

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515773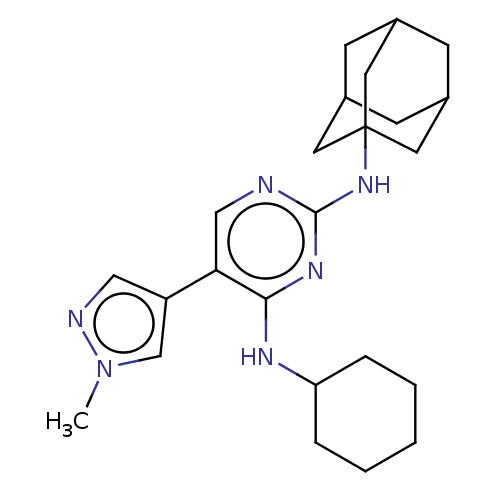 (US11053225, Compound 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515786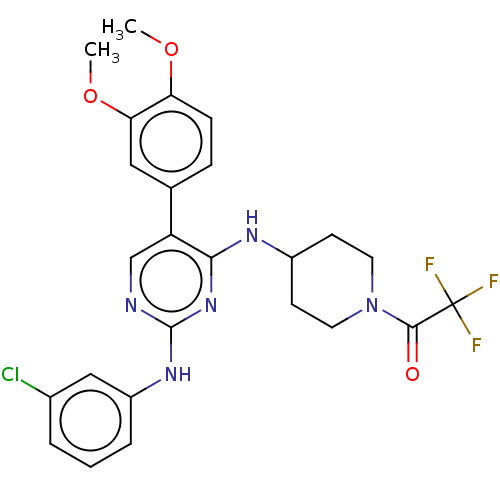 (US11053225, Compound 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384584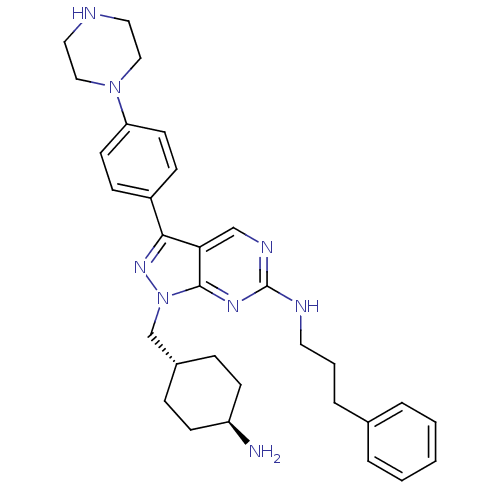 (CHEMBL2036807 | US9744172, Compound UNC607A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384583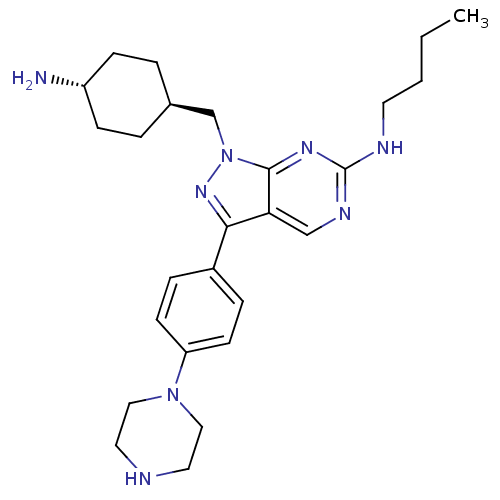 (CHEMBL2036806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515821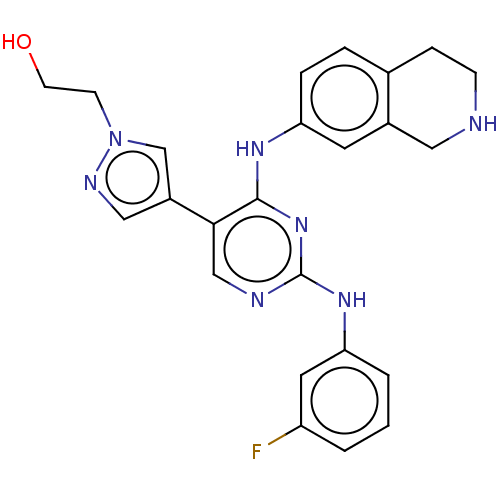 (US11053225, Compound 102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350865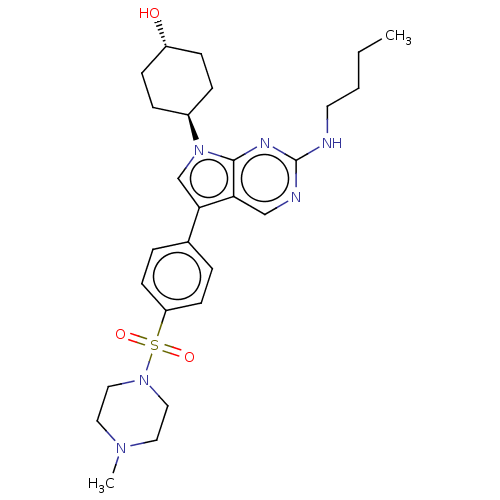 (UNC1667A | US10004755, Compound UNC1667A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Inhibition constants of MerTK, Flt3, Tyro3 and Axl kinase activity by an active compound as described herein is determined at the Km for ATP using a ... | Bioorg Med Chem 17: 7884-93 (2009) BindingDB Entry DOI: 10.7270/Q26H4KR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308226 (US9649309, Compound UNC4103A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50055496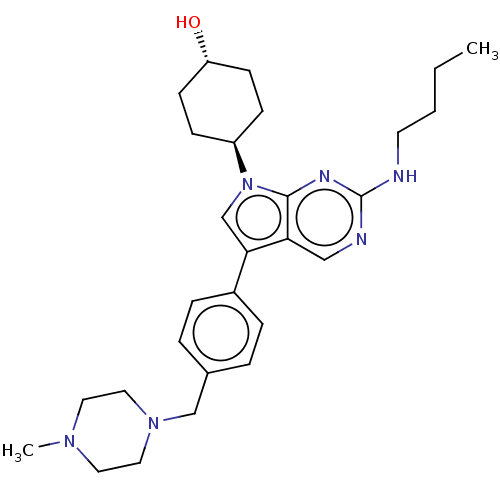 (CHEMBL3326006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human MERTK cytoplasmic domain (528 to 999 end residues) expressed in baculovirus expression system using fluorec... | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308193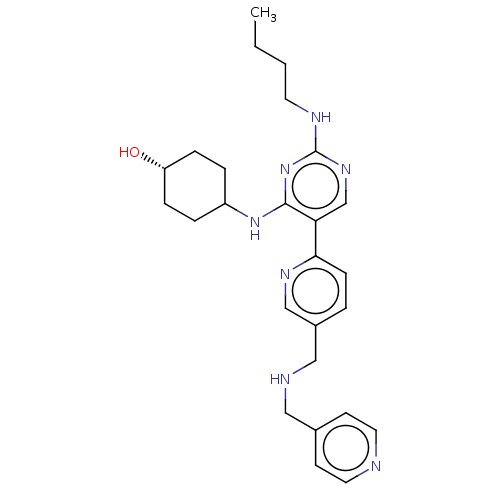 (US9649309, Compound UNC2571A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308186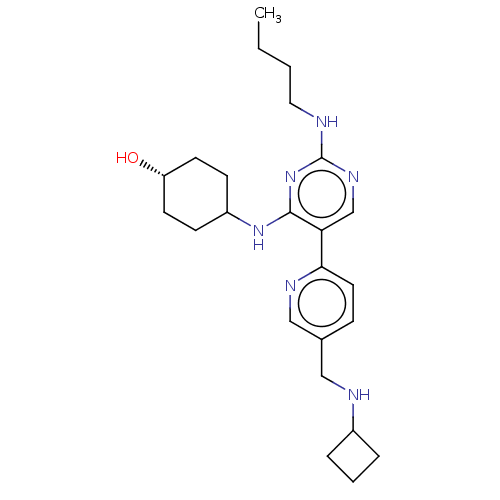 (US9649309, Compound UNC2550A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM628988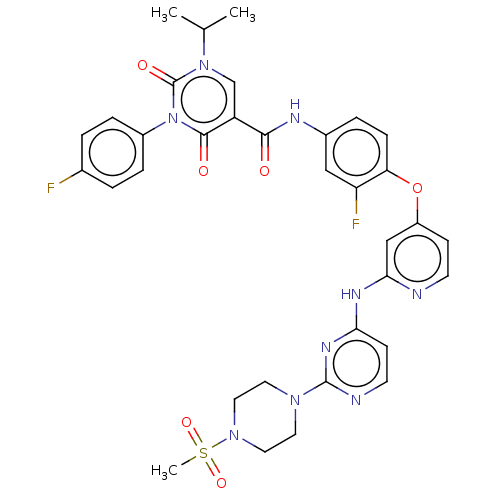 (N-{3-fluoro-4-[(2-{[2-(4-methanesulfonylpiperazin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350848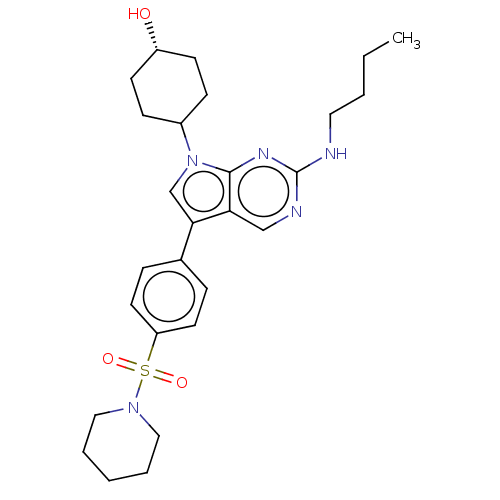 (UNC2078A | US10004755, Compound UNC2078A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Inhibition constants of MerTK, Flt3, Tyro3 and Axl kinase activity by an active compound as described herein is determined at the Km for ATP using a ... | Bioorg Med Chem 17: 7884-93 (2009) BindingDB Entry DOI: 10.7270/Q26H4KR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308189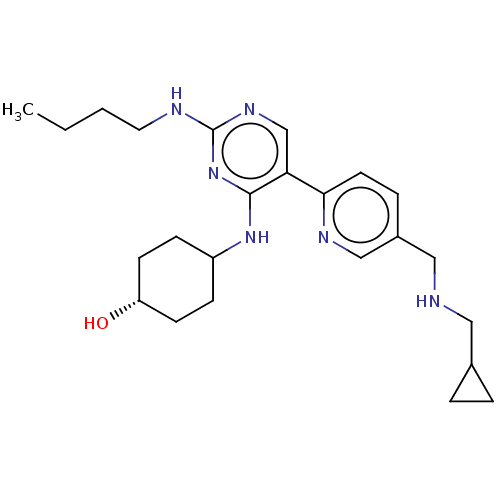 (US9649309, Compound UNC2547A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50055498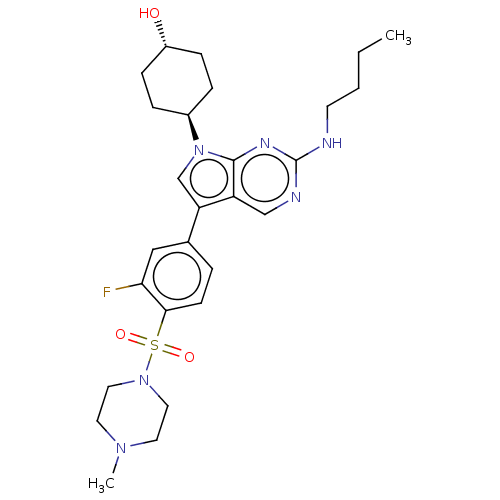 (CHEMBL3326004 | US10004755, Compound UNC1669A | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer (unknown origin) by Off-chip Mobility Shift Assay | J Med Chem 57: 7031-41 (2014) Article DOI: 10.1021/jm500749d BindingDB Entry DOI: 10.7270/Q2K075XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50055498 (CHEMBL3326004 | US10004755, Compound UNC1669A | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Inhibition constants of MerTK, Flt3, Tyro3 and Axl kinase activity by an active compound as described herein is determined at the Km for ATP using a ... | Bioorg Med Chem 17: 7884-93 (2009) BindingDB Entry DOI: 10.7270/Q26H4KR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM628989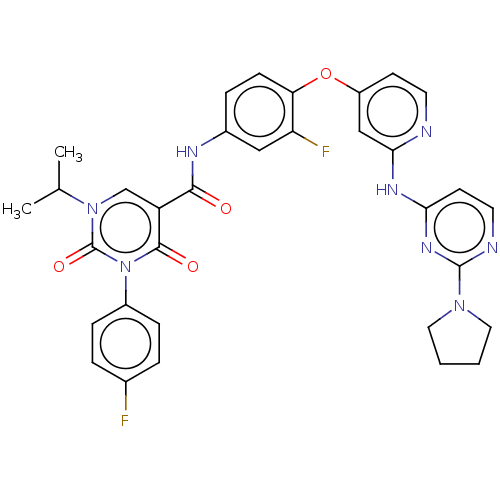 (N-{3-fluoro-4-[(2-{[2-(pyrrolidin-1-yl)pyrimidin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged human MERTK cytoplasmic domain (528 to 999 end residues) expressed in baculovirus expression system using fluorec... | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged MERTK (528 to 999 residues) (unknown origin) cytoplasmic domain expressed in Sf21 cells | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113534 BindingDB Entry DOI: 10.7270/Q2M90DGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308318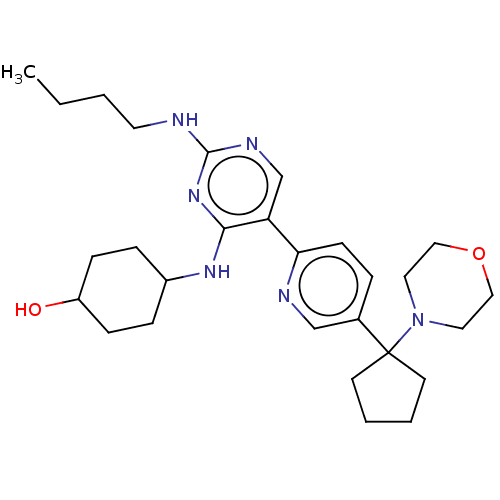 (US9649309, Compound UNC3667A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308188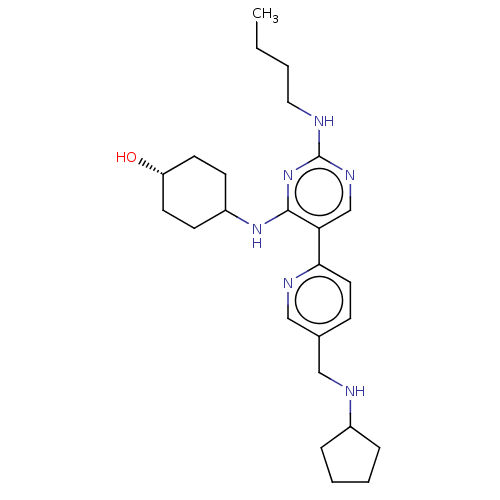 (US9649309, Compound UNC2489A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308349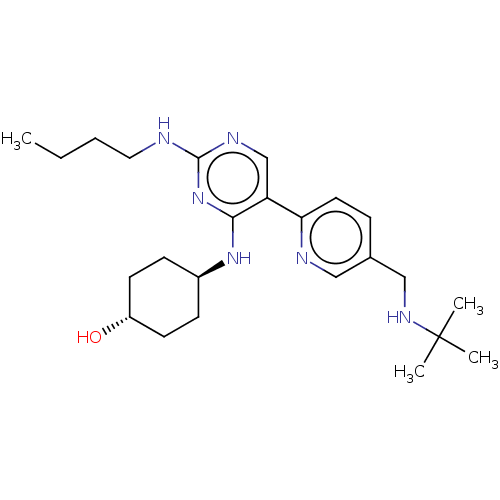 (US9649309, Compound UNC4160A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308201 (US9649309, Compound UNC2606A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444072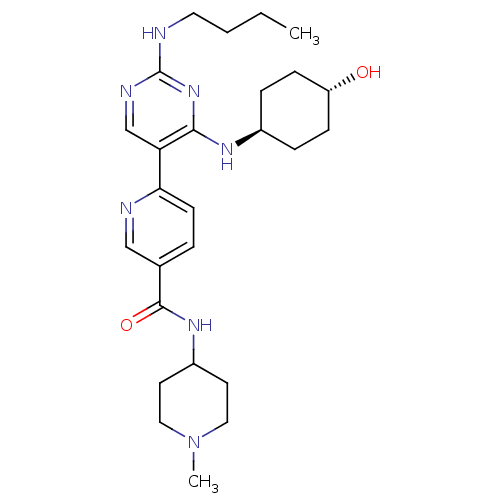 (CHEMBL3092793) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308181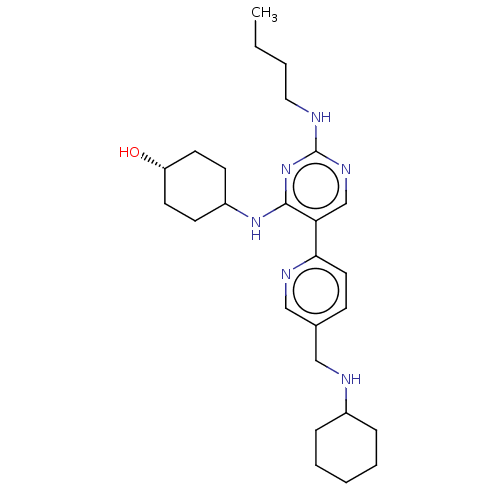 (US9649309, Compound UNC2432A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444070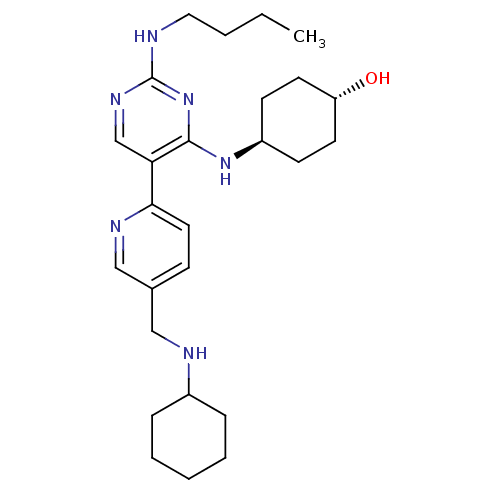 (CHEMBL3092795) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444073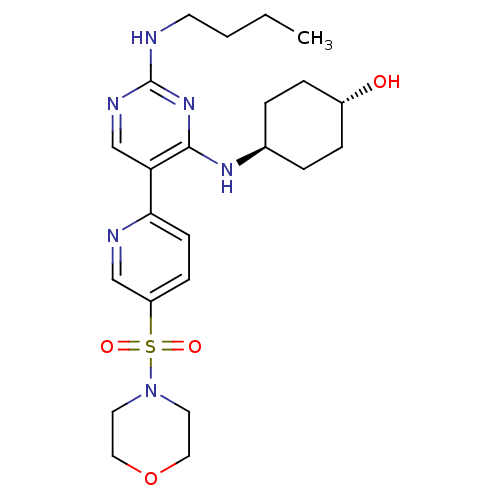 (CHEMBL3092792) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350844 (UNC1972A | US10004755, Compound UNC1972A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Inhibition constants of MerTK, Flt3, Tyro3 and Axl kinase activity by an active compound as described herein is determined at the Km for ATP using a ... | Bioorg Med Chem 17: 7884-93 (2009) BindingDB Entry DOI: 10.7270/Q26H4KR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350843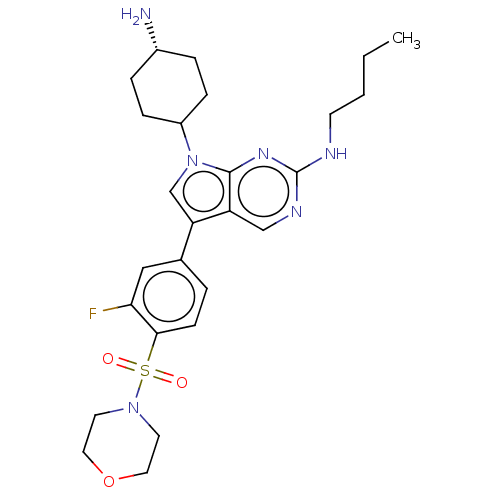 (UNC1971A | US10004755, Compound UNC1971A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Inhibition constants of MerTK, Flt3, Tyro3 and Axl kinase activity by an active compound as described herein is determined at the Km for ATP using a ... | Bioorg Med Chem 17: 7884-93 (2009) BindingDB Entry DOI: 10.7270/Q26H4KR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50055496 (CHEMBL3326006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer (unknown origin) by Off-chip Mobility Shift Assay | J Med Chem 57: 7031-41 (2014) Article DOI: 10.1021/jm500749d BindingDB Entry DOI: 10.7270/Q2K075XQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308194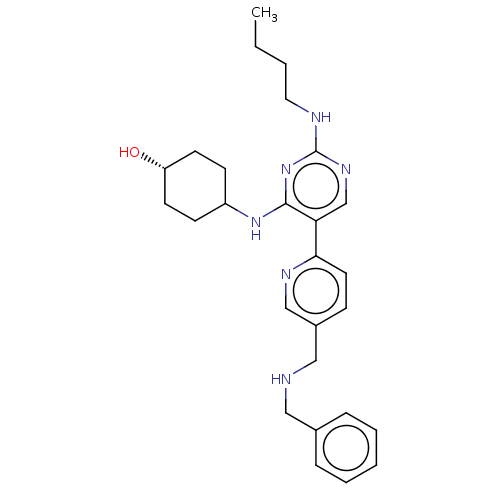 (US9649309, Compound UNC2572A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384582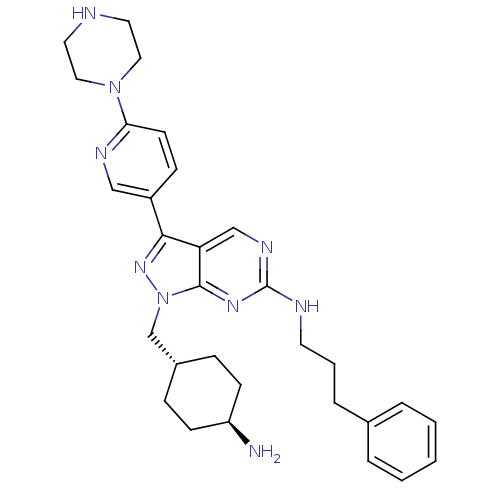 (CHEMBL2036805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer expressed in Escherichia coli BL21 (DE3) cells using EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluid capillary electr... | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM497267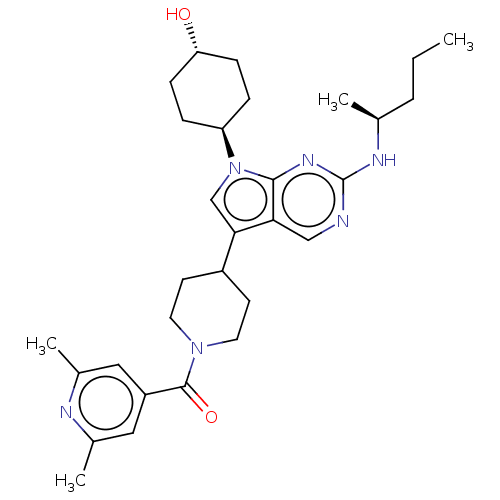 ((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal GST-tagged MERTK (528 to 999 residues) (unknown origin) cytoplasmic domain expressed in Sf21 cells | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113534 BindingDB Entry DOI: 10.7270/Q2M90DGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515836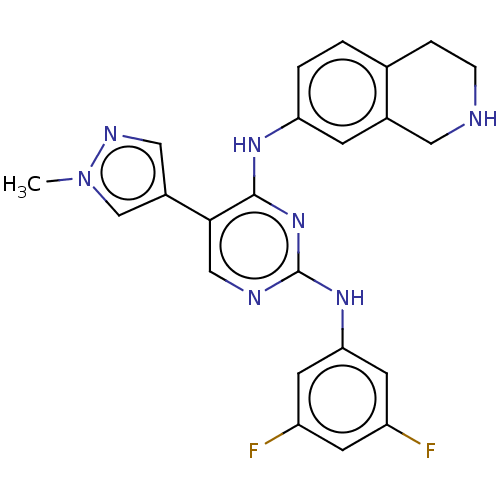 (US11053225, Compound 137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50563635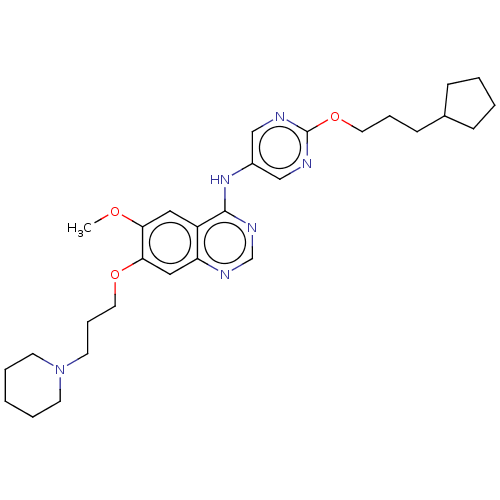 (CHEMBL4777640) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 6His/thrombin cleavage site-fused Avi-tagged dephosphorylated MER (unknown origin) (R528 to M999 residues) using Axltide (CKKSRGDYMTMQJ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50201078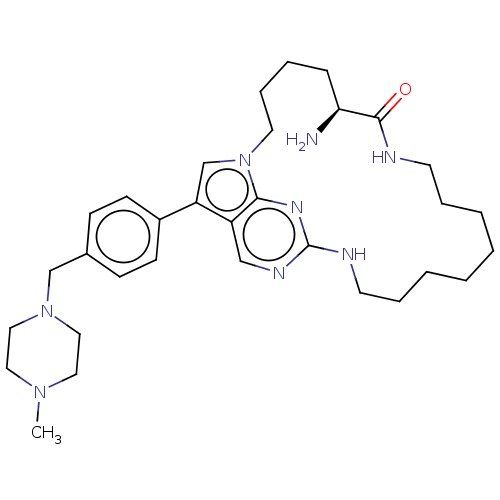 (CHEMBL3964573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of MerTK (unknown origin) by microfluidic capillary electrophoresis assay | ACS Med Chem Lett 7: 1044-1049 (2016) Article DOI: 10.1021/acsmedchemlett.6b00221 BindingDB Entry DOI: 10.7270/Q208679K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308354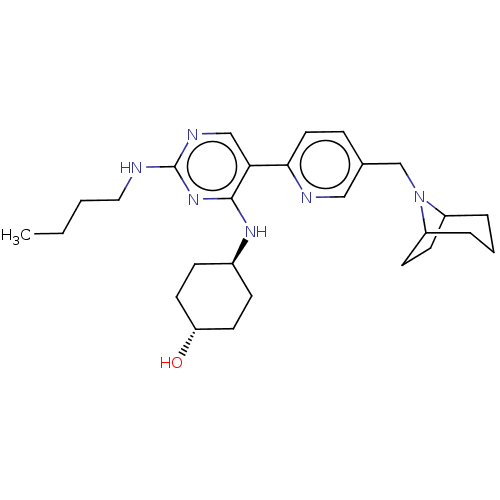 (US9649309, Compound UNC4169A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308192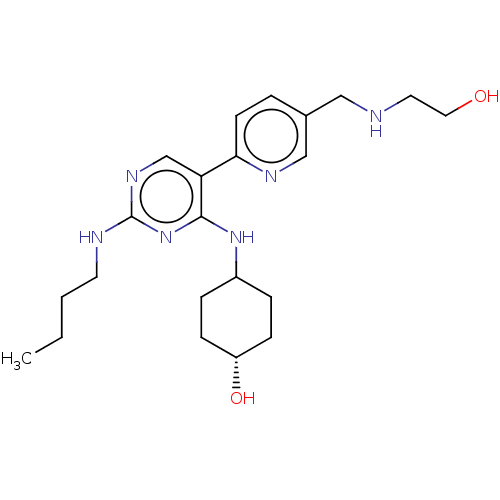 (US9649309, Compound UNC2546A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50444068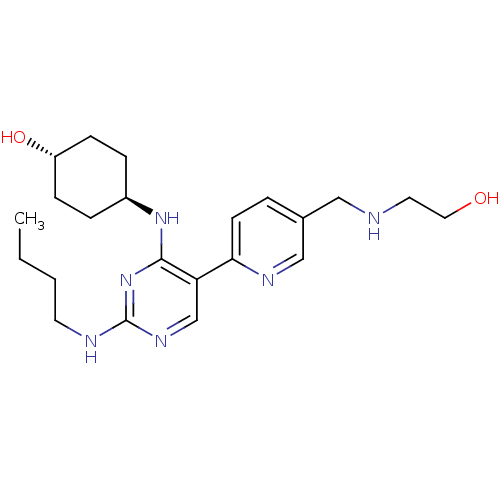 (CHEMBL3092797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer kinase (unknown origin) using 5-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by microfluidic capillary electrophoresis assay | J Med Chem 56: 9683-92 (2014) Article DOI: 10.1021/jm401387j BindingDB Entry DOI: 10.7270/Q29W0GX8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515783 (US11053225, Compound 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308229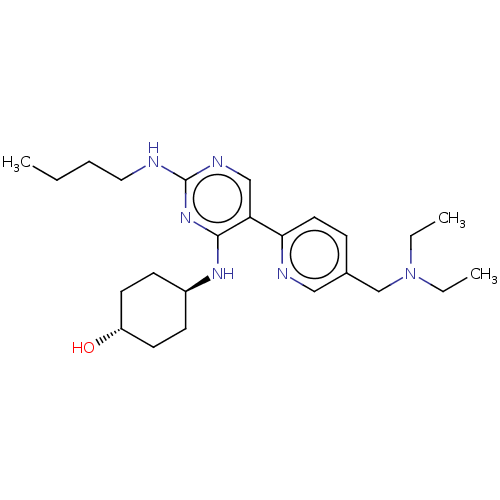 (US9649309, Compound UNC4162A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308198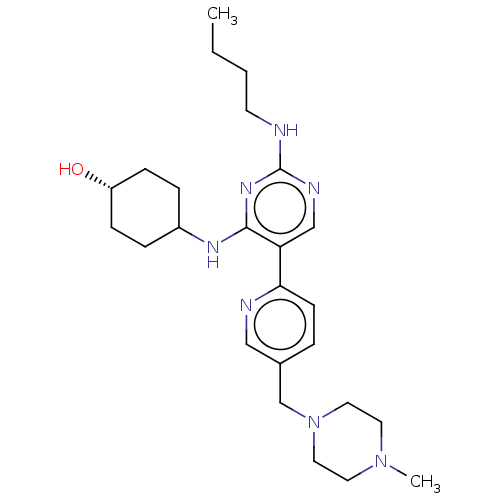 (US9649309, Compound UNC2251B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308351 (US9649309, Compound UNC4167A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350851 (UNC2123A | US10004755, Compound UNC2123A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Inhibition constants of MerTK, Flt3, Tyro3 and Axl kinase activity by an active compound as described herein is determined at the Km for ATP using a ... | Bioorg Med Chem 17: 7884-93 (2009) BindingDB Entry DOI: 10.7270/Q26H4KR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515806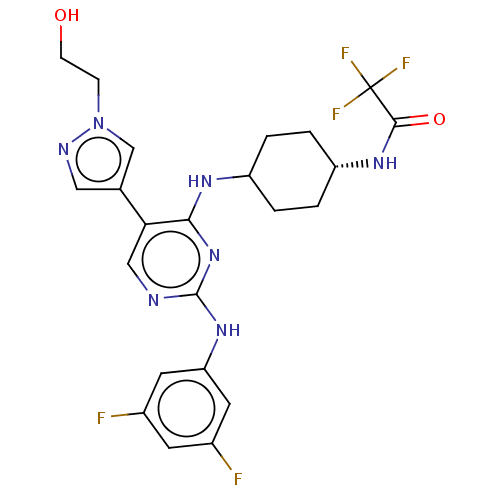 (US11053225, Compound 115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM497267 ((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MERTK (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113534 BindingDB Entry DOI: 10.7270/Q2M90DGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM497276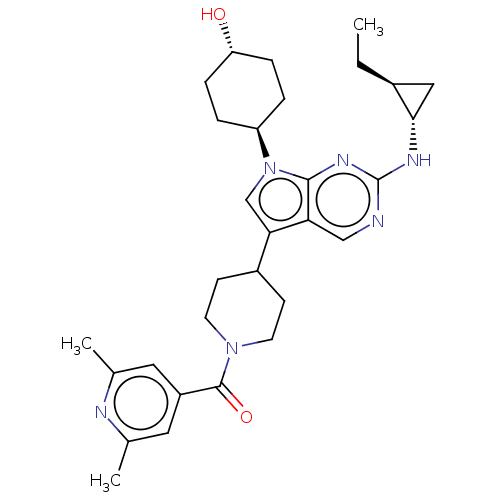 ((2,6-dimethylpyridin-4- yl)(4-(2-(((1S,2S)-2- ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MERTK (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113534 BindingDB Entry DOI: 10.7270/Q2M90DGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM515798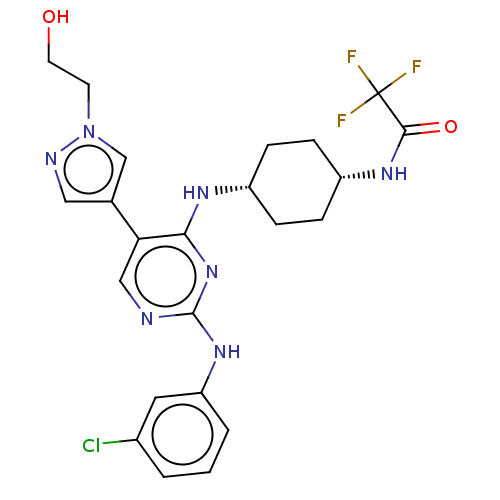 (US11053225, Compound 100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... | Citation and Details BindingDB Entry DOI: 10.7270/Q2JM2DST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM350850 (UNC2095A | US10004755, Compound UNC2095A | US97956...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Inhibition constants of MerTK, Flt3, Tyro3 and Axl kinase activity by an active compound as described herein is determined at the Km for ATP using a ... | Bioorg Med Chem 17: 7884-93 (2009) BindingDB Entry DOI: 10.7270/Q26H4KR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM401001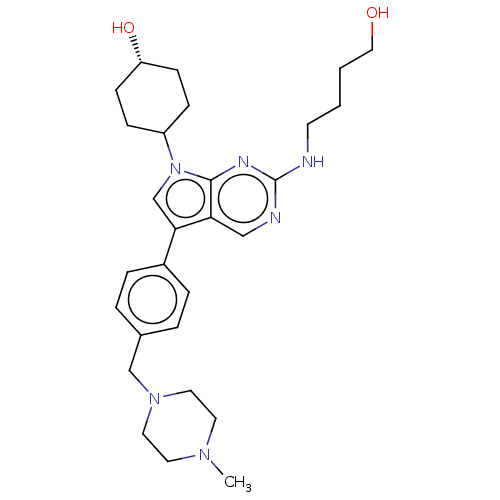 (US10004755, Compound UNC2396A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Inhibition constants of MerTK, Flt3, Tyro3 and Axl kinase activity by an active compound as described herein is determined at the Km for ATP using a ... | Bioorg Med Chem 17: 7884-93 (2009) BindingDB Entry DOI: 10.7270/Q26H4KR2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM308317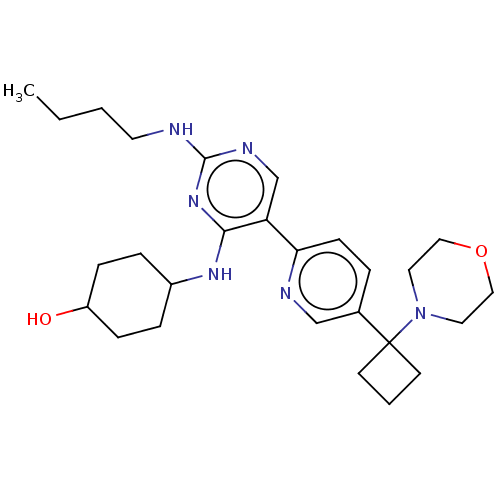 (US9649309, Compound UNC3666A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Briefly, activity assays were performed in a 384 well, polypropylene microplate in a final volume of 50 μL of 50 mM Hepes, Ph 7.4 containing 10 ... | US Patent US9649309 (2017) BindingDB Entry DOI: 10.7270/Q26Q209H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4084 total ) | Next | Last >> |