

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68208 (Type II inhibitor, 2) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68217 (Type I progenitor, 11) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68217 (Type I progenitor, 11) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM50242740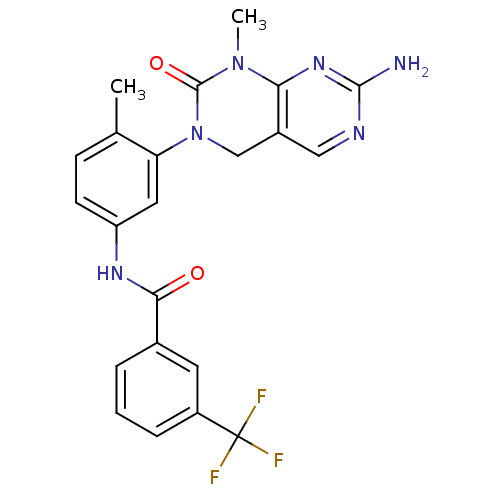 (CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68219 (Type II inhibitor, 13) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM50242740 (CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68218 (Type II inhibitor, 12) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM6568 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM6568 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68218 (Type II inhibitor, 12) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27.2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68219 (Type II inhibitor, 13) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68208 (Type II inhibitor, 2) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68214 (Type II inhibitor, 8) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68211 (Type II inhibitor, 5) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 374 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68209 (Type II inhibitor, 3) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68207 (Type I progenitor, 1) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68213 (Type I progenitor, 7) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68210 (Type I progenitor, 4) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68216 (Type II inhibitor, 10) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68212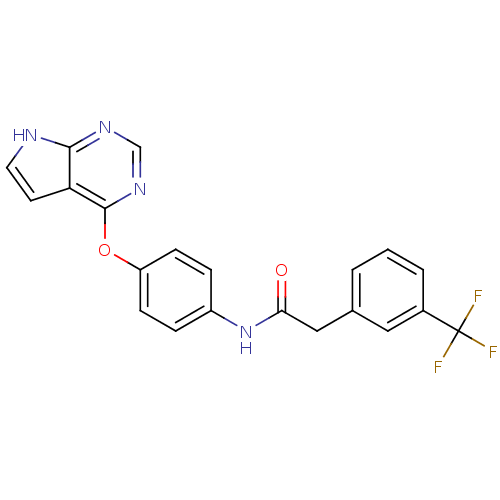 (Type II inhibitor, 6) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68211 (Type II inhibitor, 5) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68210 (Type I progenitor, 4) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68209 (Type II inhibitor, 3) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68220 (Type II inhibitor, 15) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68213 (Type I progenitor, 7) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68207 (Type I progenitor, 1) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68212 (Type II inhibitor, 6) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68214 (Type II inhibitor, 8) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68216 (Type II inhibitor, 10) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM68220 (Type II inhibitor, 15) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [1-999,Q252H] (Homo sapiens (Human)) | BDBM13530 (4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute | Assay Description An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. | Chem Biol 13: 779-86 (2006) Article DOI: 10.1016/j.chembiol.2006.05.015 BindingDB Entry DOI: 10.7270/Q2JM2836 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||