

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50396754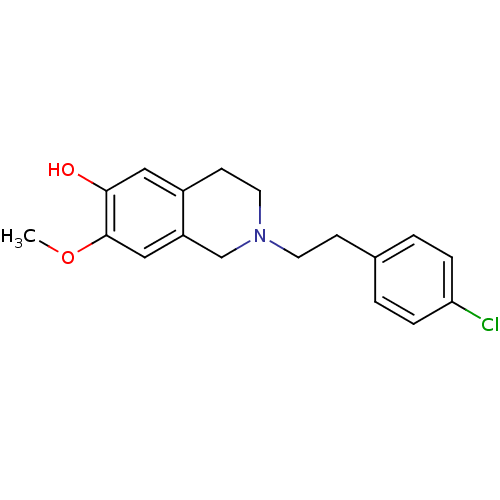 (CHEMBL2172347) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of 7-dehydroxycholesterol reductase-mediated cholesterol biosynthesis in human HL60 cells assessed as inhibition of 2-[13C]acetate after 2... | J Med Chem 55: 7614-22 (2012) Article DOI: 10.1021/jm3006096 BindingDB Entry DOI: 10.7270/Q2W95B9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50411320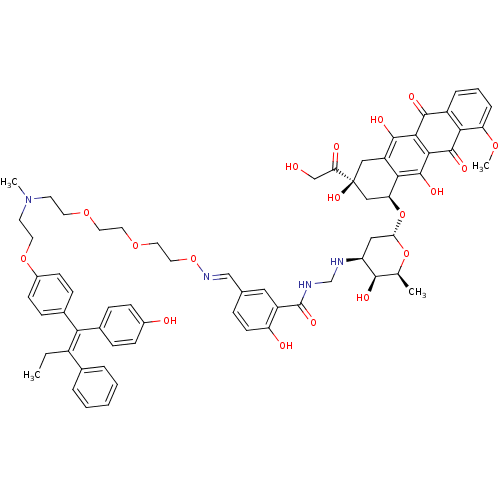 (CHEMBL222694) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of MDA-MB-231 cells containing antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50411320 (CHEMBL222694) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of MCF7 cells expressing ER and antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50411320 (CHEMBL222694) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of MDA-MB-435 cells containing antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50411319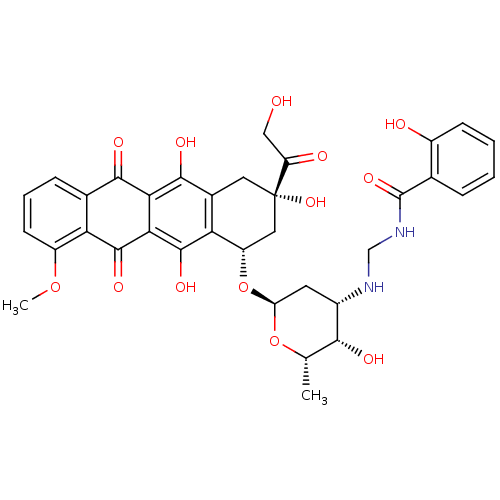 (CHEMBL266892) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of MDA-MB-435 cells containing antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50411320 (CHEMBL222694) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of multidrug resistant MCF7/Adr cells containing antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50411319 (CHEMBL266892) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of MCF7 cells expressing ER and antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50411319 (CHEMBL266892) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of MDA-MB-231 cells containing antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50396753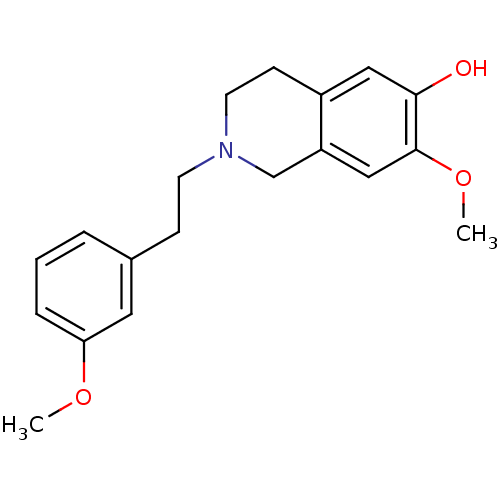 (CHEMBL2172349) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of 7-dehydroxycholesterol reductase-mediated cholesterol biosynthesis in human HL60 cells assessed as inhibition of 2-[13C]acetate after 2... | J Med Chem 55: 7614-22 (2012) Article DOI: 10.1021/jm3006096 BindingDB Entry DOI: 10.7270/Q2W95B9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM22984 ((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of MDA-MB-435 cells containing antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM22984 ((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of MCF7 cells expressing ER and antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM22984 ((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of MDA-MB-231 cells containing antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50412080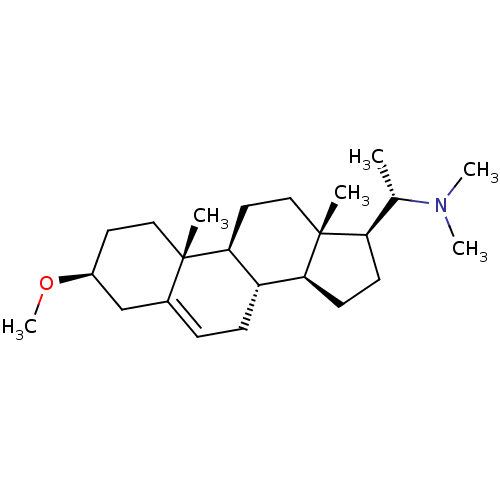 (CHEMBL342394) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting | J Nat Prod 61: 1257-62 (1998) Article DOI: 10.1021/np980162x BindingDB Entry DOI: 10.7270/Q2B27WJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50170646 (4-(2-{4-[(E)-3-(4-Chloro-phenyl)-allyl]-piperazin-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of 7-dehydroxycholesterol reductase-mediated cholesterol biosynthesis in human HL60 cells assessed as inhibition of 2-[13C]acetate after 2... | J Med Chem 55: 7614-22 (2012) Article DOI: 10.1021/jm3006096 BindingDB Entry DOI: 10.7270/Q2W95B9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50412077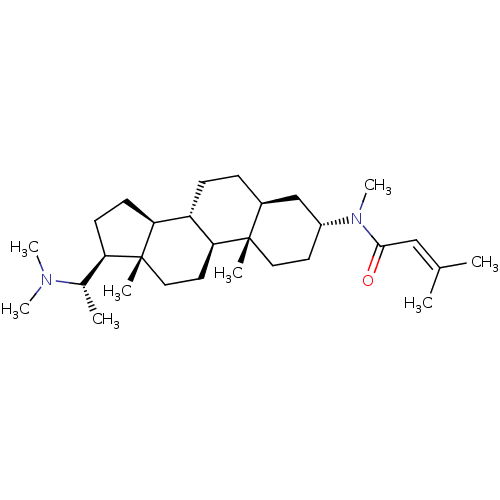 ((+)-PACHYSAMINE B) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting | J Nat Prod 61: 1257-62 (1998) Article DOI: 10.1021/np980162x BindingDB Entry DOI: 10.7270/Q2B27WJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50412082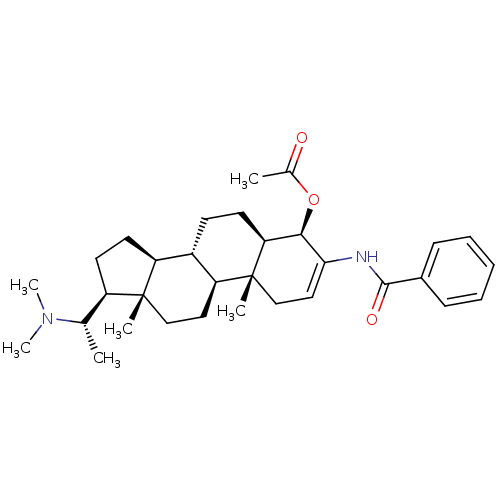 (CHEMBL455316) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting | J Nat Prod 61: 1257-62 (1998) Article DOI: 10.1021/np980162x BindingDB Entry DOI: 10.7270/Q2B27WJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50411319 (CHEMBL266892) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of multidrug resistant MCF7/Adr cells containing antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50412076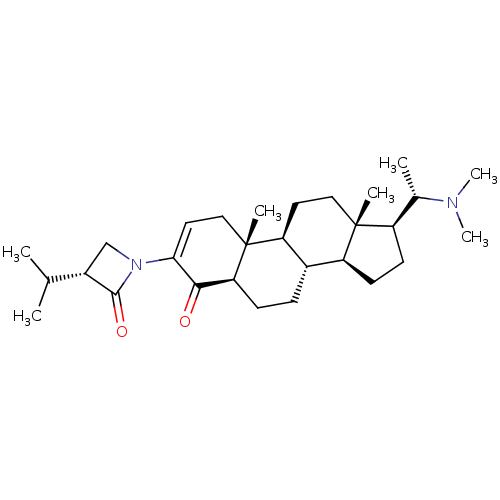 (CHEMBL457817) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting | J Nat Prod 61: 1257-62 (1998) Article DOI: 10.1021/np980162x BindingDB Entry DOI: 10.7270/Q2B27WJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50412083 (CHEMBL458033) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting | J Nat Prod 61: 1257-62 (1998) Article DOI: 10.1021/np980162x BindingDB Entry DOI: 10.7270/Q2B27WJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50412081 (CHEMBL505436) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting | J Nat Prod 61: 1257-62 (1998) Article DOI: 10.1021/np980162x BindingDB Entry DOI: 10.7270/Q2B27WJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50396752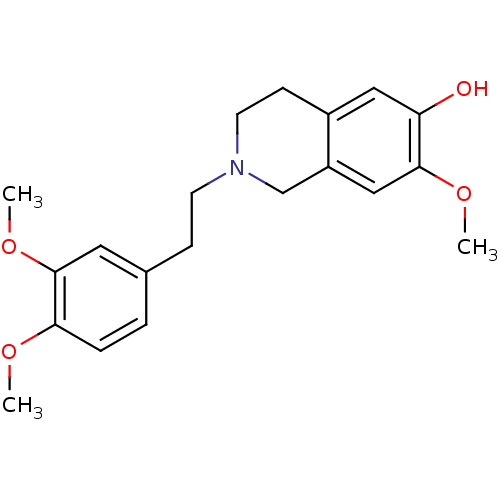 (CHEMBL2172350) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther-Universit£t Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of 7-dehydroxycholesterol reductase-mediated cholesterol biosynthesis in human HL60 cells assessed as inhibition of 2-[13C]acetate after 2... | J Med Chem 55: 7614-22 (2012) Article DOI: 10.1021/jm3006096 BindingDB Entry DOI: 10.7270/Q2W95B9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50412079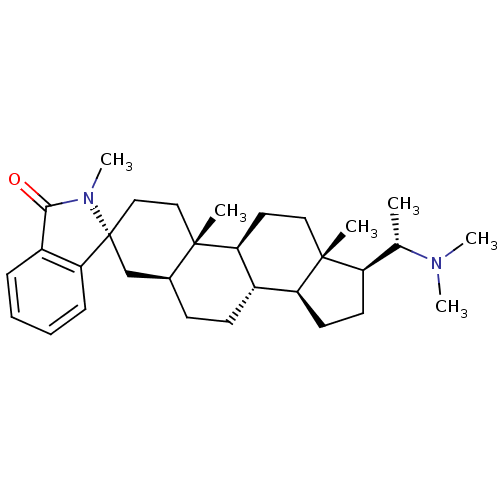 (CHEMBL456512) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting | J Nat Prod 61: 1257-62 (1998) Article DOI: 10.1021/np980162x BindingDB Entry DOI: 10.7270/Q2B27WJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50135147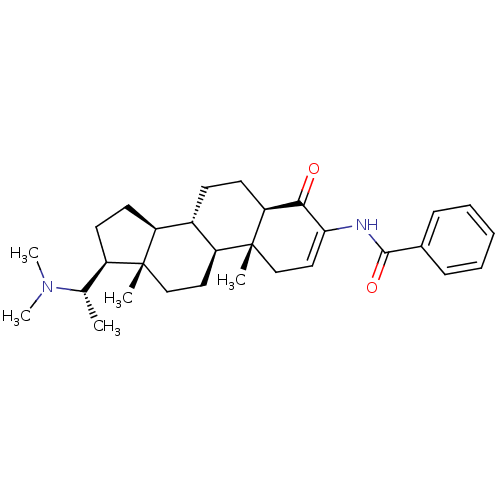 ((+)-axillaridine A | 14-(1-dimethylaminoethyl)-2,1...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting | J Nat Prod 61: 1257-62 (1998) Article DOI: 10.1021/np980162x BindingDB Entry DOI: 10.7270/Q2B27WJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM22984 ((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado Curated by ChEMBL | Assay Description Growth inhibition of multidrug resistant MCF7/Adr cells containing antiestrogen binding sites | J Med Chem 47: 6509-18 (2004) Article DOI: 10.1021/jm049496b BindingDB Entry DOI: 10.7270/Q23R0V3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 7-dehydrocholesterol reductase (Homo sapiens (Human)) | BDBM50412078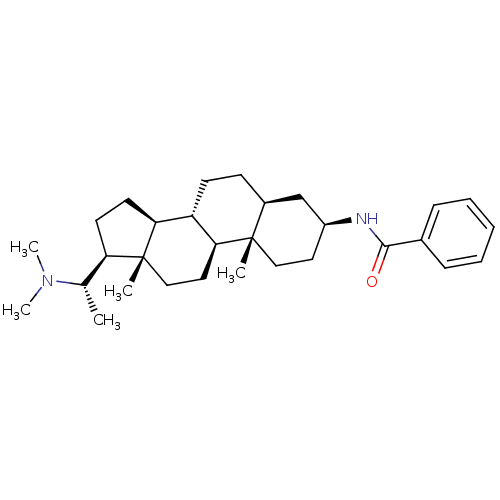 (EPIPACHYSAMINE D) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting | J Nat Prod 61: 1257-62 (1998) Article DOI: 10.1021/np980162x BindingDB Entry DOI: 10.7270/Q2B27WJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||