

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myeloblastin (Homo sapiens (Human)) | BDBM50063047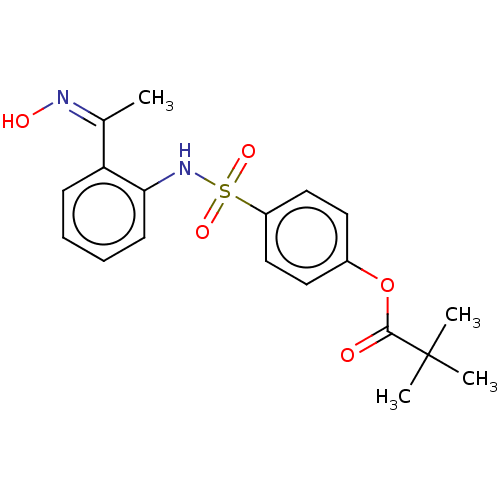 (CHEMBL3398148) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50063053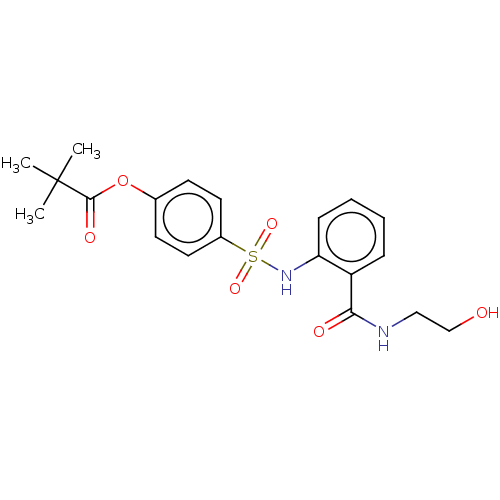 (CHEMBL3398164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50084637 (2,2-Dimethyl-propionic acid 4-[2-(carboxymethyl-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50063050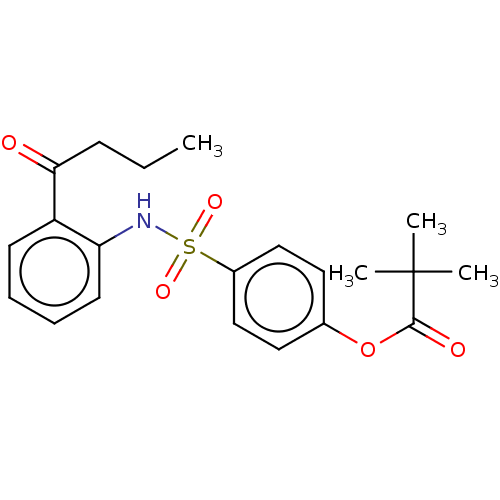 (CHEMBL3398145) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50063052 (CHEMBL3398143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101988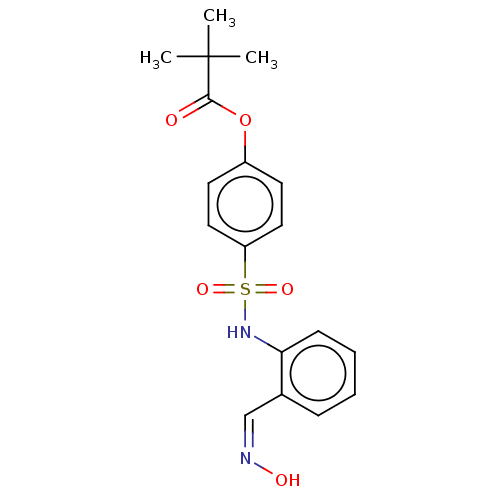 (CHEMBL3398153) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50031630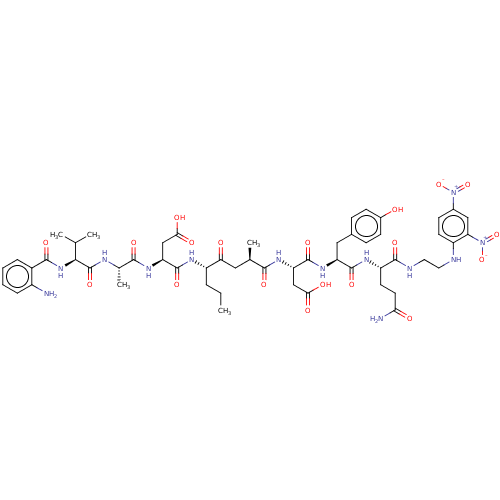 (CHEMBL3359765) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bergen Curated by ChEMBL | Assay Description Inhibition of human PR3 using keto-D-DY-FRET as substrate after 30 mins by HPLC analysis | J Med Chem 57: 9396-408 (2014) Article DOI: 10.1021/jm500782s BindingDB Entry DOI: 10.7270/Q2VQ349F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM166437 (US9073833, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50063054 (CHEMBL3398165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50063051 (CHEMBL3398144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50384111 (CHEMBL2029556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of proteinase 3 using N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide as substrate after 60 mins | Bioorg Med Chem Lett 22: 3993-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.093 BindingDB Entry DOI: 10.7270/Q2NZ88PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101980 (CHEMBL3398150) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50031630 (CHEMBL3359765) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bergen Curated by ChEMBL | Assay Description Inhibition of human PR3 using D-DY-FRET as substrate after 30 mins by HPLC analysis | J Med Chem 57: 9396-408 (2014) Article DOI: 10.1021/jm500782s BindingDB Entry DOI: 10.7270/Q2VQ349F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50063048 (CHEMBL3398147) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101989 (CHEMBL3398154) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50063049 (CHEMBL3398146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM166438 (US9073833, 6) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50063046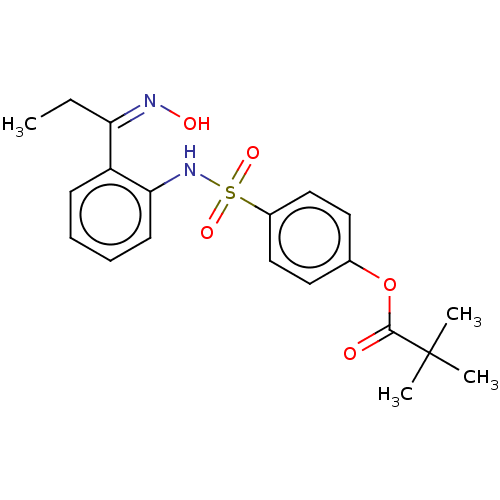 (CHEMBL3398149) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101983 (CHEMBL3398155) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101984 (CHEMBL3398156) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101978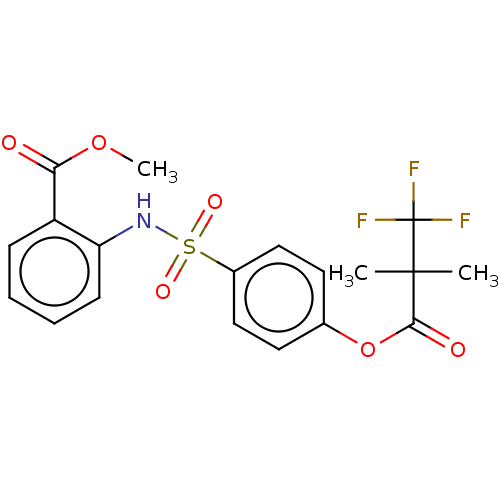 (CHEMBL3398160) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50384104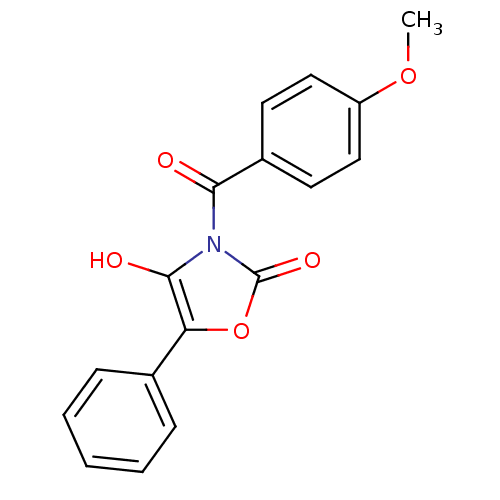 (CHEMBL2029559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of proteinase 3 using N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide as substrate after 60 mins | Bioorg Med Chem Lett 22: 3993-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.093 BindingDB Entry DOI: 10.7270/Q2NZ88PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50444297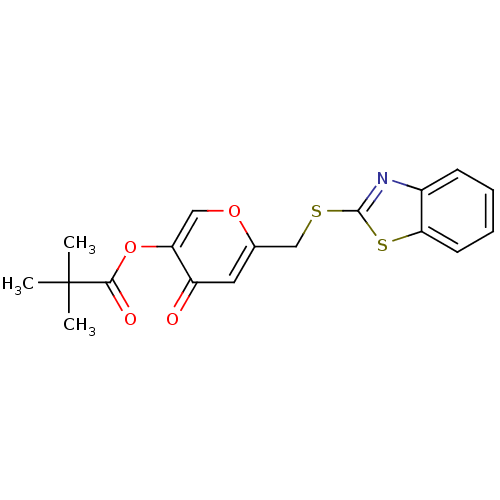 (CHEMBL3093815) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human proteinase 3 using N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide as substrate measured for 30 mins by spectrophotometry | J Med Chem 56: 9802-6 (2014) Article DOI: 10.1021/jm4011725 BindingDB Entry DOI: 10.7270/Q2RX9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101977 (CHEMBL3398159) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101985 (CHEMBL3398157) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101981 (CHEMBL3398161) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101982 (CHEMBL3398162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101987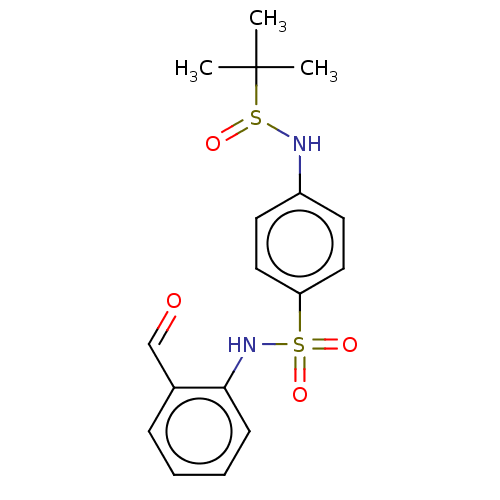 (CHEMBL3398163) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50101986 (CHEMBL3398158) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chang Gung University Curated by ChEMBL | Assay Description Inhibition of Pr3 (unknown origin) using t-butyloxycarbonyl-Ala-Ala-Nva-thiobenzyl ester as substrate measured for 180 mins by spectrophotometry | Bioorg Med Chem 23: 1123-34 (2015) Article DOI: 10.1016/j.bmc.2014.12.056 BindingDB Entry DOI: 10.7270/Q2CJ8G5M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50031631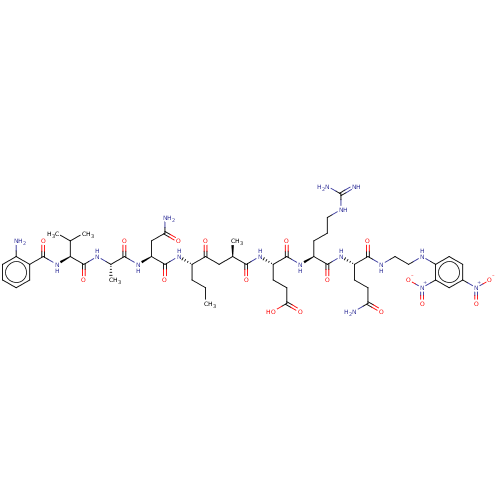 (CHEMBL3359767) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bergen Curated by ChEMBL | Assay Description Inhibition of human PR3 using D-DY-FRET as substrate after 30 mins by HPLC analysis | J Med Chem 57: 9396-408 (2014) Article DOI: 10.1021/jm500782s BindingDB Entry DOI: 10.7270/Q2VQ349F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50384108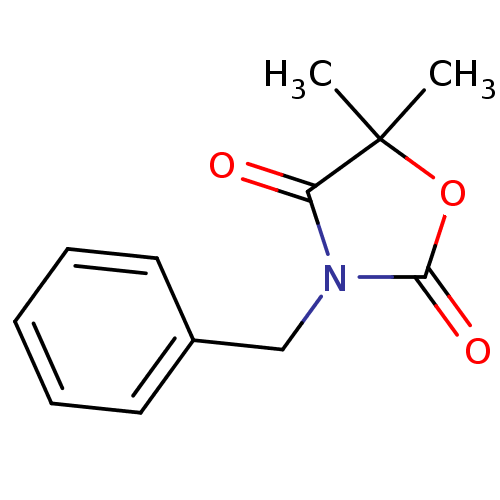 (CHEMBL2029554) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of proteinase 3 using N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide as substrate after 60 mins | Bioorg Med Chem Lett 22: 3993-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.093 BindingDB Entry DOI: 10.7270/Q2NZ88PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50384112 (CHEMBL2029557) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of proteinase 3 using N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide as substrate after 60 mins | Bioorg Med Chem Lett 22: 3993-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.093 BindingDB Entry DOI: 10.7270/Q2NZ88PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50171289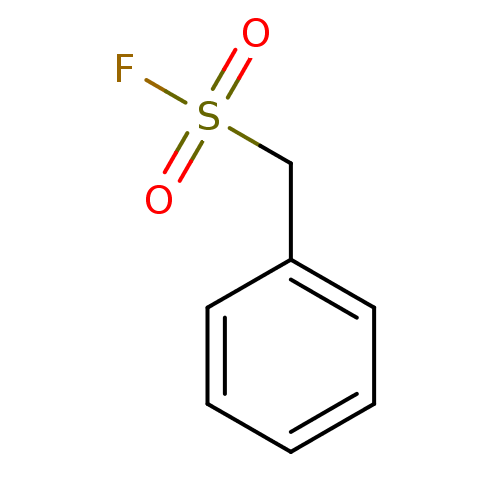 (Benzenemethanesulfonyl fluoride | CHEMBL190503 | P...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bergen Curated by ChEMBL | Assay Description Inhibition of human PR3 using D-DY-FRET as substrate after 30 mins by HPLC analysis | J Med Chem 57: 9396-408 (2014) Article DOI: 10.1021/jm500782s BindingDB Entry DOI: 10.7270/Q2VQ349F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50444296 (CHEMBL3093814) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human proteinase 3 using N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide as substrate measured for 30 mins by spectrophotometry | J Med Chem 56: 9802-6 (2014) Article DOI: 10.1021/jm4011725 BindingDB Entry DOI: 10.7270/Q2RX9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50270029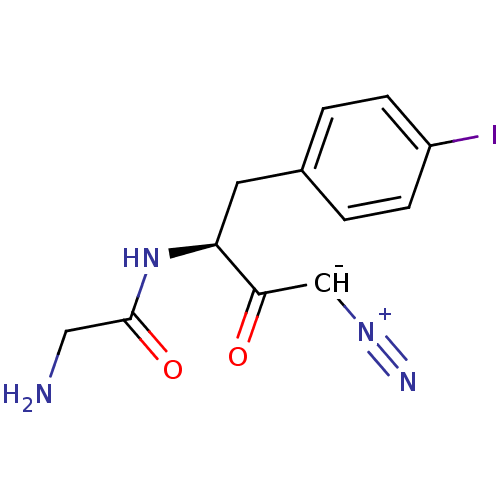 (2-Amino-N-[(S)-3-diazo-1-(4-iodo-benzyl)-2-oxo-pro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of proteinase-3 in human U937 cells | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50310690 (CHEMBL1078411 | Cis-(S)-2-amino-N-((1R,2R)-1-cyano...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Canada& Co. Curated by ChEMBL | Assay Description Inhibition of human Proteinase-3 | Bioorg Med Chem Lett 19: 5392-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.114 BindingDB Entry DOI: 10.7270/Q298874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50270040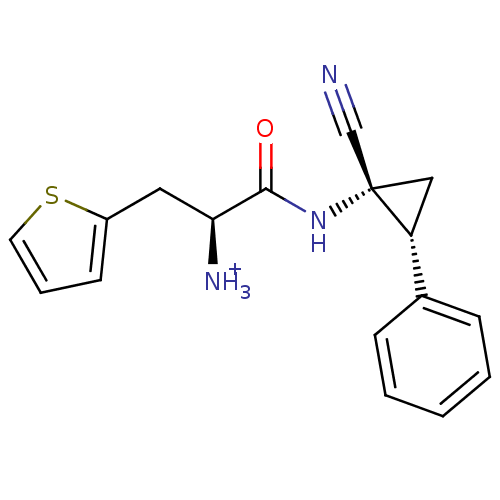 ((S)-1-((1R,2R)-1-cyano-2-phenylcyclopropylamino)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of proteinase-3 in human U937 cells | J Biol Chem 282: 20836-46 (2007) Article DOI: 10.1074/jbc.M702615200 BindingDB Entry DOI: 10.7270/Q2125TJM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50260049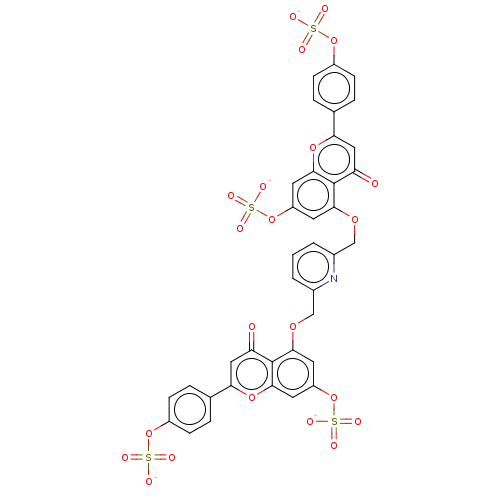 (CHEMBL4104474) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University Curated by ChEMBL | Assay Description Inhibition of human neutrophil proteinase 3 using M4765 as substrate preincubated for 5 mins followed by substrate addition | J Med Chem 62: 5501-5511 (2019) Article DOI: 10.1021/acs.jmedchem.9b00379 BindingDB Entry DOI: 10.7270/Q2DR2ZR4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50444295 (CHEMBL3093806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human proteinase 3 using N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide as substrate measured for 30 mins by spectrophotometry | J Med Chem 56: 9802-6 (2014) Article DOI: 10.1021/jm4011725 BindingDB Entry DOI: 10.7270/Q2RX9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloblastin (Homo sapiens (Human)) | BDBM50084637 (2,2-Dimethyl-propionic acid 4-[2-(carboxymethyl-ca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de Lisboa Curated by ChEMBL | Assay Description Inhibition of human proteinase 3 using N-MeOSuc-Ala-Ala-Pro-Val-p-nitroanilide as substrate measured for 30 mins by spectrophotometry | J Med Chem 56: 9802-6 (2014) Article DOI: 10.1021/jm4011725 BindingDB Entry DOI: 10.7270/Q2RX9DJ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||