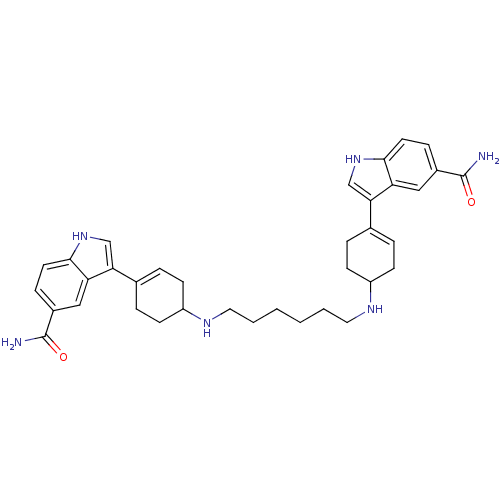 (3-(4-{6-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1E receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50285318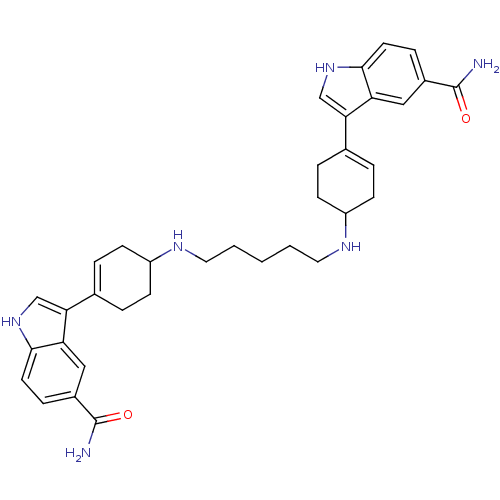 (3-(4-{5-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1E receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50285312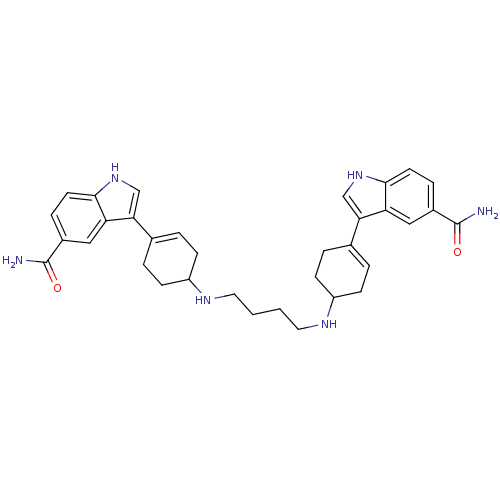 (3-(4-{4-[4-(5-carbamoyl-1H-3-indolyl)-3-cyclohexen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against 5-hydroxytryptamine 1E receptor subtype | Bioorg Med Chem Lett 5: 123-126 (1995) Article DOI: 10.1016/0960-894X(94)00470-Z BindingDB Entry DOI: 10.7270/Q2KP824F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31072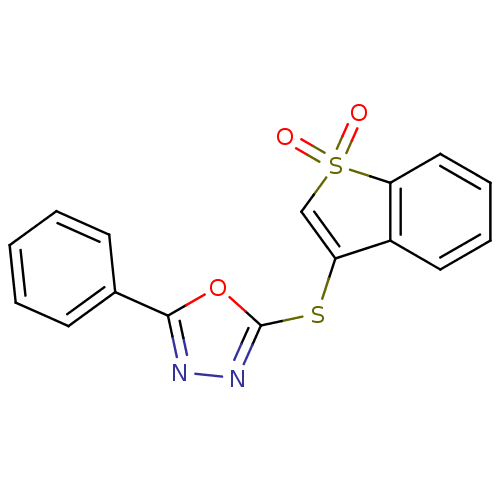 (2-[(1,1-dioxido-1-benzothien-3-yl)thio]-5-phenyl-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31361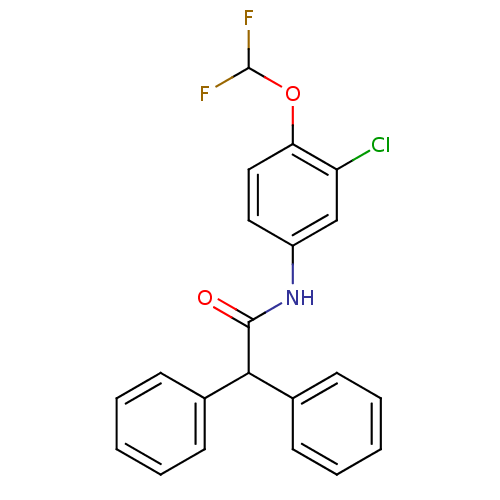 (MLS000391019 | N-[3-chloro-4-(difluoromethoxy)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31347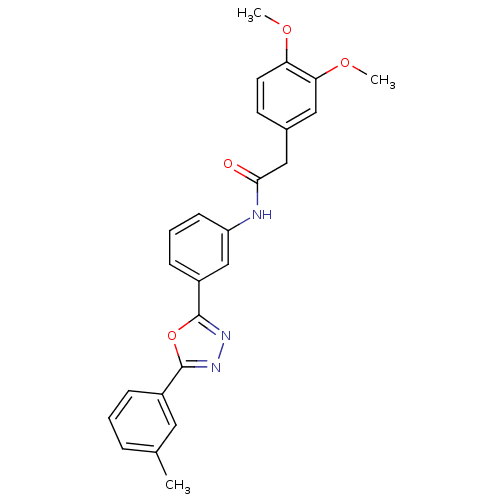 (2-(3,4-dimethoxyphenyl)-N-[3-[5-(3-methylphenyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.76E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31053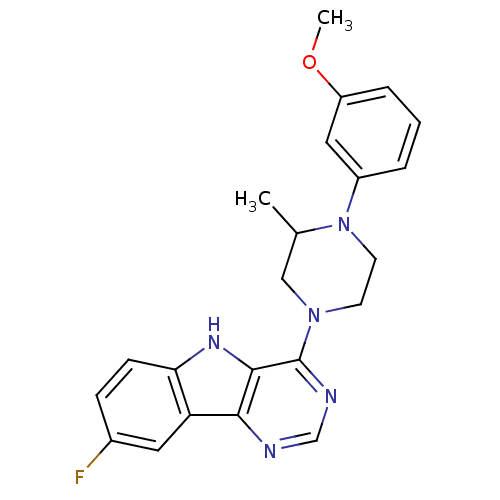 (8-fluoranyl-4-[4-(3-methoxyphenyl)-3-methyl-pipera...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.82E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31352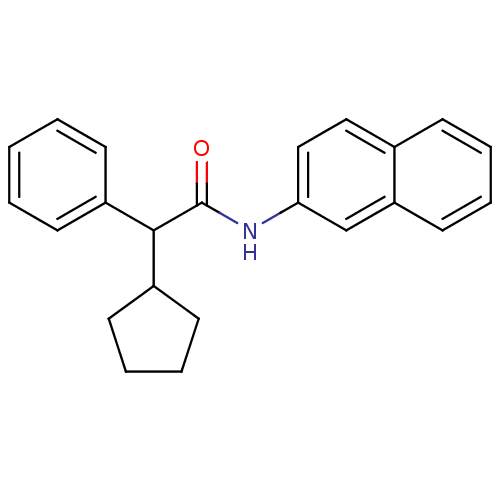 (2-Cyclopentyl-N-naphthalen-2-yl-2-phenyl-acetamide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31054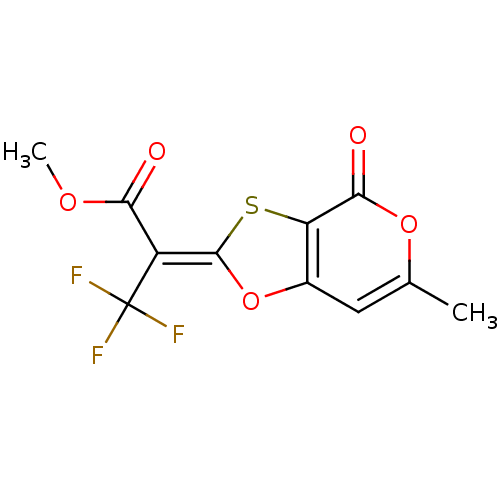 ((2E)-3,3,3-trifluoro-2-(4-keto-6-methyl-[1,3]oxath...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 1.87E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31068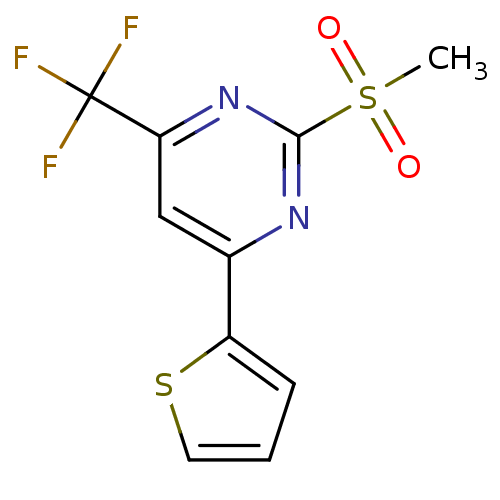 (2-(methylsulfonyl)-4-thien-2-yl-6-(trifluoromethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.93E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31364 ((1S,2S)-2-Phenyl-cyclopropanecarboxylic acid [1-[5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31042 ((5-acetamido-4-acetyloxy-2,1,3-benzoxadiazol-7-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31063 (2-(methylsulfonyl)-4-phenyl-6-(trifluoromethyl)pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.13E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31064 (4-(2-furanyl)-2-methylsulfonyl-6-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31082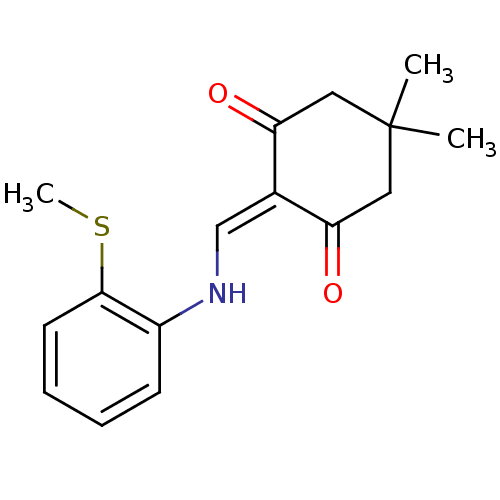 (5,5-dimethyl-2-[(2-methylsulfanylanilino)methylide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.36E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31363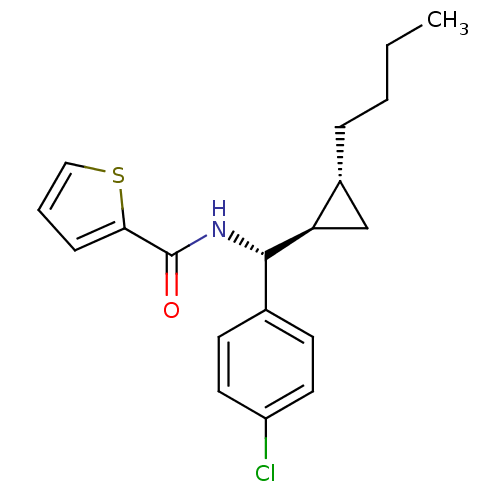 (MLS000563797 | N-[(R)-[(1R,2R)-2-butylcyclopropyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 2.47E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31372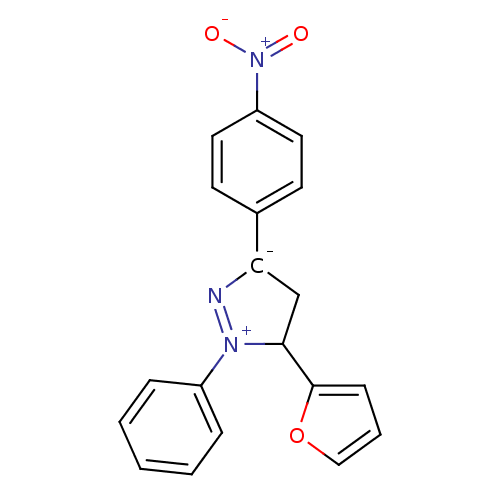 (3-(2-furanyl)-5-(4-nitrophenyl)-2-phenyl-3,4-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31076 (2-[1-(4-methoxyphenyl)tetrazol-5-yl]sulfonyl-1-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 2.61E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31083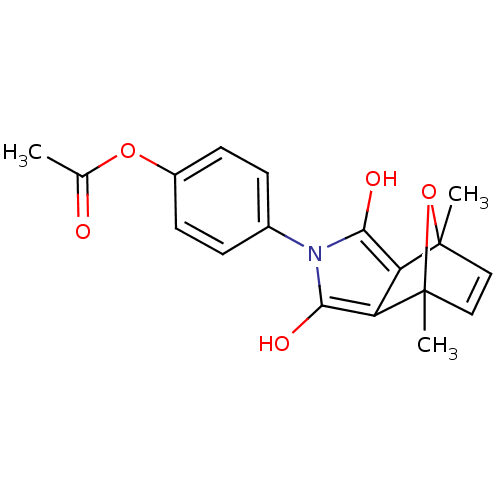 (MLS000107813 | SMR000103777 | [4-(4,7-dimethyl-1,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.62E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31066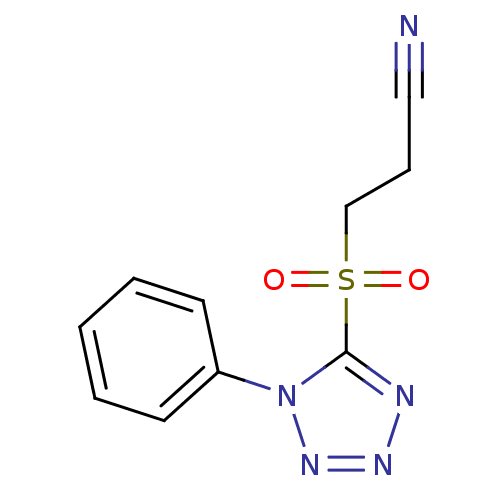 (3-(1-phenyltetrazol-5-yl)sulfonylpropanenitrile | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31062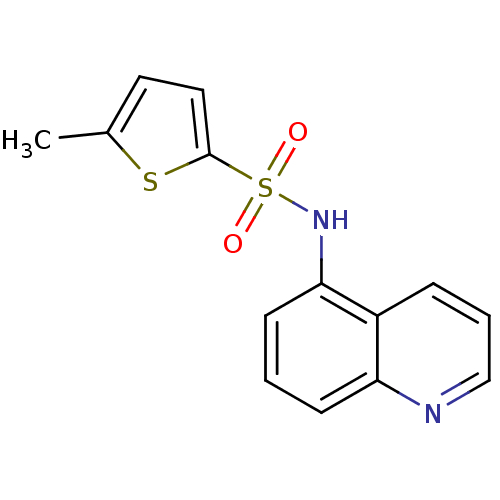 (5-methyl-N-(5-quinolinyl)-2-thiophenesulfonamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31067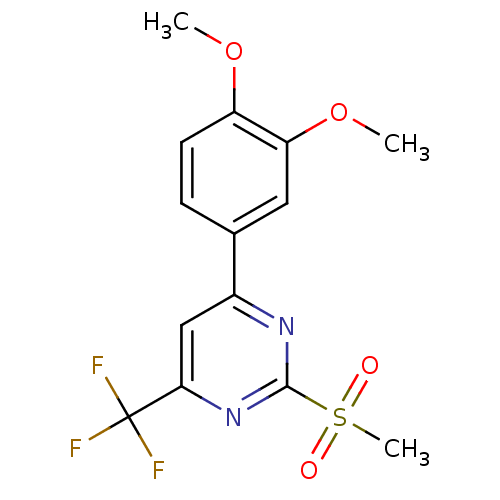 (4-(3,4-dimethoxyphenyl)-2-(methylsulfonyl)-6-(trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31061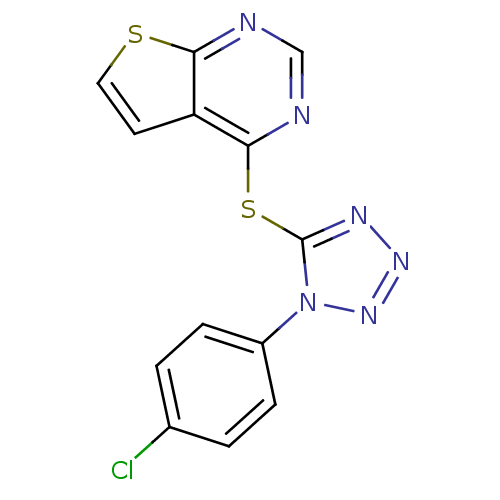 (4-[1-(4-chlorophenyl)tetrazol-5-yl]sulfanylthieno[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31060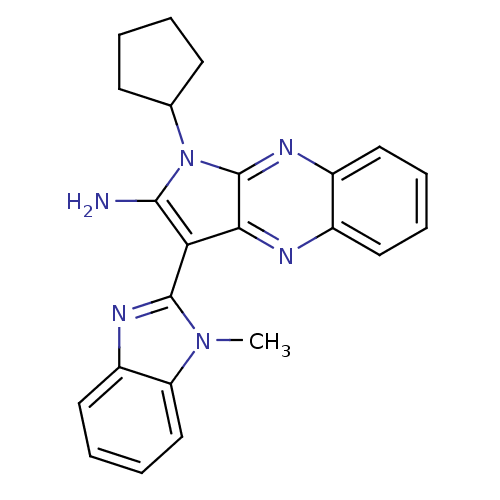 (1-cyclopentyl-3-(1-methyl-2-benzimidazolyl)-2-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31074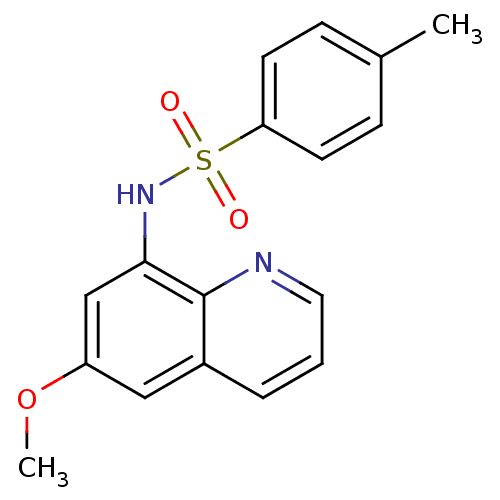 (MLS000062611 | N-(6-methoxy-8-quinolinyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31371 (3-(tert-butyl)-N-(4-fluorophenyl)-1-(3-methylbenzy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31043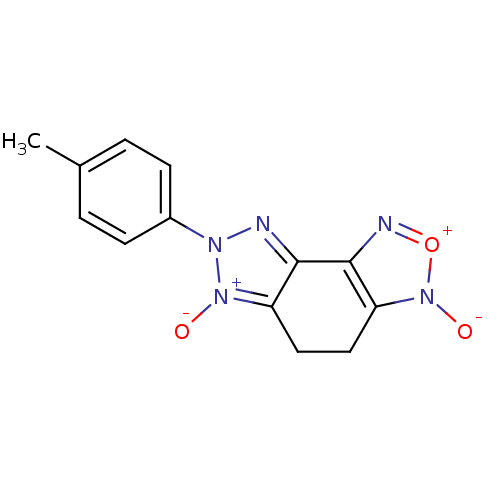 (3,6-dioxido-7-(p-tolyl)-4,5-dihydrotriazolo[4,5-e]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PCBioAssay | n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31081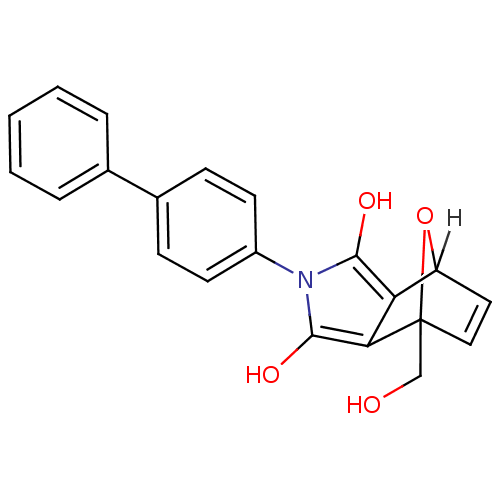 (7-(hydroxymethyl)-2-(4-phenylphenyl)-4,7a-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.91E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31071 ((E)-3-[5-(1-azepanyl)-2-furanyl]-2-(4-fluorophenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31362 (2-chlorobenzoic acid [2-(3-chloro-4-methyl-anilino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 5.28E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31355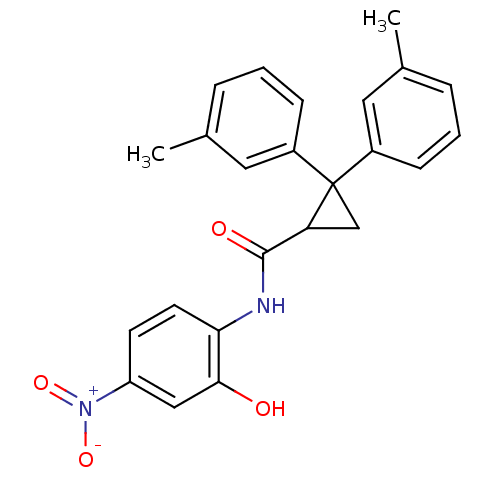 (2,2-Di-m-tolyl-cyclopropanecarboxylic acid (2-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 5.46E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity at 5-hydroxytryptamine 1E receptor | J Med Chem 40: 3501-3 (1997) Article DOI: 10.1021/jm9704560 BindingDB Entry DOI: 10.7270/Q2DZ07D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31080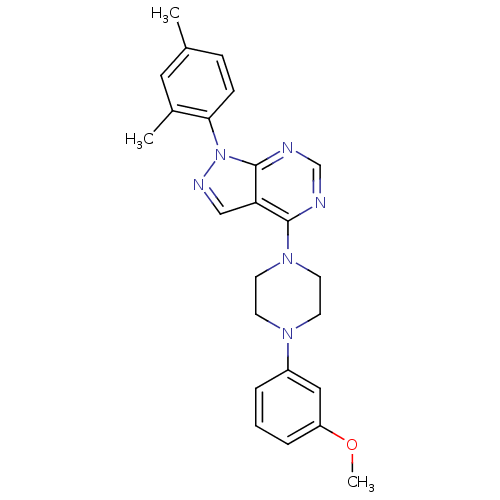 (1-(2,4-dimethylphenyl)-4-[4-(3-methoxyphenyl)-1-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 6.99E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31353 (2-Cyclopentyl-2-phenyl-N-p-tolyl-acetamide | 2-cyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 7.06E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31051 (4-[4-(3-methoxyphenyl)-3-methyl-1-piperazinyl]-5H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 8.24E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31046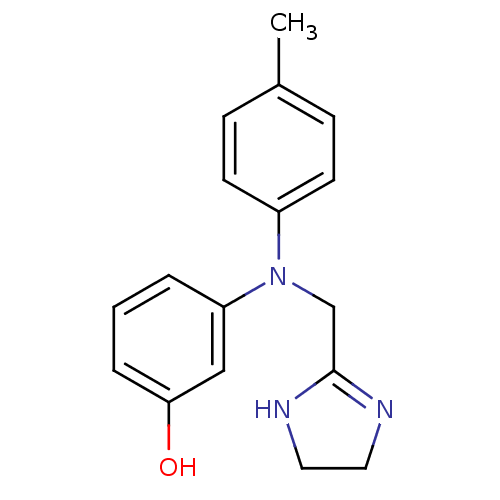 (3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 9.22E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31350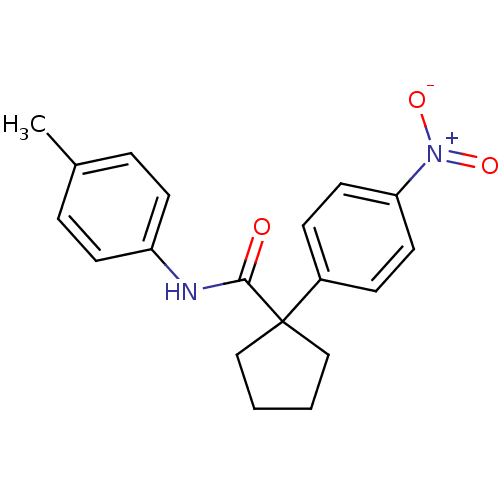 (1-(4-nitrophenyl)-N-(p-tolyl)cyclopentanecarboxami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 9.48E+3 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description The CHO cell line was cultured in T-175 flasks at 37 degrees C and 95% relative humidity (RH). The growth media consisted of Dulbecco's Modified ... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2FQ9TZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50304075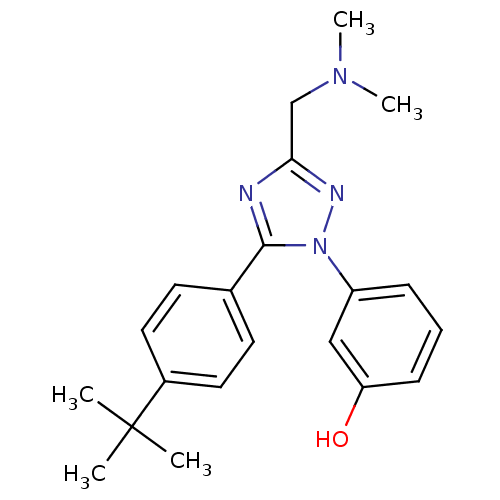 (3-(5-(4-tert-butylphenyl)-3-((dimethylamino)methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and the Informatics Institute of UMDNJ Curated by ChEMBL | Assay Description Inhibition of 5HT1E receptor | Bioorg Med Chem 17: 6442-50 (2009) Article DOI: 10.1016/j.bmc.2009.07.007 BindingDB Entry DOI: 10.7270/Q2QZ2B14 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50060429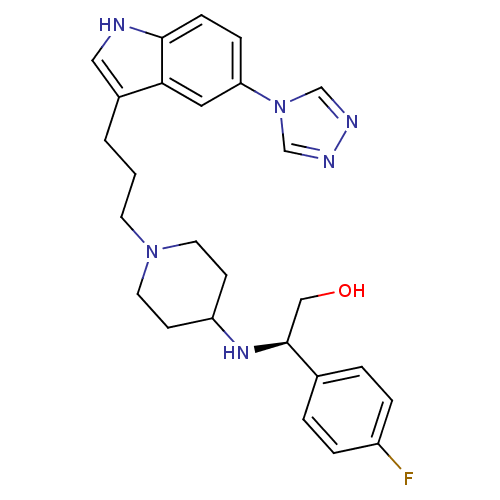 ((R)-2-(4-Fluoro-phenyl)-2-{1-[3-(5-[1,2,4]triazol-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was tested for the displacement of [3H]-5-HT binding to cloned human 5-hydroxytryptamine 1E receptor stably expressed in CHO cells | J Med Chem 42: 4981-5001 (2000) BindingDB Entry DOI: 10.7270/Q20P0Z77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM50060437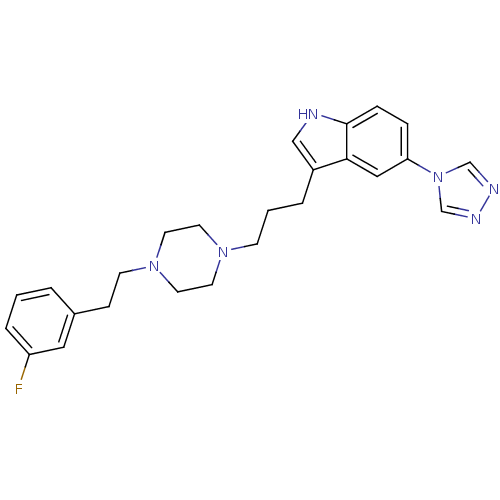 (3-(3-{4-[2-(3-Fluoro-phenyl)-ethyl]-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the affinity at 5-hydroxytryptamine 1E receptor | J Med Chem 40: 3501-3 (1997) Article DOI: 10.1021/jm9704560 BindingDB Entry DOI: 10.7270/Q2DZ07D8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31052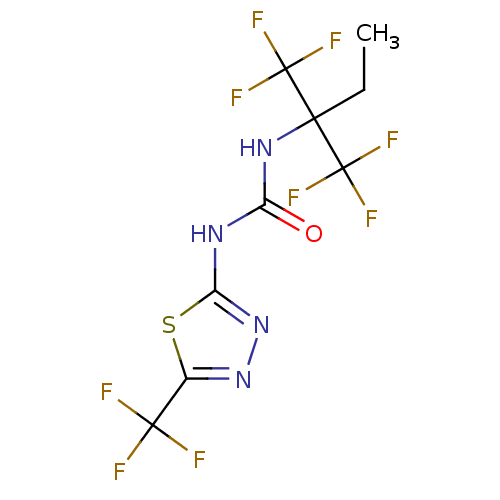 (1-[1,1-bis(trifluoromethyl)propyl]-3-[5-(trifluoro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1E (Homo sapiens (Human)) | BDBM31046 (3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | 7.4 | 23 |
The Scripps Research Institute Molecular Screening Center Curated by PubChem BioAssay | Assay Description To measure 5-HT1E activity, a Chinese Hamster Ovary (CHO) cell line was developed at the Scripps Molecular Research Institute Screening center. This ... | PubChem Bioassay (2007) BindingDB Entry DOI: 10.7270/Q2KH0KNM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |