 Found 73 hits of ic50 data for polymerid = 50000853,50004670
Found 73 hits of ic50 data for polymerid = 50000853,50004670 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50469238
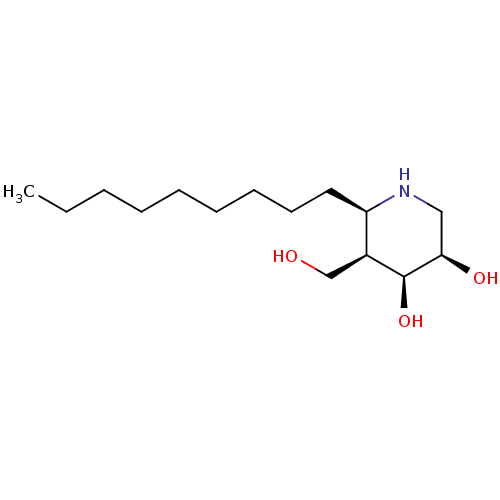
(CHEMBL4288931)Show SMILES CCCCCCCCC[C@H]1NC[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C15H31NO3/c1-2-3-4-5-6-7-8-9-13-12(11-17)15(19)14(18)10-16-13/h12-19H,2-11H2,1H3/t12-,13+,14+,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS
Curated by ChEMBL
| Assay Description
Inhibition of beta-galactosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-galactopyranoside as s... |
Bioorg Med Chem 26: 5462-5469 (2018)
Article DOI: 10.1016/j.bmc.2018.09.023
BindingDB Entry DOI: 10.7270/Q2G73HFJ |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50235812
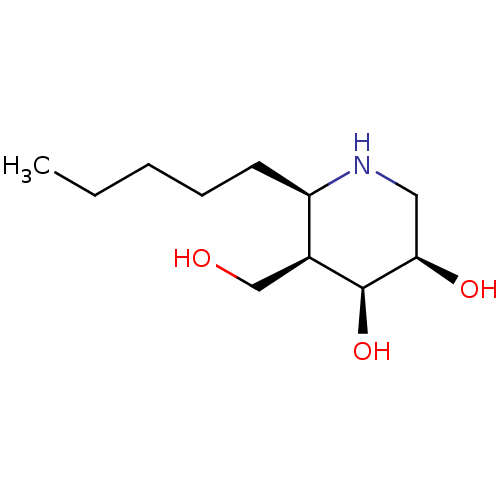
(CHEMBL4085739)Show SMILES CCCCC[C@H]1NC[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23NO3/c1-2-3-4-5-9-8(7-13)11(15)10(14)6-12-9/h8-15H,2-7H2,1H3/t8-,9+,10+,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Eur J Med Chem 126: 160-170 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.095
BindingDB Entry DOI: 10.7270/Q21G0PHR |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50469239
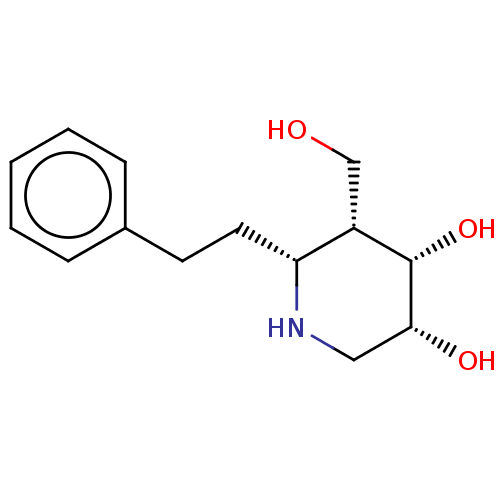
(CHEMBL4284436)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)CN[C@@H]1CCc1ccccc1 |r| Show InChI InChI=1S/C14H21NO3/c16-9-11-12(15-8-13(17)14(11)18)7-6-10-4-2-1-3-5-10/h1-5,11-18H,6-9H2/t11-,12+,13+,14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS
Curated by ChEMBL
| Assay Description
Inhibition of beta-galactosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-galactopyranoside as s... |
Bioorg Med Chem 26: 5462-5469 (2018)
Article DOI: 10.1016/j.bmc.2018.09.023
BindingDB Entry DOI: 10.7270/Q2G73HFJ |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50235812

(CHEMBL4085739)Show SMILES CCCCC[C@H]1NC[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23NO3/c1-2-3-4-5-9-8(7-13)11(15)10(14)6-12-9/h8-15H,2-7H2,1H3/t8-,9+,10+,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS
Curated by ChEMBL
| Assay Description
Inhibition of beta-galactosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-galactopyranoside as s... |
Bioorg Med Chem 26: 5462-5469 (2018)
Article DOI: 10.1016/j.bmc.2018.09.023
BindingDB Entry DOI: 10.7270/Q2G73HFJ |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50358321
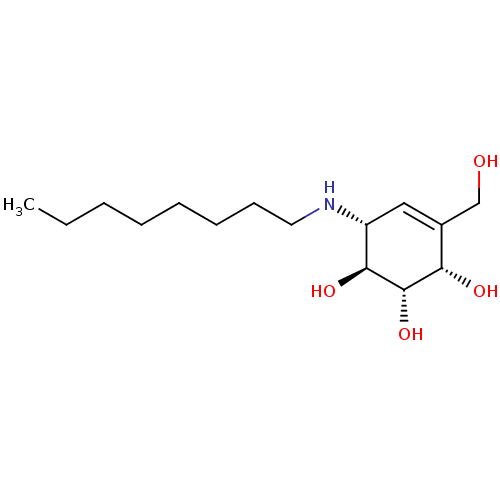
(CHEMBL1922579 | CHEMBL1922581)Show SMILES CCCCCCCCN[C@@H]1C=C(CO)[C@H](O)[C@H](O)[C@H]1O |r,t:10| Show InChI InChI=1S/C15H29NO4/c1-2-3-4-5-6-7-8-16-12-9-11(10-17)13(18)15(20)14(12)19/h9,12-20H,2-8,10H2,1H3/t12-,13+,14+,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human lysosomal beta-galactosidase using 4-MU beta-gal as substrate incubated for 96 hrs by fluorescence assay |
Bioorg Med Chem Lett 26: 1438-42 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.059
BindingDB Entry DOI: 10.7270/Q2HQ41SR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50235812

(CHEMBL4085739)Show SMILES CCCCC[C@H]1NC[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23NO3/c1-2-3-4-5-9-8(7-13)11(15)10(14)6-12-9/h8-15H,2-7H2,1H3/t8-,9+,10+,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Eur J Med Chem 126: 160-170 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.095
BindingDB Entry DOI: 10.7270/Q21G0PHR |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50150470
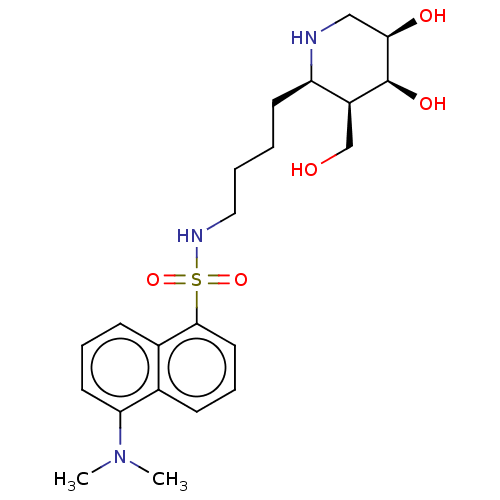
(CHEMBL3771185)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCC[C@H]1NC[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C22H33N3O5S/c1-25(2)19-10-5-8-16-15(19)7-6-11-21(16)31(29,30)24-12-4-3-9-18-17(14-26)22(28)20(27)13-23-18/h5-8,10-11,17-18,20,22-24,26-28H,3-4,9,12-14H2,1-2H3/t17-,18+,20+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human lysosomal beta-galactosidase using 4-MU beta-gal as substrate incubated for 96 hrs by fluorescence assay |
Bioorg Med Chem Lett 26: 1438-42 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.059
BindingDB Entry DOI: 10.7270/Q2HQ41SR |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50350758
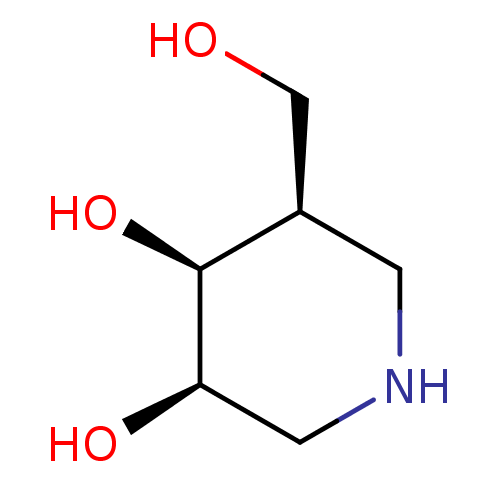
(CHEMBL1818433)Show InChI InChI=1S/C6H13NO3/c8-3-4-1-7-2-5(9)6(4)10/h4-10H,1-3H2/t4-,5-,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Eur J Med Chem 126: 160-170 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.095
BindingDB Entry DOI: 10.7270/Q21G0PHR |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50469237
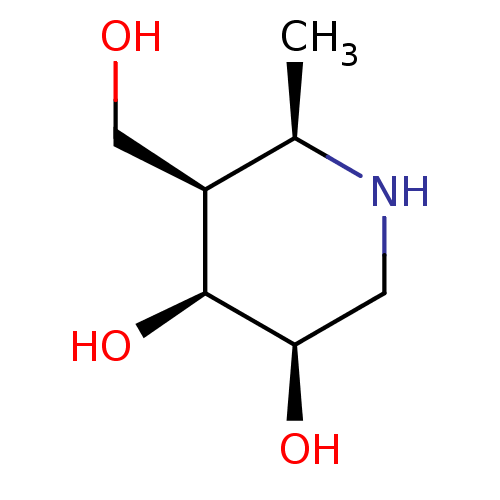
(CHEMBL4281031)Show InChI InChI=1S/C7H15NO3/c1-4-5(3-9)7(11)6(10)2-8-4/h4-11H,2-3H2,1H3/t4-,5+,6-,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS
Curated by ChEMBL
| Assay Description
Inhibition of beta-galactosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-galactopyranoside as s... |
Bioorg Med Chem 26: 5462-5469 (2018)
Article DOI: 10.1016/j.bmc.2018.09.023
BindingDB Entry DOI: 10.7270/Q2G73HFJ |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50150471
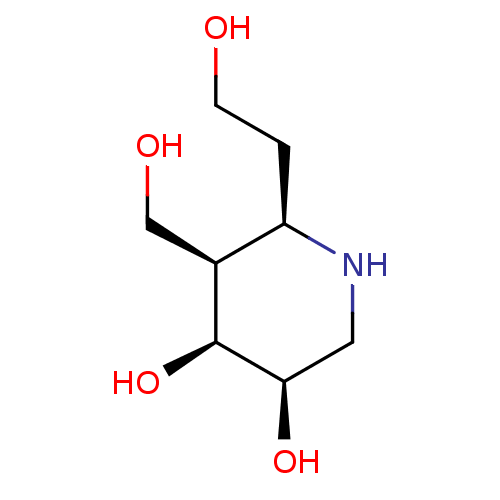
(CHEMBL3770764)Show InChI InChI=1S/C8H17NO4/c10-2-1-6-5(4-11)8(13)7(12)3-9-6/h5-13H,1-4H2/t5-,6+,7+,8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Graz University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human lysosomal beta-galactosidase using 4-MU beta-gal as substrate incubated for 96 hrs by fluorescence assay |
Bioorg Med Chem Lett 26: 1438-42 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.059
BindingDB Entry DOI: 10.7270/Q2HQ41SR |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50520992

(CHEMBL4438366)Show SMILES CCCC[C@@H]1C[C@@H](O)[C@@H](O)[C@@H](O)CN1 |r| Show InChI InChI=1S/C10H21NO3/c1-2-3-4-7-5-8(12)10(14)9(13)6-11-7/h7-14H,2-6H2,1H3/t7-,8-,9+,10-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of beta-galactosidase (unknown origin) |
Eur J Med Chem 162: 465-494 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.031
BindingDB Entry DOI: 10.7270/Q2154MGW |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50246569
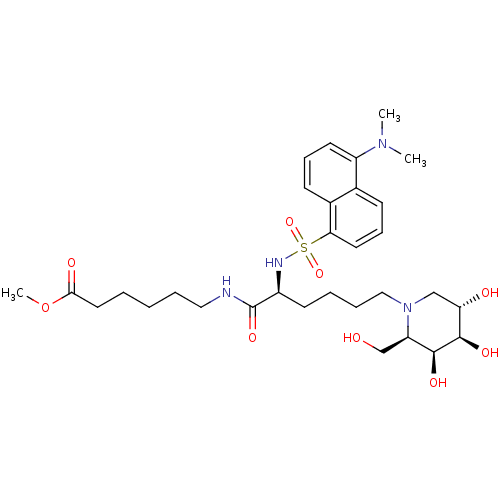
(CHEMBL505422 | Methyl 6-[N2-dansyl-N6-(1,5-dideoxy...)Show SMILES COC(=O)CCCCCNC(=O)[C@H](CCCCN1C[C@H](O)[C@@H](O)[C@@H](O)[C@H]1CO)NS(=O)(=O)c1cccc2c(cccc12)N(C)C |r| Show InChI InChI=1S/C31H48N4O9S/c1-34(2)24-14-9-12-22-21(24)11-10-15-27(22)45(42,43)33-23(31(41)32-17-7-4-5-16-28(38)44-3)13-6-8-18-35-19-26(37)30(40)29(39)25(35)20-36/h9-12,14-15,23,25-26,29-30,33,36-37,39-40H,4-8,13,16-20H2,1-3H3,(H,32,41)/t23-,25+,26-,29-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universität Graz
Curated by ChEMBL
| Assay Description
Inhibition of human lysosomal beta galactosidase |
Bioorg Med Chem 16: 10216-20 (2008)
Article DOI: 10.1016/j.bmc.2008.10.054
BindingDB Entry DOI: 10.7270/Q2DZ085J |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50075942
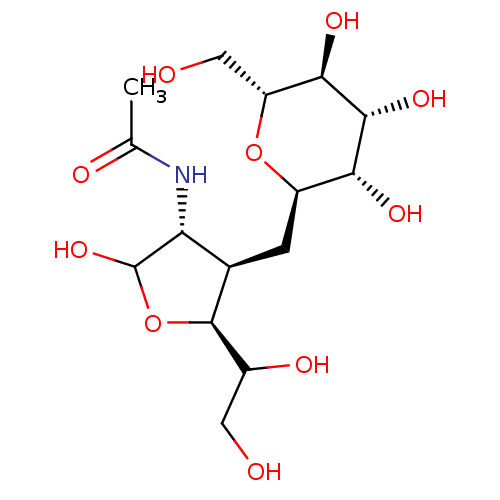
(CHEMBL165192 | N-[2,5-Dihydroxy-6-hydroxymethyl-4-...)Show SMILES CC(=O)N[C@H]1C(O)O[C@H](C(O)CO)[C@@H]1C[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O Show InChI InChI=1S/C15H27NO10/c1-5(19)16-10-6(14(7(20)3-17)26-15(10)24)2-8-11(21)13(23)12(22)9(4-18)25-8/h6-15,17-18,20-24H,2-4H2,1H3,(H,16,19)/t6-,7?,8-,9-,10-,11-,12-,13-,14+,15?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de chimie organique de l'Université de Lausanne
Curated by ChEMBL
| Assay Description
Concentration required to inhibit beta-galactosidase enzyme by 50% from jack bean was reported |
Bioorg Med Chem Lett 9: 793-6 (1999)
BindingDB Entry DOI: 10.7270/Q2K073FM |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50182801
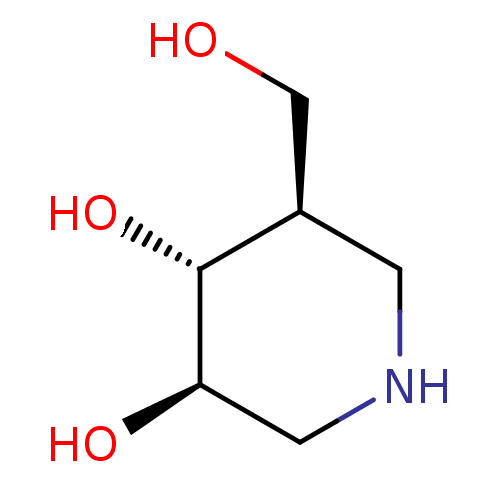
((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...)Show InChI InChI=1S/C6H13NO3/c8-3-4-1-7-2-5(9)6(4)10/h4-10H,1-3H2/t4-,5-,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS
Curated by ChEMBL
| Assay Description
Inhibition of beta-galactosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-galactopyranoside as s... |
Bioorg Med Chem 26: 5462-5469 (2018)
Article DOI: 10.1016/j.bmc.2018.09.023
BindingDB Entry DOI: 10.7270/Q2G73HFJ |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50403936
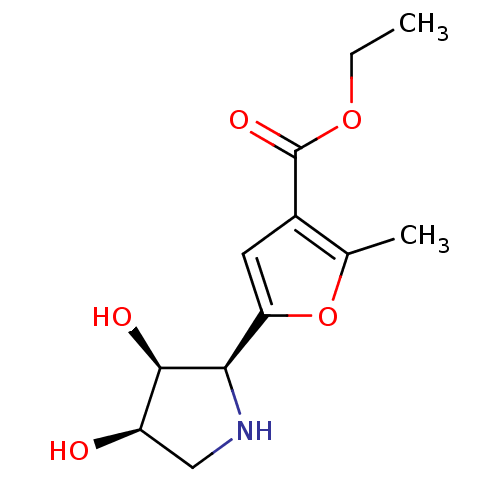
(CHEMBL2114148)Show SMILES CCOC(=O)c1cc(oc1C)[C@H]1NC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H17NO5/c1-3-17-12(16)7-4-9(18-6(7)2)10-11(15)8(14)5-13-10/h4,8,10-11,13-15H,3,5H2,1-2H3/t8-,10-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla
Curated by ChEMBL
| Assay Description
Inhibition constant (Competitive) of the compound against alpha-l-Fucosidase from Bovine epididymis |
Bioorg Med Chem Lett 12: 2335-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GB23CB |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50403936

(CHEMBL2114148)Show SMILES CCOC(=O)c1cc(oc1C)[C@H]1NC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C12H17NO5/c1-3-17-12(16)7-4-9(18-6(7)2)10-11(15)8(14)5-13-10/h4,8,10-11,13-15H,3,5H2,1-2H3/t8-,10-,11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla
Curated by ChEMBL
| Assay Description
Inhibitor concentration of compound against beta-Galactosidase from Jack beans |
Bioorg Med Chem Lett 12: 2335-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GB23CB |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50350758

(CHEMBL1818433)Show InChI InChI=1S/C6H13NO3/c8-3-4-1-7-2-5(9)6(4)10/h4-10H,1-3H2/t4-,5-,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Eur J Med Chem 126: 160-170 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.095
BindingDB Entry DOI: 10.7270/Q21G0PHR |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50235815
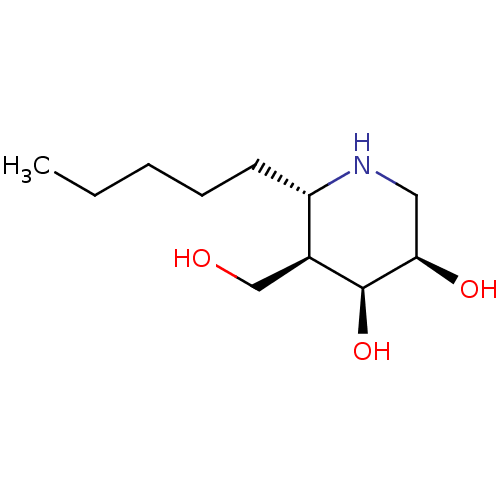
(CHEMBL4099495)Show SMILES CCCCC[C@@H]1NC[C@@H](O)[C@@H](O)[C@H]1CO |r| Show InChI InChI=1S/C11H23NO3/c1-2-3-4-5-9-8(7-13)11(15)10(14)6-12-9/h8-15H,2-7H2,1H3/t8-,9-,10+,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Eur J Med Chem 126: 160-170 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.095
BindingDB Entry DOI: 10.7270/Q21G0PHR |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50111607

(3,4-Dichloro-N-[5-(5-chloro-benzothiazol-2-ylsulfa...)Show SMILES Clc1ccc2sc(Sc3nnc(NC(=O)c4ccc(Cl)c(Cl)c4)s3)nc2c1 Show InChI InChI=1S/C16H7Cl3N4OS3/c17-8-2-4-12-11(6-8)20-15(25-12)27-16-23-22-14(26-16)21-13(24)7-1-3-9(18)10(19)5-7/h1-6H,(H,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50403937
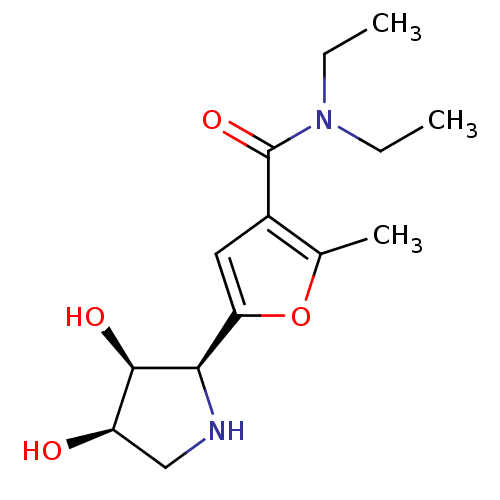
(CHEMBL2114149)Show SMILES CCN(CC)C(=O)c1cc(oc1C)[C@H]1NC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H22N2O4/c1-4-16(5-2)14(19)9-6-11(20-8(9)3)12-13(18)10(17)7-15-12/h6,10,12-13,15,17-18H,4-5,7H2,1-3H3/t10-,12-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla
Curated by ChEMBL
| Assay Description
Inhibitor concentration of the compound against Beta-galactosidase from Aspergillus niger |
Bioorg Med Chem Lett 12: 2335-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GB23CB |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50299749

((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:19.20.25:26,THB:21:20:26:27.22.23,23:22:19:25.24.26,23:24:19:27.21.22| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20-,21-,22?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50182798
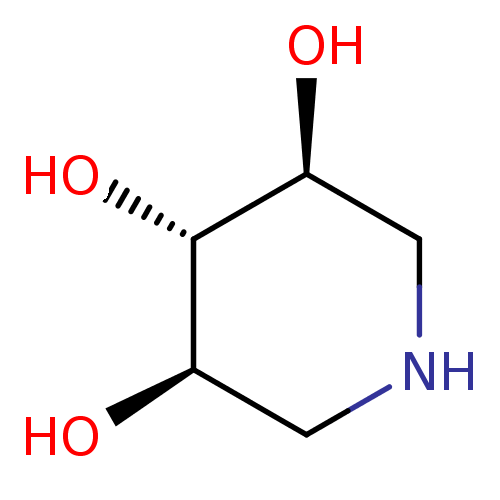
((3R,4r,5S)-piperidine-3,4,5-triol | 1,5-Dideoxy-1,...)Show InChI InChI=1S/C5H11NO3/c7-3-1-6-2-4(8)5(3)9/h3-9H,1-2H2/t3-,4+,5+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-galactosidase from aspergillus oryzae (sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50288855

(1-Butyl-piperidine-3,4,5-triol | CHEMBL152232)Show InChI InChI=1S/C9H19NO3/c1-2-3-4-10-5-7(11)9(13)8(12)6-10/h7-9,11-13H,2-6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of beta-galactosidase from aspergillus oryzae (sigma G 7256). |
Bioorg Med Chem Lett 6: 553-558 (1996)
Article DOI: 10.1016/0960-894X(96)00068-6
BindingDB Entry DOI: 10.7270/Q2ZS2WHG |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50065297

(3-(4-Isopropyl-benzylidene)-1,3-dihydro-indol-2-on...)Show InChI InChI=1S/C18H17NO/c1-12(2)14-9-7-13(8-10-14)11-16-15-5-3-4-6-17(15)19-18(16)20/h3-12H,1-2H3,(H,19,20)/b16-11+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50403937

(CHEMBL2114149)Show SMILES CCN(CC)C(=O)c1cc(oc1C)[C@H]1NC[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C14H22N2O4/c1-4-16(5-2)14(19)9-6-11(20-8(9)3)12-13(18)10(17)7-15-12/h6,10,12-13,15,17-18H,4-5,7H2,1-3H3/t10-,12-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla
Curated by ChEMBL
| Assay Description
Inhibitor concentration of the compound against Beta-galactosidase from Aspergillus niger was tested at a dose of 1 mM |
Bioorg Med Chem Lett 12: 2335-9 (2002)
BindingDB Entry DOI: 10.7270/Q2GB23CB |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50111606
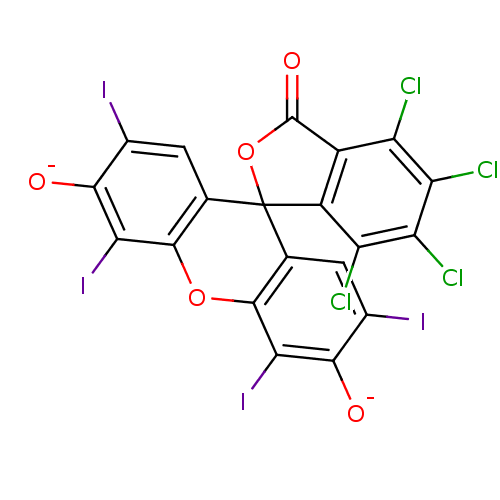
(4,5,6,7-tetrachloro-2',4',5',7'-tetraiodo-3-oxospi...)Show SMILES [O-]c1c(I)cc2c(Oc3c(I)c([O-])c(I)cc3C22OC(=O)c3c2c(Cl)c(Cl)c(Cl)c3Cl)c1I Show InChI InChI=1S/C20H4Cl4I4O5/c21-9-7-8(10(22)12(24)11(9)23)20(33-19(7)31)3-1-5(25)15(29)13(27)17(3)32-18-4(20)2-6(26)16(30)14(18)28/h1-2,29-30H/p-2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50111585

(4-(4-Bromo-phenylazo)-phenol | 4-bromophenylazophe...)Show InChI InChI=1S/C12H9BrN2O/c13-9-1-3-10(4-2-9)14-15-11-5-7-12(16)8-6-11/h1-8,16H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50163440
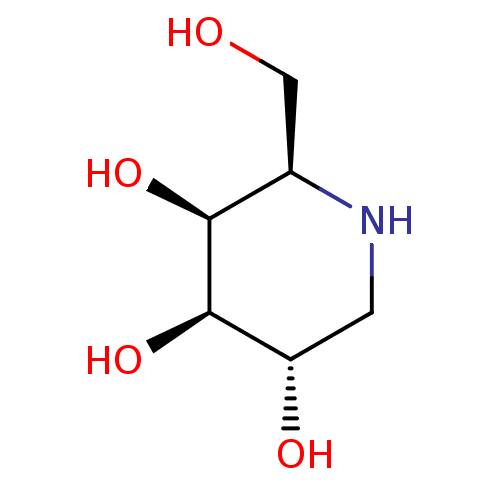
((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5+,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human beta-galactosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50079267
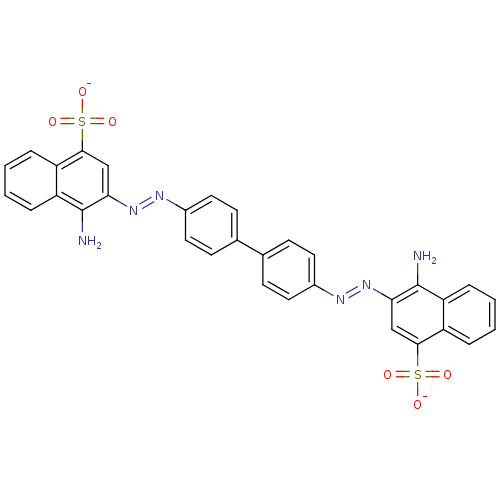
(Congo Red | Direct red 28 | Kongorot | Sodium diph...)Show SMILES Nc1c(cc(c2ccccc12)S([O-])(=O)=O)\N=N\c1ccc(cc1)-c1ccc(cc1)\N=N\c1cc(c2ccccc2c1N)S([O-])(=O)=O Show InChI InChI=1S/C32H24N6O6S2/c33-31-25-7-3-1-5-23(25)29(45(39,40)41)17-27(31)37-35-21-13-9-19(10-14-21)20-11-15-22(16-12-20)36-38-28-18-30(46(42,43)44)24-6-2-4-8-26(24)32(28)34/h1-18H,33-34H2,(H,39,40,41)(H,42,43,44)/p-2/b37-35+,38-36+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335394
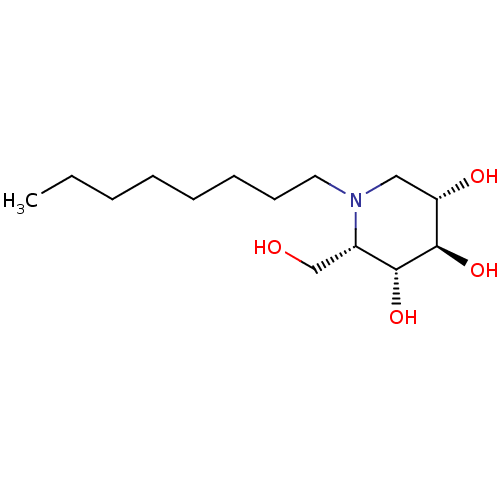
(CHEMBL1651626 | N-Octyl-L-ido-1-deoxynojirimycin)Show SMILES CCCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@@H]1CO |r| Show InChI InChI=1S/C14H29NO4/c1-2-3-4-5-6-7-8-15-9-12(17)14(19)13(18)11(15)10-16/h11-14,16-19H,2-10H2,1H3/t11-,12-,13+,14+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50104296

(2-(2,2-Dihydroxy-ethyl)-pyrrolidine-3,4-diol | CHE...)Show InChI InChI=1S/C6H13NO4/c8-4-2-7-3(6(4)11)1-5(9)10/h3-11H,1-2H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Section de Chimie de l'Universit£ de Lausanne
Curated by ChEMBL
| Assay Description
Inhibitory activity towards Beta-galactosidase from Jack bean |
Bioorg Med Chem Lett 11: 2489-93 (2001)
BindingDB Entry DOI: 10.7270/Q2H70GBZ |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50073992
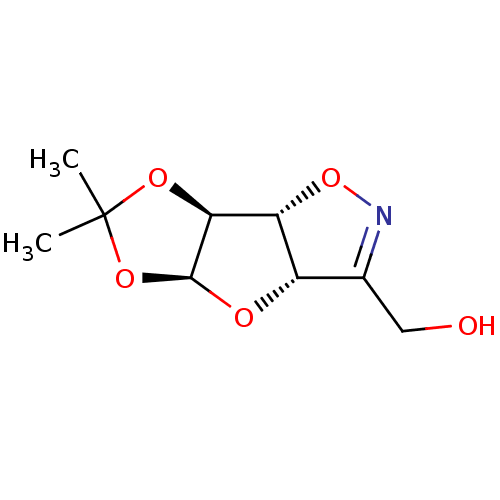
(((3aS,4aS,7aS,7bR)-6,6-Dimethyl-3a,4a,7a,7b-tetrah...)Show SMILES CC1(C)O[C@@H]2O[C@@H]3[C@@H](ON=C3CO)[C@@H]2O1 |c:9| Show InChI InChI=1S/C9H13NO5/c1-9(2)13-7-6-5(12-8(7)14-9)4(3-11)10-15-6/h5-8,11H,3H2,1-2H3/t5-,6+,7-,8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de chimie organique de l'Université de Lausanne
Curated by ChEMBL
| Assay Description
Inhibition of beta-galactosidase from Aspergillus orizae |
Bioorg Med Chem Lett 9: 277-8 (1999)
BindingDB Entry DOI: 10.7270/Q28051T5 |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50369776
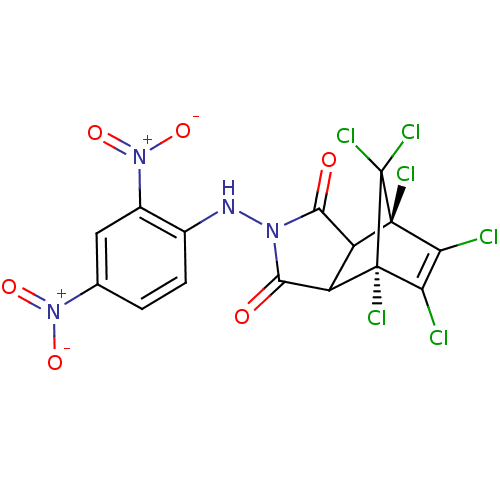
(CHEMBL298301)Show SMILES [O-][N+](=O)c1ccc(NN2C(=O)C3C(C2=O)[C@@]2(Cl)C(Cl)=C(Cl)[C@]3(Cl)C2(Cl)Cl)c(c1)[N+]([O-])=O |t:19,TLB:9:11:23:19.17,20:19:23:11.12,THB:13:12:23:19.17,18:17:23:11.12| Show InChI InChI=1S/C15H6Cl6N4O6/c16-9-10(17)14(19)8-7(13(9,18)15(14,20)21)11(26)23(12(8)27)22-5-2-1-4(24(28)29)3-6(5)25(30)31/h1-3,7-8,22H/t7?,8?,13-,14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50235814
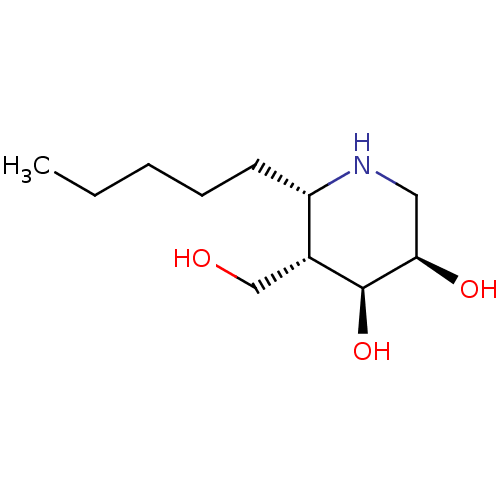
(CHEMBL4081472)Show SMILES CCCCC[C@@H]1NC[C@@H](O)[C@@H](O)[C@@H]1CO |r| Show InChI InChI=1S/C11H23NO3/c1-2-3-4-5-9-8(7-13)11(15)10(14)6-12-9/h8-15H,2-7H2,1H3/t8-,9+,10-,11+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS
Curated by ChEMBL
| Assay Description
Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E |
Eur J Med Chem 126: 160-170 (2017)
Article DOI: 10.1016/j.ejmech.2016.09.095
BindingDB Entry DOI: 10.7270/Q21G0PHR |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50111596
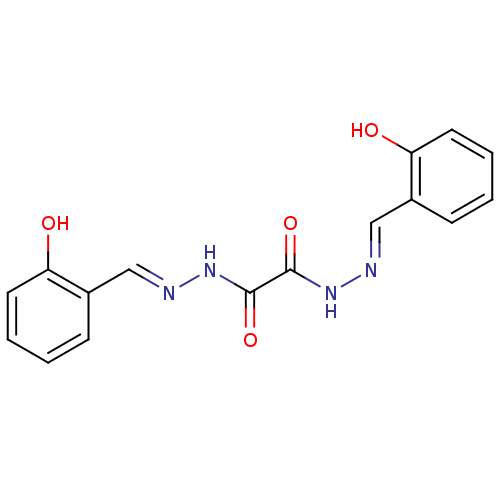
(CHEMBL46666 | N'1,N'2-bis(2-hydroxybenzylidene)oxa...)Show InChI InChI=1S/C16H14N4O4/c21-13-7-3-1-5-11(13)9-17-19-15(23)16(24)20-18-10-12-6-2-4-8-14(12)22/h1-10,21-22H,(H,19,23)(H,20,24)/b17-9+,18-10+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50111589
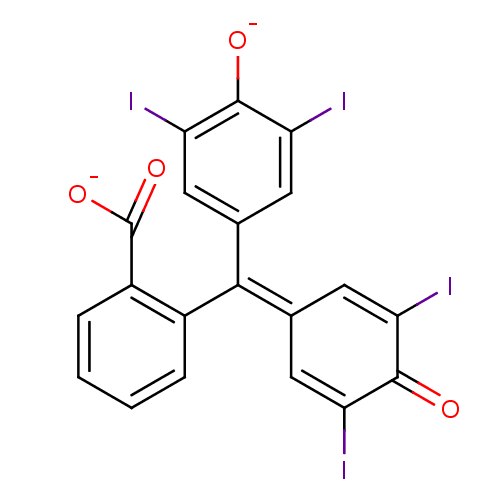
(2-[(3,5-diiodo-4-oxidophenyl)(3,5-diiodo-4-oxocycl...)Show SMILES [#8-]-[#6](=O)-c1ccccc1\[#6](=[#6]-1\[#6]=[#6](I)-[#6](=O)-[#6](I)=[#6]-1)-c1cc(I)c(-[#8-])c(I)c1 |c:18,t:12| Show InChI InChI=1S/C20H10I4O4/c21-13-5-9(6-14(22)18(13)25)17(10-7-15(23)19(26)16(24)8-10)11-3-1-2-4-12(11)20(27)28/h1-8,25H,(H,27,28)/p-2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM18358
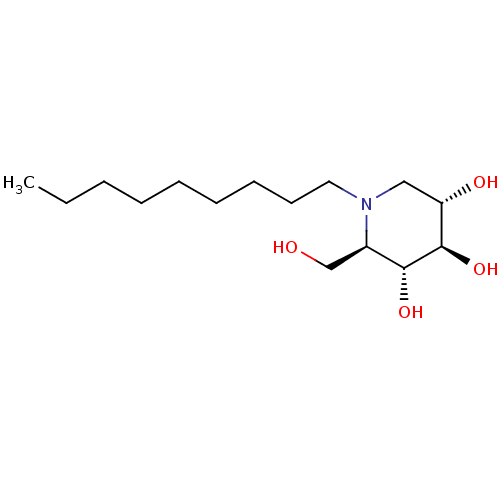
((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...)Show SMILES CCCCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C15H31NO4/c1-2-3-4-5-6-7-8-9-16-10-13(18)15(20)14(19)12(16)11-17/h12-15,17-20H,2-11H2,1H3/t12-,13+,14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335395
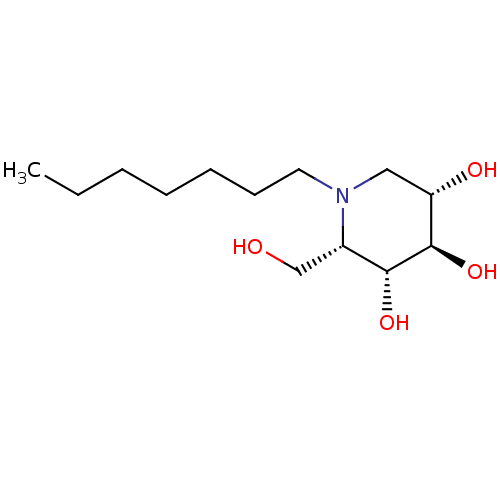
(CHEMBL1651555 | N-Heptyl-L-ido-1-deoxynojirimycin)Show SMILES CCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@@H]1CO |r| Show InChI InChI=1S/C13H27NO4/c1-2-3-4-5-6-7-14-8-11(16)13(18)12(17)10(14)9-15/h10-13,15-18H,2-9H2,1H3/t10-,11-,12+,13+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335390

(CHEMBL1651630 | N-Hexoxypentyl-1-deoxynojirimycin)Show SMILES CCCCCCOCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C17H35NO5/c1-2-3-4-7-10-23-11-8-5-6-9-18-12-15(20)17(22)16(21)14(18)13-19/h14-17,19-22H,2-13H2,1H3/t14-,15+,16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335388
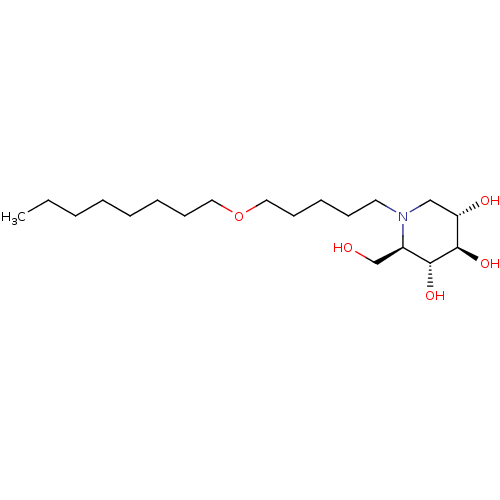
(CHEMBL1651632 | N-Octoxypentyl-1-deoxynojirimycin)Show SMILES CCCCCCCCOCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C19H39NO5/c1-2-3-4-5-6-9-12-25-13-10-7-8-11-20-14-17(22)19(24)18(23)16(20)15-21/h16-19,21-24H,2-15H2,1H3/t16-,17+,18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335385

(CHEMBL1651635 | N-Pentoxypentyl-L-ido-1-deoxynojir...)Show SMILES CCCCCOCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@@H]1CO |r| Show InChI InChI=1S/C16H33NO5/c1-2-3-6-9-22-10-7-4-5-8-17-11-14(19)16(21)15(20)13(17)12-18/h13-16,18-21H,2-12H2,1H3/t13-,14-,15+,16+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335384
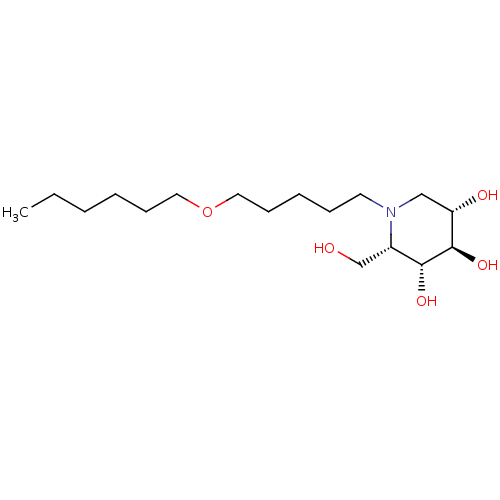
(CHEMBL1651636 | N-Hexoxypentyl-L-ido-1-deoxynojiri...)Show SMILES CCCCCCOCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@@H]1CO |r| Show InChI InChI=1S/C17H35NO5/c1-2-3-4-7-10-23-11-8-5-6-9-18-12-15(20)17(22)16(21)14(18)13-19/h14-17,19-22H,2-13H2,1H3/t14-,15-,16+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335383

(CHEMBL1651637 | N-Heptoxypentyl-L-ido-1-deoxynojir...)Show SMILES CCCCCCCOCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@@H]1CO |r| Show InChI InChI=1S/C18H37NO5/c1-2-3-4-5-8-11-24-12-9-6-7-10-19-13-16(21)18(23)17(22)15(19)14-20/h15-18,20-23H,2-14H2,1H3/t15-,16-,17+,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335389

(CHEMBL1651631 | N-Heptoxypentyl-1-deoxynojirimycin)Show SMILES CCCCCCCOCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C18H37NO5/c1-2-3-4-5-8-11-24-12-9-6-7-10-19-13-16(21)18(23)17(22)15(19)14-20/h15-18,20-23H,2-14H2,1H3/t15-,16+,17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335382
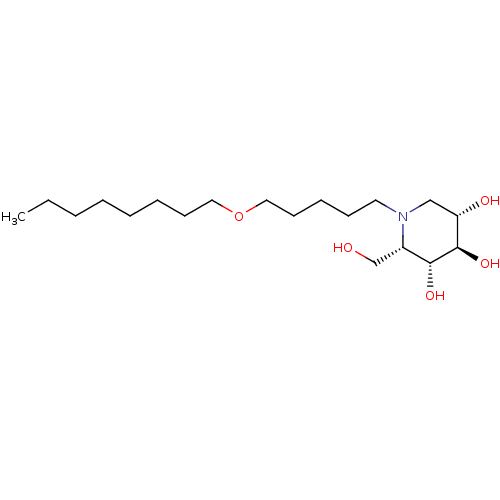
(CHEMBL1651638 | N-Octoxypentyl-L-ido-1-deoxynojiri...)Show SMILES CCCCCCCCOCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@@H]1CO |r| Show InChI InChI=1S/C19H39NO5/c1-2-3-4-5-6-9-12-25-13-10-7-8-11-20-14-17(22)19(24)18(23)16(20)15-21/h16-19,21-24H,2-15H2,1H3/t16-,17-,18+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50111592
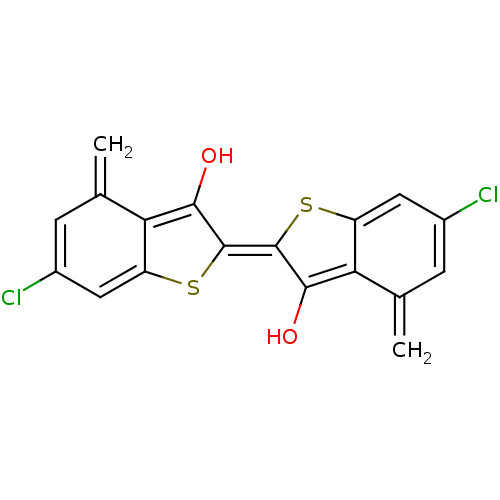
(6,6'-Dichloro-4,4'-dimethyl-[2,2']bi[benzo[b]thiop...)Show SMILES Oc1c2c(cc(Cl)cc2=C)s\c1=c1\sc2cc(Cl)cc(=C)c2c1O Show InChI InChI=1S/C18H10Cl2O2S2/c1-7-3-9(19)5-11-13(7)15(21)17(23-11)18-16(22)14-8(2)4-10(20)6-12(14)24-18/h3-6,21-22H,1-2H2/b18-17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335392

(CHEMBL1651628 | N-Butoxypentyl-1-deoxynojirimycin)Show SMILES CCCCOCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C15H31NO5/c1-2-3-8-21-9-6-4-5-7-16-10-13(18)15(20)14(19)12(16)11-17/h12-15,17-20H,2-11H2,1H3/t12-,13+,14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM50335391
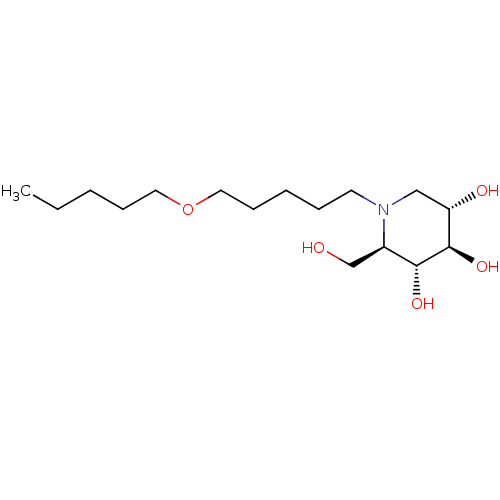
(CHEMBL1651629 | N-Pentoxypentyl-1-deoxynojirimycin)Show SMILES CCCCCOCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO |r| Show InChI InChI=1S/C16H33NO5/c1-2-3-6-9-22-10-7-4-5-8-17-11-14(19)16(21)15(20)13(17)12-18/h13-16,18-21H,2-12H2,1H3/t13-,14+,15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Mus musculus) | BDBM18357

((2R,3R,4R,5S)-2-(hydroxymethyl)-1-octylpiperidine-...)Show SMILES CCCCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@H]1CO Show InChI InChI=1S/C14H29NO4/c1-2-3-4-5-6-7-8-15-9-12(17)14(19)13(18)11(15)10-16/h11-14,16-19H,2-10H2,1H3/t11-,12+,13-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of lactase in mouse intestinal input by glucose release assay |
ACS Med Chem Lett 2: 119-123 (2011)
Article DOI: 10.1021/ml100192b
BindingDB Entry DOI: 10.7270/Q21C1X5H |
More data for this
Ligand-Target Pair | |
Beta-galactosidase
(Homo sapiens (Human)) | BDBM50111591
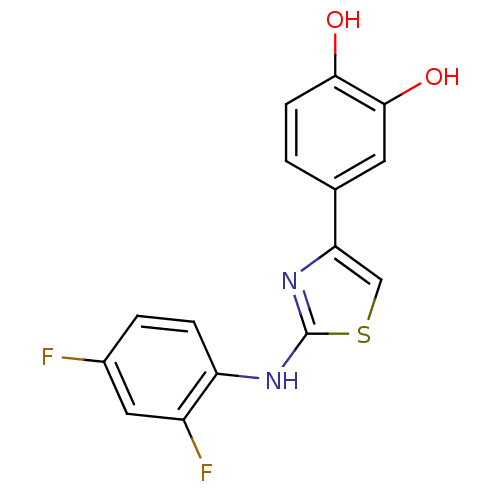
(4-[2-(2,4-Difluoro-phenylamino)-thiazol-4-yl]-benz...)Show InChI InChI=1S/C15H10F2N2O2S/c16-9-2-3-11(10(17)6-9)18-15-19-12(7-22-15)8-1-4-13(20)14(21)5-8/h1-7,20-21H,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of Beta-galactosidase |
J Med Chem 45: 1712-22 (2002)
BindingDB Entry DOI: 10.7270/Q20C4WHD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data