 Found 1469 hits of ic50 data for polymerid = 4370,4371
Found 1469 hits of ic50 data for polymerid = 4370,4371 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Activin receptor type-1
(Homo sapiens (Human)) | BDBM621073
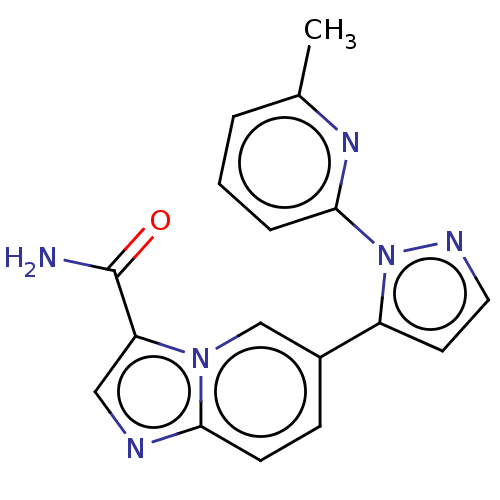
(Synthesis of 5-(3-(4-fluorobenzylamino)-1-(6-methy...) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM518186
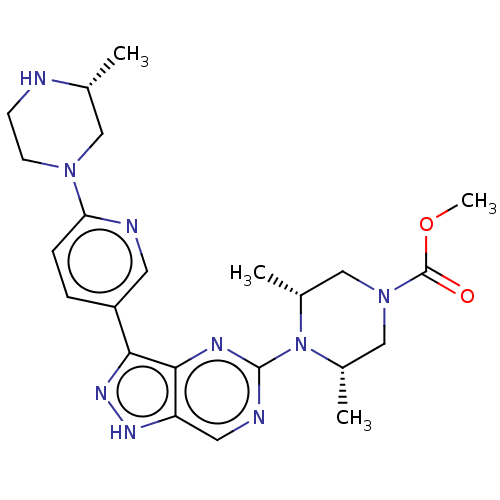
(US11111247, Example 17)Show SMILES COC(=O)N1C[C@H](C)N([C@H](C)C1)c1ncc2[nH]nc(-c3ccc(nc3)N3CCN[C@H](C)C3)c2n1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00206
BindingDB Entry DOI: 10.7270/Q2R78K72 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM518192
(US11111247, Example 22)Show SMILES COC(=O)N1C[C@H](C)N([C@H](C)C1)c1ncc2[nH]nc(-c3ccc(cc3)N3CCN(C)CC3)c2n1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00206
BindingDB Entry DOI: 10.7270/Q2R78K72 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50560457
(CHEMBL4790396)Show SMILES CN1CCN(CC1)c1ccc(cc1)-c1ccn2c(cnc2c1)-c1csc2nccnc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALK2 using casein as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127418
BindingDB Entry DOI: 10.7270/Q2WW7NDJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM603776
(US11654147, Compound 213)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cnn2cc(cnc12)N1CCN(CC1)C1CCNCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27085CT |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM102619
(K02288a | US10688093, Compound 382_0087_0284 | US1...)Show InChI InChI=1S/C20H20N2O4/c1-24-17-9-13(10-18(25-2)19(17)26-3)16-8-14(11-22-20(16)21)12-5-4-6-15(23)7-12/h4-11,23H,1-3H3,(H2,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128452
BindingDB Entry DOI: 10.7270/Q2JS9VGJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM518173
(US11111247, Example 9)Show SMILES C[C@@H]1CN(CCN1)c1ccc(cn1)-c1n[nH]c2cnc(nc12)N1[C@@H](C)CN(C[C@H]1C)C(=O)N1CCCC1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00206
BindingDB Entry DOI: 10.7270/Q2R78K72 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM518194
(US11111247, Example 24)Show SMILES COC(=O)N1C[C@H](C)N([C@H](C)C1)c1ncc2[nH]nc(-c3ccc(nc3)N3CCN(C)CC3)c2n1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00206
BindingDB Entry DOI: 10.7270/Q2R78K72 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM518193
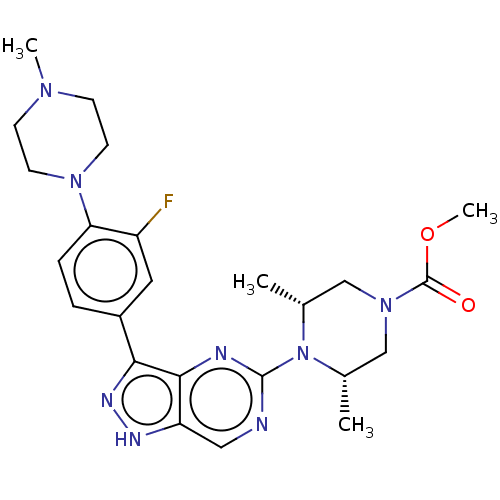
(US11111247, Example 23)Show SMILES COC(=O)N1C[C@H](C)N([C@H](C)C1)c1ncc2[nH]nc(-c3ccc(N4CCN(C)CC4)c(F)c3)c2n1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00206
BindingDB Entry DOI: 10.7270/Q2R78K72 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM603863
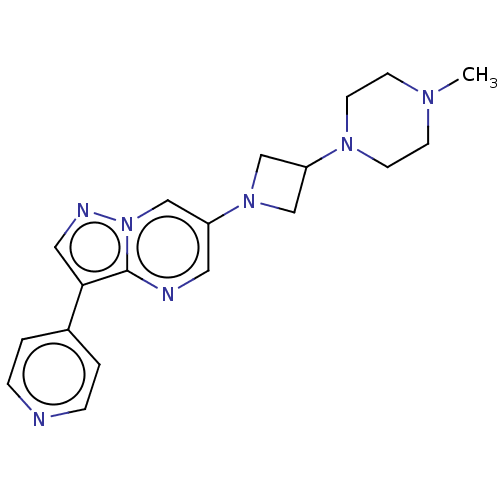
(US11654147, Compound 313 | US11654147, Compound 33...)Show SMILES CN1CCN(CC1)C1CN(C1)c1cnc2c(cnn2c1)-c1ccncc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27085CT |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528195
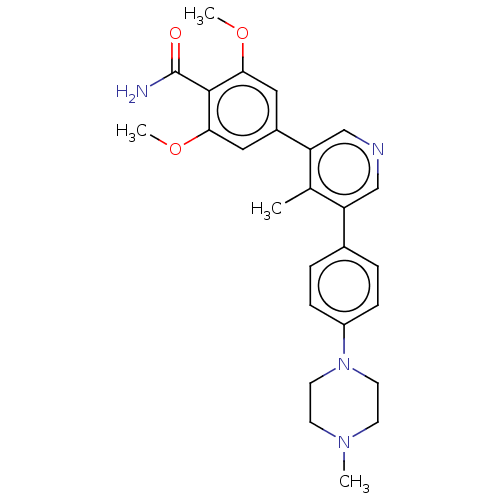
(CHEMBL4435320)Show SMILES COc1cc(cc(OC)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C26H30N4O3/c1-17-21(18-5-7-20(8-6-18)30-11-9-29(2)10-12-30)15-28-16-22(17)19-13-23(32-3)25(26(27)31)24(14-19)33-4/h5-8,13-16H,9-12H2,1-4H3,(H2,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of ALK2 R258G mutant (unknown origin) |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528195

(CHEMBL4435320)Show SMILES COc1cc(cc(OC)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C26H30N4O3/c1-17-21(18-5-7-20(8-6-18)30-11-9-29(2)10-12-30)15-28-16-22(17)19-13-23(32-3)25(26(27)31)24(14-19)33-4/h5-8,13-16H,9-12H2,1-4H3,(H2,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of ALK2 G328V mutant (unknown origin) |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528203
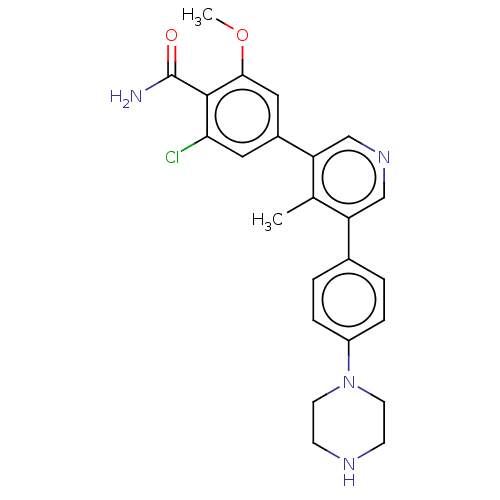
(CHEMBL4434694)Show SMILES COc1cc(cc(Cl)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C24H25ClN4O2/c1-15-19(16-3-5-18(6-4-16)29-9-7-27-8-10-29)13-28-14-20(15)17-11-21(25)23(24(26)30)22(12-17)31-2/h3-6,11-14,27H,7-10H2,1-2H3,(H2,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of ALK2 G328V mutant (unknown origin) |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM621068
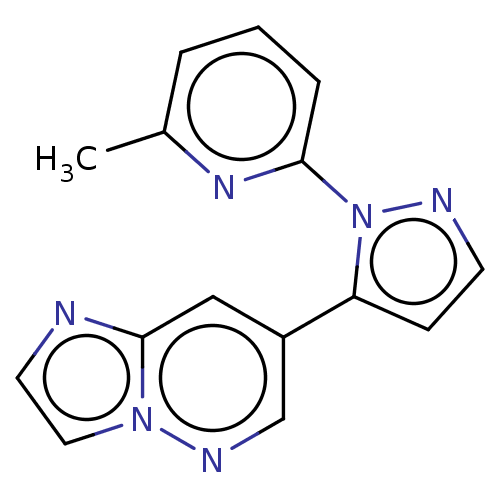
(US20230303562, Example 6) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM518135
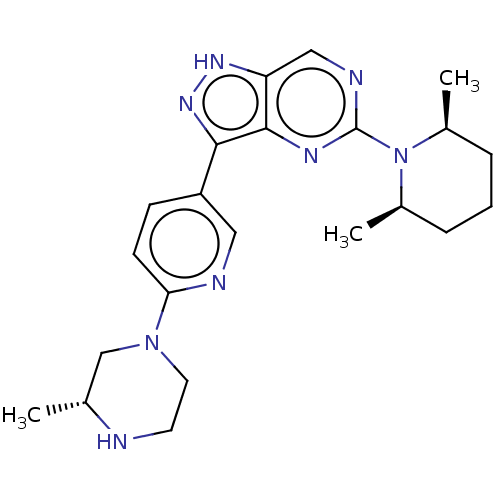
(US11111247, Example 1)Show SMILES C[C@@H]1CN(CCN1)c1ccc(cn1)-c1n[nH]c2cnc(nc12)N1[C@@H](C)CCC[C@H]1C |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00206
BindingDB Entry DOI: 10.7270/Q2R78K72 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50596223
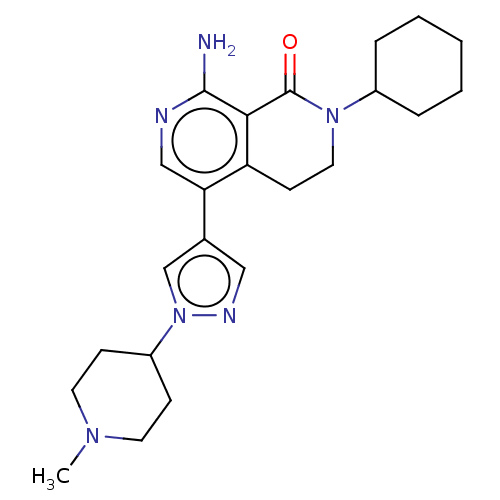
(CHEMBL5200241)Show SMILES CN1CCC(CC1)n1cc(cn1)-c1cnc(N)c2C(=O)N(CCc12)C1CCCCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128452
BindingDB Entry DOI: 10.7270/Q2JS9VGJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM603862

(US11654147, Compound 312 | US11654147, Compound 33...)Show SMILES CN1CCN(CC1)C1CN(C1)c1cnc2c(cnn2c1)-c1cc(C)cc2ccc(F)cc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27085CT |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM603850

(US11654147, Compound 298)Show SMILES CN1CC2(C1)CN(C2)c1cnc2c(cnn2c1)-c1cc(C)nc2ccccc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27085CT |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528190
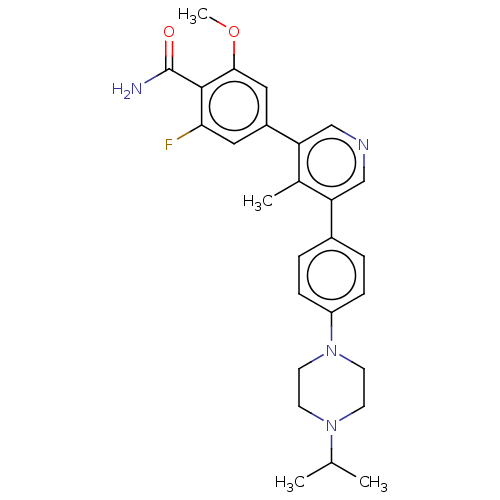
(CHEMBL4548795)Show SMILES COc1cc(cc(F)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCN(CC1)C(C)C Show InChI InChI=1S/C27H31FN4O2/c1-17(2)31-9-11-32(12-10-31)21-7-5-19(6-8-21)22-15-30-16-23(18(22)3)20-13-24(28)26(27(29)33)25(14-20)34-4/h5-8,13-17H,9-12H2,1-4H3,(H2,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of ALK2 G328V mutant (unknown origin) |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528209
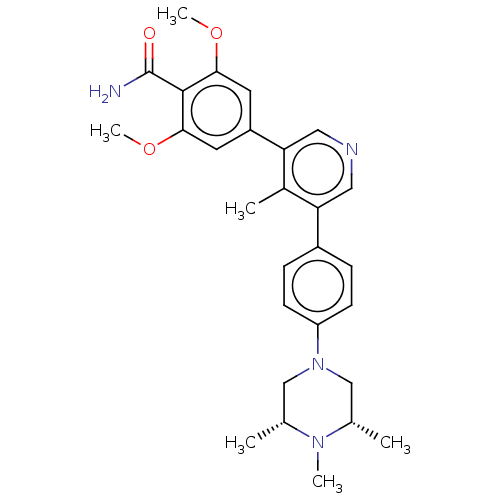
(CHEMBL4526828)Show SMILES COc1cc(cc(OC)c1C(N)=O)-c1cncc(-c2ccc(cc2)N2C[C@H](C)N(C)[C@H](C)C2)c1C |r| Show InChI InChI=1S/C28H34N4O3/c1-17-15-32(16-18(2)31(17)4)22-9-7-20(8-10-22)23-13-30-14-24(19(23)3)21-11-25(34-5)27(28(29)33)26(12-21)35-6/h7-14,17-18H,15-16H2,1-6H3,(H2,29,33)/t17-,18+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50544399
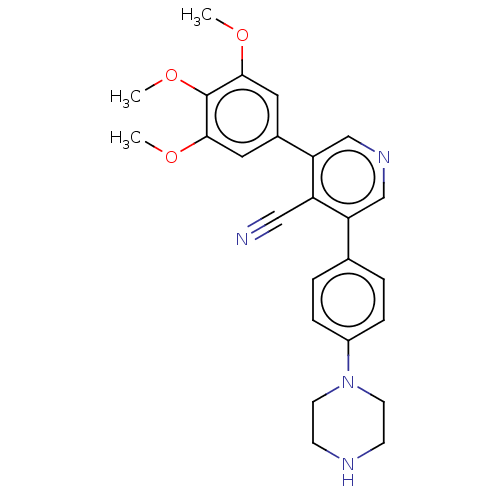
(CHEMBL4638273)Show SMILES COc1cc(cc(OC)c1OC)-c1cncc(-c2ccc(cc2)N2CCNCC2)c1C#N Show InChI InChI=1S/C25H26N4O3/c1-30-23-12-18(13-24(31-2)25(23)32-3)22-16-28-15-21(20(22)14-26)17-4-6-19(7-5-17)29-10-8-27-9-11-29/h4-7,12-13,15-16,27H,8-11H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay |
J Med Chem 63: 10061-10085 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01199
BindingDB Entry DOI: 10.7270/Q2VM4GV3 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528195

(CHEMBL4435320)Show SMILES COc1cc(cc(OC)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C26H30N4O3/c1-17-21(18-5-7-20(8-6-18)30-11-9-29(2)10-12-30)15-28-16-22(17)19-13-23(32-3)25(26(27)31)24(14-19)33-4/h5-8,13-16H,9-12H2,1-4H3,(H2,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528196
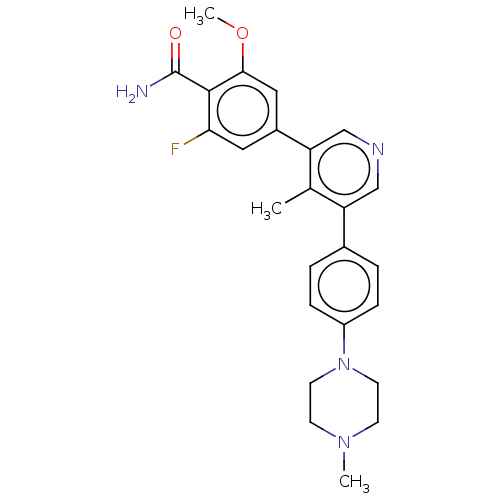
(CHEMBL4575655)Show SMILES COc1cc(cc(F)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C25H27FN4O2/c1-16-20(17-4-6-19(7-5-17)30-10-8-29(2)9-11-30)14-28-15-21(16)18-12-22(26)24(25(27)31)23(13-18)32-3/h4-7,12-15H,8-11H2,1-3H3,(H2,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50596221
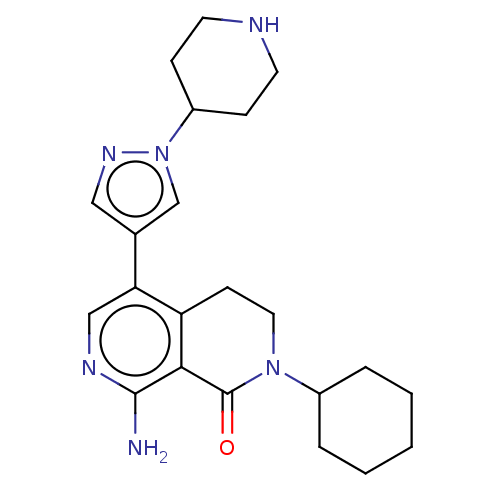
(CHEMBL5186575)Show SMILES Nc1ncc(-c2cnn(c2)C2CCNCC2)c2CCN(C3CCCCC3)C(=O)c12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128452
BindingDB Entry DOI: 10.7270/Q2JS9VGJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50262079
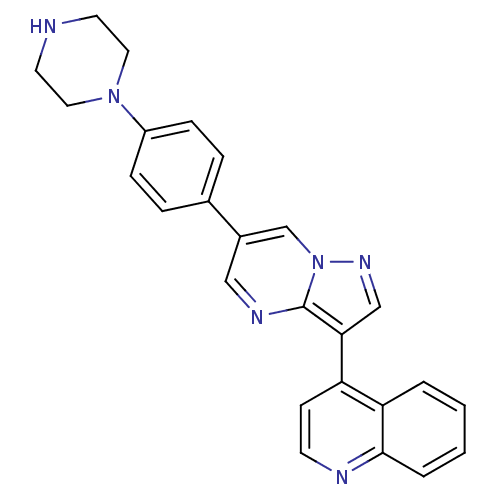
(4-(6-(4-(piperazin-1-yl)phenyl)pyrazolo[1,5-a]pyri...)Show SMILES C1CN(CCN1)c1ccc(cc1)-c1cnc2c(cnn2c1)-c1ccnc2ccccc12 Show InChI InChI=1S/C25H22N6/c1-2-4-24-22(3-1)21(9-10-27-24)23-16-29-31-17-19(15-28-25(23)31)18-5-7-20(8-6-18)30-13-11-26-12-14-30/h1-10,15-17,26H,11-14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of 10 uM ATP by radiometric kinase assay |
J Med Chem 60: 1495-1508 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01679
BindingDB Entry DOI: 10.7270/Q2TQ63S4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50596220
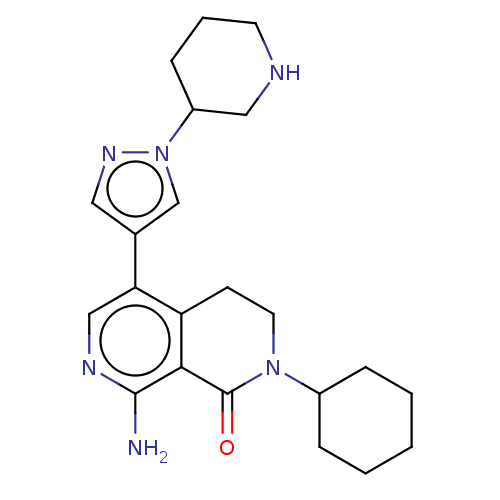
(CHEMBL5205781)Show SMILES Nc1ncc(-c2cnn(c2)C2CCCNC2)c2CCN(C3CCCCC3)C(=O)c12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128452
BindingDB Entry DOI: 10.7270/Q2JS9VGJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM488104
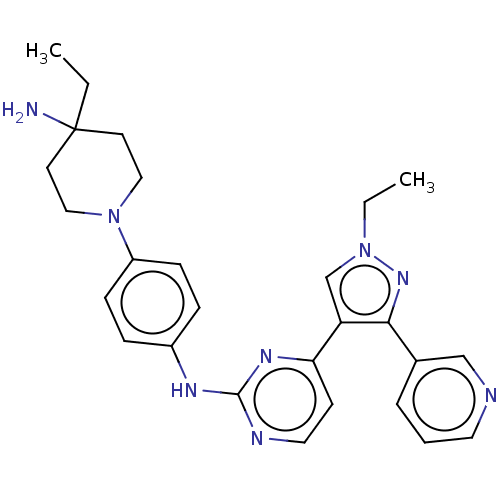
(N-(4-(4-Amino-4- ethylpiperidin-1- yl)phenyl)-4-(1...)Show SMILES CCn1cc(c(n1)-c1cccnc1)-c1ccnc(Nc2ccc(cc2)N2CCC(N)(CC)CC2)n1 Show InChI InChI=1S/C27H32N8/c1-3-27(28)12-16-34(17-13-27)22-9-7-21(8-10-22)31-26-30-15-11-24(32-26)23-19-35(4-2)33-25(23)20-6-5-14-29-18-20/h5-11,14-15,18-19H,3-4,12-13,16-17,28H2,1-2H3,(H,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN; THE UNIVERSITY OF TOKYO
US Patent
| Assay Description
TABLE 5: An overview of evaluation of an inhibitory effect of a compound of the present invention with respect to ALK2 is as follows. In a buffer sol... |
US Patent US10954216 (2021)
BindingDB Entry DOI: 10.7270/Q2P84G0W |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM451816
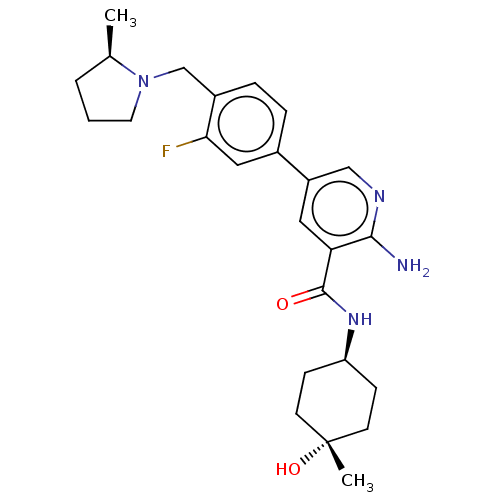
(US10710980, Example 46 | US10947218, Example 46)Show SMILES C[C@@H]1CCCN1Cc1ccc(cc1F)-c1cnc(N)c(c1)C(=O)N[C@H]1CC[C@](C)(O)CC1 |r,wU:24.26,27.30,1.0,wD:27.31,(4.77,-2.71,;6.24,-3.19,;6.71,-4.65,;8.25,-4.65,;8.73,-3.19,;7.48,-2.28,;7.48,-.74,;6.15,.03,;4.81,-.74,;3.48,.03,;3.48,1.57,;4.81,2.34,;6.15,1.57,;7.48,2.34,;2.15,2.34,;2.15,3.88,;.81,4.65,;-.52,3.88,;-1.85,4.65,;-.52,2.34,;.81,1.57,;-1.85,1.57,;-1.85,.03,;-3.19,2.34,;-4.52,1.57,;-4.52,.03,;-5.85,-.74,;-7.19,.03,;-7.96,-1.3,;-8.73,.03,;-7.19,1.57,;-5.85,2.34,)| Show InChI InChI=1S/C25H33FN4O2/c1-16-4-3-11-30(16)15-18-6-5-17(13-22(18)26)19-12-21(23(27)28-14-19)24(31)29-20-7-9-25(2,32)10-8-20/h5-6,12-14,16,20,32H,3-4,7-11,15H2,1-2H3,(H2,27,28)(H,29,31)/t16-,20-,25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128452
BindingDB Entry DOI: 10.7270/Q2JS9VGJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM603851
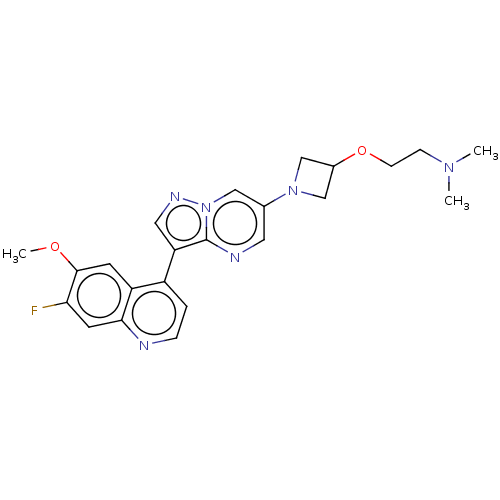
(US11654147, Compound 299 | US11654147, Compound 30...)Show SMILES COc1cc2c(ccnc2cc1F)-c1cnn2cc(cnc12)N1CC(C1)OCCN(C)C | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27085CT |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM4077
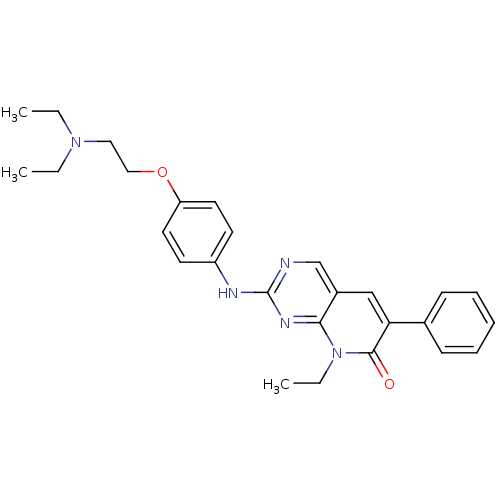
(2-({4-[2-(diethylamino)ethoxy]phenyl}amino)-8-ethy...)Show SMILES CCN(CC)CCOc1ccc(Nc2ncc3cc(-c4ccccc4)c(=O)n(CC)c3n2)cc1 Show InChI InChI=1S/C27H31N5O2/c1-4-31(5-2)16-17-34-23-14-12-22(13-15-23)29-27-28-19-21-18-24(20-10-8-7-9-11-20)26(33)32(6-3)25(21)30-27/h7-15,18-19H,4-6,16-17H2,1-3H3,(H,28,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALK2 using casein as substrate incubated for 30 mins in presence of [gamma33P]ATP by radiometric hotspot kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113252
BindingDB Entry DOI: 10.7270/Q21Z4863 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528195

(CHEMBL4435320)Show SMILES COc1cc(cc(OC)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C26H30N4O3/c1-17-21(18-5-7-20(8-6-18)30-11-9-29(2)10-12-30)15-28-16-22(17)19-13-23(32-3)25(26(27)31)24(14-19)33-4/h5-8,13-16H,9-12H2,1-4H3,(H2,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 R206H mutant using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528203

(CHEMBL4434694)Show SMILES COc1cc(cc(Cl)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C24H25ClN4O2/c1-15-19(16-3-5-18(6-4-16)29-9-7-27-8-10-29)13-28-14-20(15)17-11-21(25)23(24(26)30)22(12-17)31-2/h3-6,11-14,27H,7-10H2,1-2H3,(H2,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of ALK2 R258G mutant (unknown origin) |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50544408
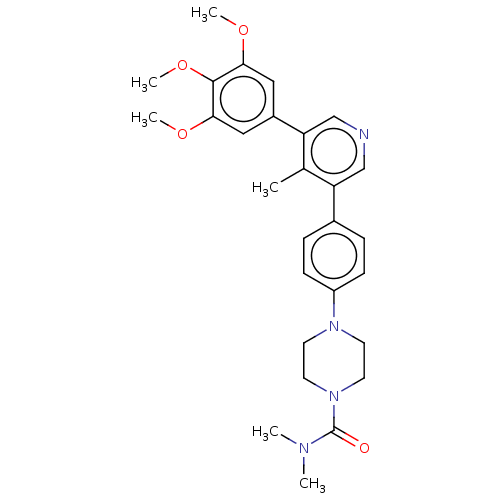
(CHEMBL4641207)Show SMILES COc1cc(cc(OC)c1OC)-c1cncc(-c2ccc(cc2)N2CCN(CC2)C(=O)N(C)C)c1C Show InChI InChI=1S/C28H34N4O4/c1-19-23(17-29-18-24(19)21-15-25(34-4)27(36-6)26(16-21)35-5)20-7-9-22(10-8-20)31-11-13-32(14-12-31)28(33)30(2)3/h7-10,15-18H,11-14H2,1-6H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay |
J Med Chem 63: 10061-10085 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01199
BindingDB Entry DOI: 10.7270/Q2VM4GV3 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528208
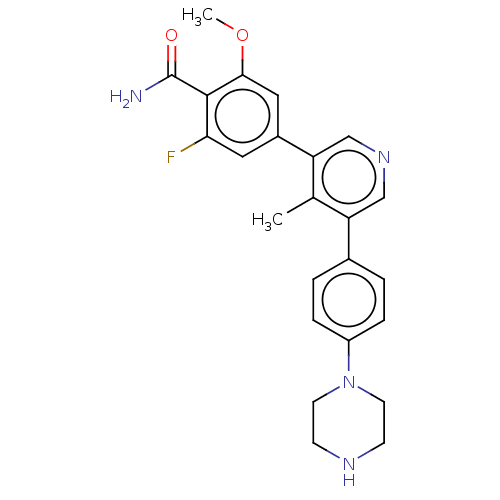
(CHEMBL4440168)Show SMILES COc1cc(cc(F)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C24H25FN4O2/c1-15-19(16-3-5-18(6-4-16)29-9-7-27-8-10-29)13-28-14-20(15)17-11-21(25)23(24(26)30)22(12-17)31-2/h3-6,11-14,27H,7-10H2,1-2H3,(H2,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of ALK2 G328V mutant (unknown origin) |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50596219
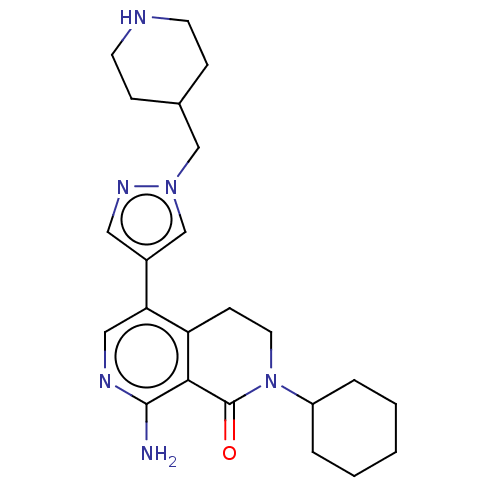
(CHEMBL5189461)Show SMILES Nc1ncc(-c2cnn(CC3CCNCC3)c2)c2CCN(C3CCCCC3)C(=O)c12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128452
BindingDB Entry DOI: 10.7270/Q2JS9VGJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM621072
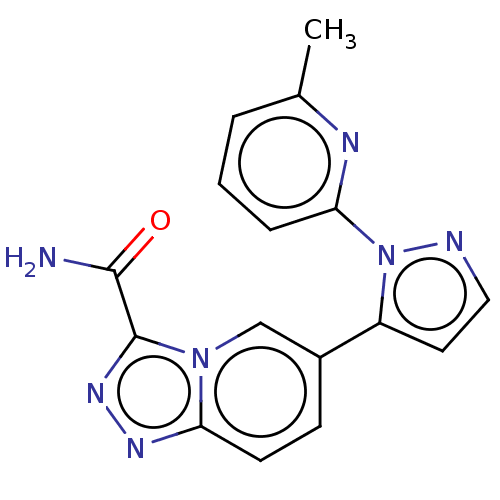
(US20230303562, Example 10) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4.82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM518180
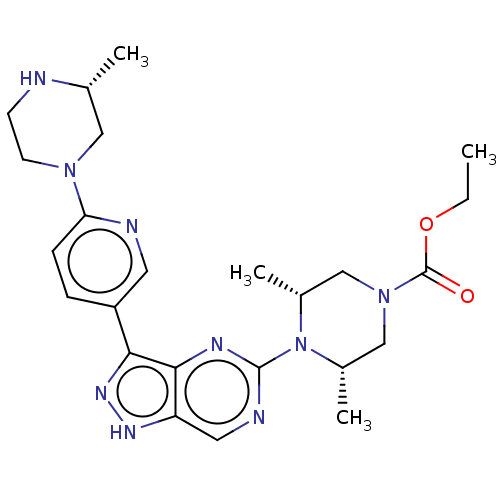
(US11111247, Example 11)Show SMILES CCOC(=O)N1C[C@H](C)N([C@H](C)C1)c1ncc2[nH]nc(-c3ccc(nc3)N3CCN[C@H](C)C3)c2n1 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00206
BindingDB Entry DOI: 10.7270/Q2R78K72 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM603695
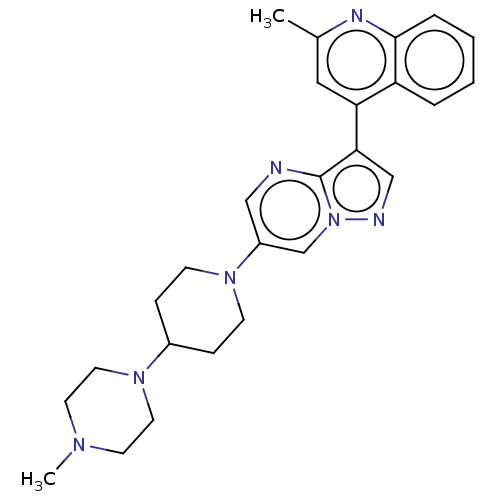
(US11654147, Compound 8)Show SMILES CN1CCN(CC1)C1CCN(CC1)c1cnc2c(cnn2c1)-c1cc(C)nc2ccccc12 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q27085CT |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528203

(CHEMBL4434694)Show SMILES COc1cc(cc(Cl)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C24H25ClN4O2/c1-15-19(16-3-5-18(6-4-16)29-9-7-27-8-10-29)13-28-14-20(15)17-11-21(25)23(24(26)30)22(12-17)31-2/h3-6,11-14,27H,7-10H2,1-2H3,(H2,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 R206H mutant using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528204
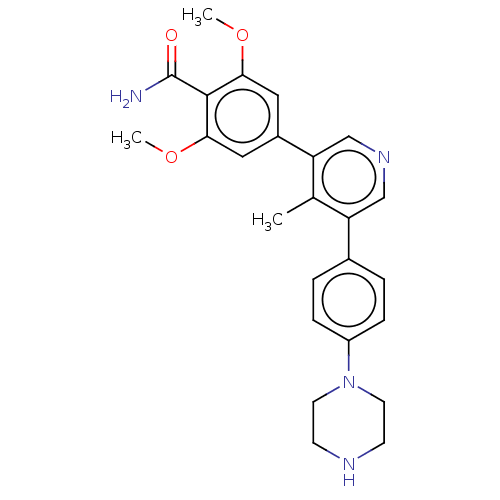
(CHEMBL4565968)Show SMILES COc1cc(cc(OC)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCNCC1 Show InChI InChI=1S/C25H28N4O3/c1-16-20(17-4-6-19(7-5-17)29-10-8-27-9-11-29)14-28-15-21(16)18-12-22(31-2)24(25(26)30)23(13-18)32-3/h4-7,12-15,27H,8-11H2,1-3H3,(H2,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50564975
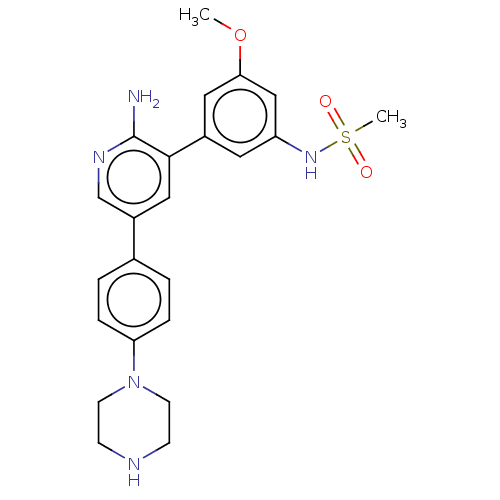
(CHEMBL4784430)Show SMILES COc1cc(NS(C)(=O)=O)cc(c1)-c1cc(cnc1N)-c1ccc(cc1)N1CCNCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112417
BindingDB Entry DOI: 10.7270/Q2M0495P |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50564978
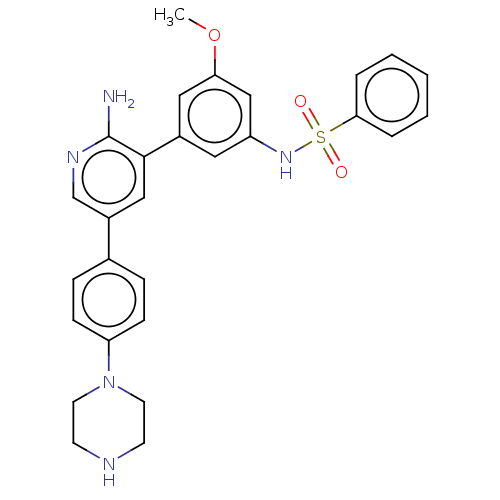
(CHEMBL4776631)Show SMILES COc1cc(NS(=O)(=O)c2ccccc2)cc(c1)-c1cc(cnc1N)-c1ccc(cc1)N1CCNCC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by kinase assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112417
BindingDB Entry DOI: 10.7270/Q2M0495P |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50560477
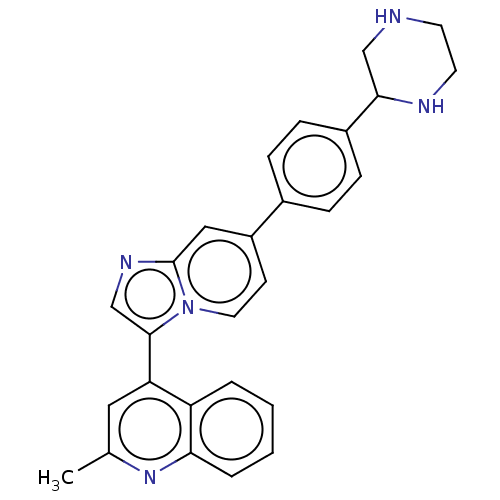
(CHEMBL4742906)Show SMILES Cc1cc(-c2cnc3cc(ccn23)-c2ccc(cc2)C2CNCCN2)c2ccccc2n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALK2 using casein as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127418
BindingDB Entry DOI: 10.7270/Q2WW7NDJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50560478
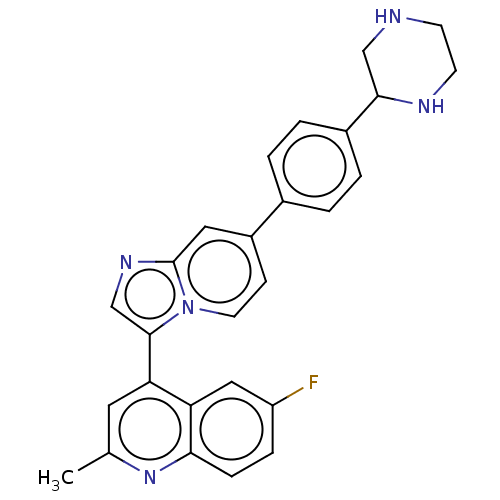
(CHEMBL4790557)Show SMILES Cc1cc(-c2cnc3cc(ccn23)-c2ccc(cc2)C2CNCCN2)c2cc(F)ccc2n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALK2 using casein as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127418
BindingDB Entry DOI: 10.7270/Q2WW7NDJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50560479
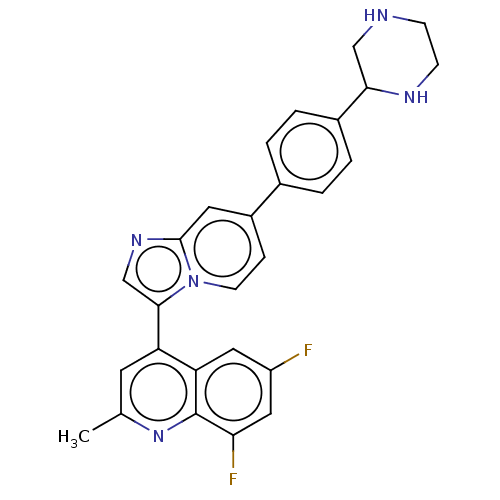
(CHEMBL4778971)Show SMILES Cc1cc(-c2cnc3cc(ccn23)-c2ccc(cc2)C2CNCCN2)c2cc(F)cc(F)c2n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALK2 using casein as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127418
BindingDB Entry DOI: 10.7270/Q2WW7NDJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50560480
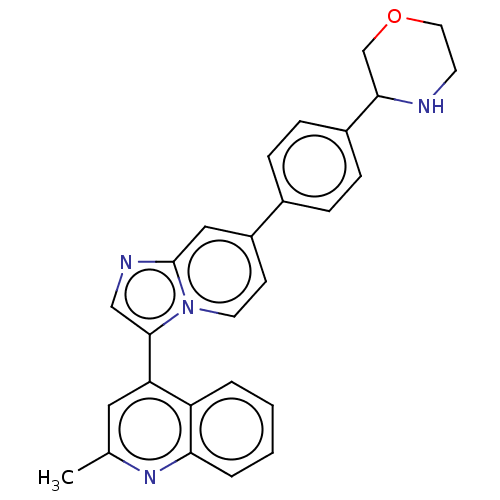
(CHEMBL4779300)Show SMILES Cc1cc(-c2cnc3cc(ccn23)-c2ccc(cc2)C2COCCN2)c2ccccc2n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALK2 using casein as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127418
BindingDB Entry DOI: 10.7270/Q2WW7NDJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50560481
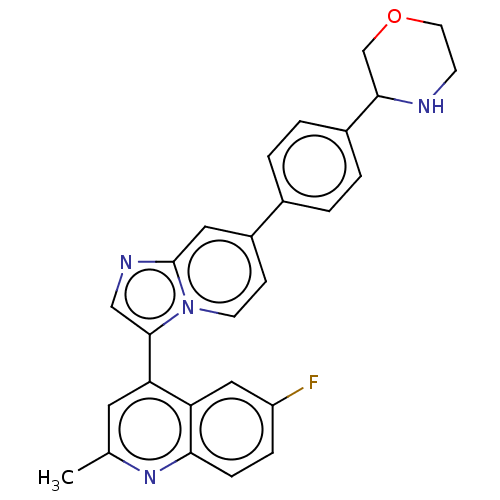
(CHEMBL4746124)Show SMILES Cc1cc(-c2cnc3cc(ccn23)-c2ccc(cc2)C2COCCN2)c2cc(F)ccc2n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ALK2 using casein as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127418
BindingDB Entry DOI: 10.7270/Q2WW7NDJ |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50544414
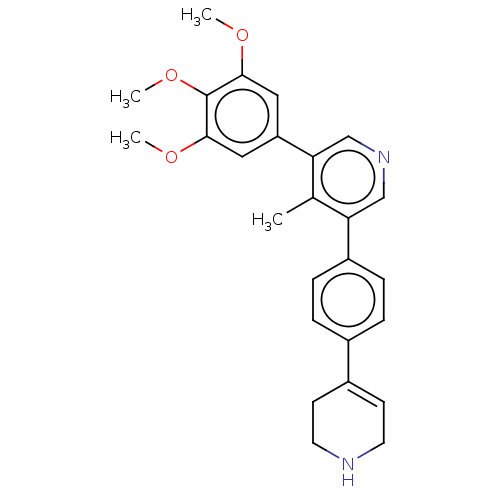
(CHEMBL4641530)Show SMILES COc1cc(cc(OC)c1OC)-c1cncc(c1C)-c1ccc(cc1)C1=CCNCC1 |t:28| Show InChI InChI=1S/C26H28N2O3/c1-17-22(20-7-5-18(6-8-20)19-9-11-27-12-10-19)15-28-16-23(17)21-13-24(29-2)26(31-4)25(14-21)30-3/h5-9,13-16,27H,10-12H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ontario Institute for Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of [gamma-33P]-ATP by radiometric hotspot assay |
J Med Chem 63: 10061-10085 (2020)
Article DOI: 10.1021/acs.jmedchem.0c01199
BindingDB Entry DOI: 10.7270/Q2VM4GV3 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528190

(CHEMBL4548795)Show SMILES COc1cc(cc(F)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCN(CC1)C(C)C Show InChI InChI=1S/C27H31FN4O2/c1-17(2)31-9-11-32(12-10-31)21-7-5-19(6-8-21)22-15-30-16-23(18(22)3)20-13-24(28)26(27(29)33)25(14-20)34-4/h5-8,13-17H,9-12H2,1-4H3,(H2,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |
Activin receptor type-1
(Homo sapiens (Human)) | BDBM50528205
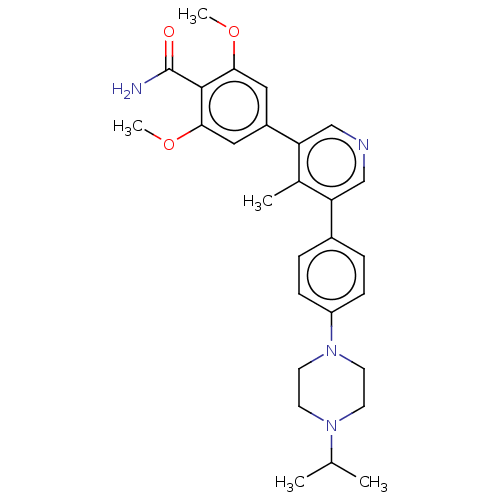
(CHEMBL4468389)Show SMILES COc1cc(cc(OC)c1C(N)=O)-c1cncc(-c2ccc(cc2)N2CCN(CC2)C(C)C)c1C Show InChI InChI=1S/C28H34N4O3/c1-18(2)31-10-12-32(13-11-31)22-8-6-20(7-9-22)23-16-30-17-24(19(23)3)21-14-25(34-4)27(28(29)33)26(15-21)35-5/h6-9,14-18H,10-13H2,1-5H3,(H2,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by ChEMBL
| Assay Description
Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay |
J Med Chem 63: 4978-4996 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00395
BindingDB Entry DOI: 10.7270/Q2MK6HB1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data