 Found 21553 hits of ic50 data for polymerid = 50003688,520
Found 21553 hits of ic50 data for polymerid = 50003688,520 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM350321
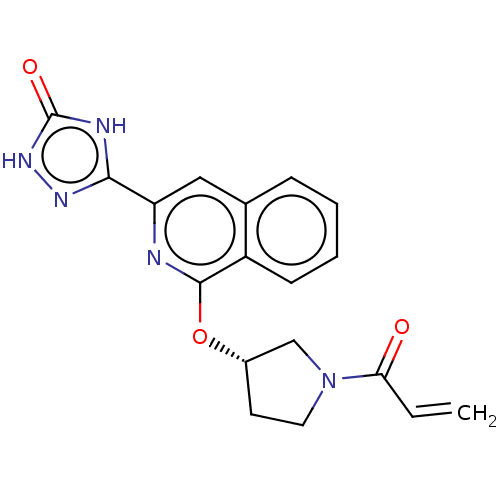
((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human EGFR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Mer
(Homo sapiens (Human)) | BDBM515773
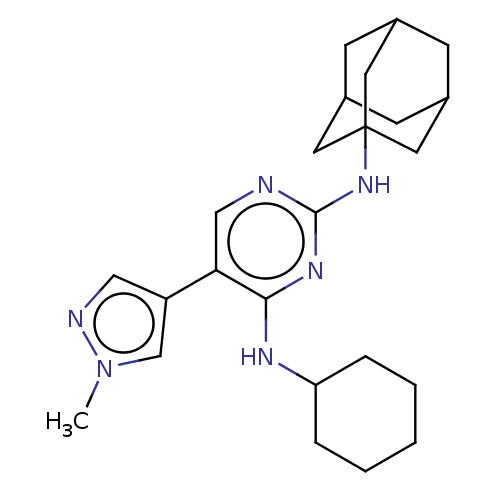
(US11053225, Compound 23)Show SMILES Cn1cc(cn1)-c1cnc(NC23CC4CC(CC(C4)C2)C3)nc1NC1CCCCC1 |TLB:20:11:18:14.15.16,THB:10:11:18:14.15.16,20:15:18:11.19.12,19:11:14:18.17.16,19:17:14:11.20.12| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
For evaluation of inhibitory activities of the compounds according to the present invention on Tyro 3, Axl, and Mer, the following test was carried o... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JM2DST |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal GST tagged EGFR L858R/T790M double mutant (669 to 1210 residues) expressed in insect expression system usi... |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3585
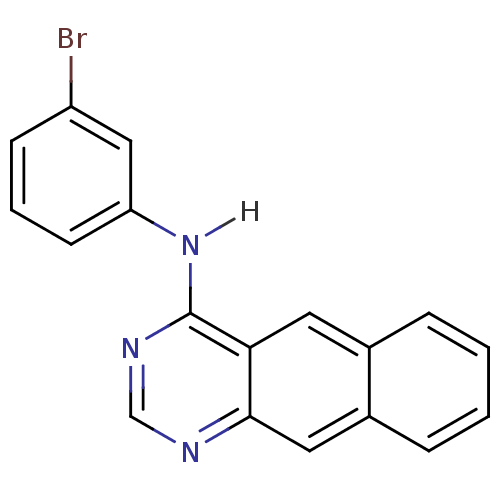
(4-[(3-Bromophenyl)amino]benzo[g]quinazoline | Benz...)Show InChI InChI=1S/C18H12BrN3/c19-14-6-3-7-15(10-14)22-18-16-8-12-4-1-2-5-13(12)9-17(16)20-11-21-18/h1-11H,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 918-28 (1996)
Article DOI: 10.1021/jm950692f
BindingDB Entry DOI: 10.7270/Q2222RZB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3556
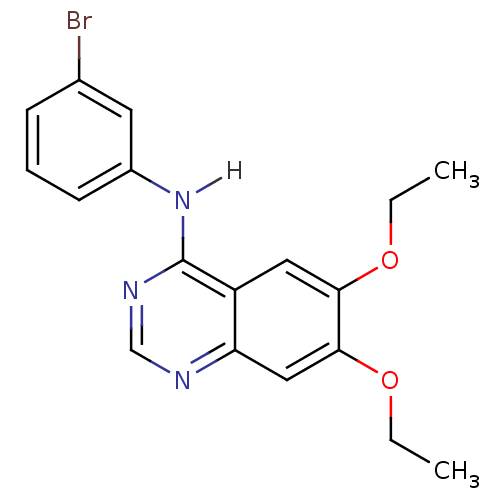
(4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...)Show InChI InChI=1S/C18H18BrN3O2/c1-3-23-16-9-14-15(10-17(16)24-4-2)20-11-21-18(14)22-13-7-5-6-12(19)8-13/h5-11H,3-4H2,1-2H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR |
Proc Natl Acad Sci USA 104: 20523-8 (2007)
Checked by Author
Article DOI: 10.1073/pnas.0708800104
BindingDB Entry DOI: 10.7270/Q2DB82RH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3556

(4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...)Show InChI InChI=1S/C18H18BrN3O2/c1-3-23-16-9-14-15(10-17(16)24-4-2)20-11-21-18(14)22-13-7-5-6-12(19)8-13/h5-11H,3-4H2,1-2H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 267-76 (1996)
Article DOI: 10.1021/jm9503613
BindingDB Entry DOI: 10.7270/Q25T3HPR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3604
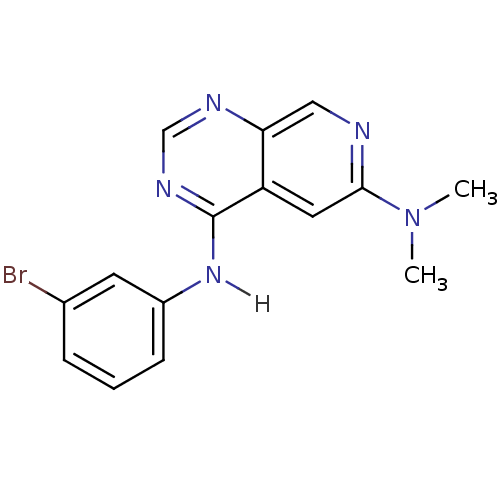
(4-N-(3-bromophenyl)-6-N,6-N-dimethylpyrido[3,4-d]p...)Show InChI InChI=1S/C15H14BrN5/c1-21(2)14-7-12-13(8-17-14)18-9-19-15(12)20-11-5-3-4-10(16)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3604

(4-N-(3-bromophenyl)-6-N,6-N-dimethylpyrido[3,4-d]p...)Show InChI InChI=1S/C15H14BrN5/c1-21(2)14-7-12-13(8-17-14)18-9-19-15(12)20-11-5-3-4-10(16)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 41: 742-51 (1998)
Article DOI: 10.1021/jm970641d
BindingDB Entry DOI: 10.7270/Q2DB800T |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3556

(4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...)Show InChI InChI=1S/C18H18BrN3O2/c1-3-23-16-9-14-15(10-17(16)24-4-2)20-11-21-18(14)22-13-7-5-6-12(19)8-13/h5-11H,3-4H2,1-2H3,(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00603 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zurich
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
J Med Chem 51: 1179-88 (2008)
Article DOI: 10.1021/jm070654j
BindingDB Entry DOI: 10.7270/Q29Z95RD |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3570
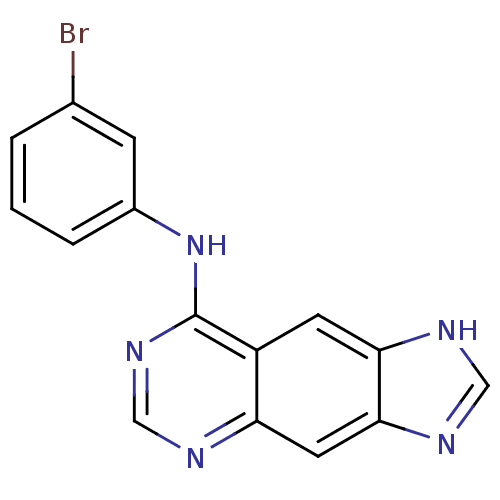
(8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...)Show InChI InChI=1S/C15H10BrN5/c16-9-2-1-3-10(4-9)21-15-11-5-13-14(19-7-18-13)6-12(11)17-8-20-15/h1-8H,(H,18,19)(H,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 42: 5464-74 (1999)
Article DOI: 10.1021/jm9903949
BindingDB Entry DOI: 10.7270/Q28K7783 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3603

(4-N-(3-bromophenyl)-6-N-methylpyrido[3,4-d]pyrimid...)Show InChI InChI=1S/C14H12BrN5/c1-16-13-6-11-12(7-17-13)18-8-19-14(11)20-10-4-2-3-9(15)5-10/h2-8H,1H3,(H,16,17)(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Parke-Davis Pharmaceutical Research
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 42: 5464-74 (1999)
Article DOI: 10.1021/jm9903949
BindingDB Entry DOI: 10.7270/Q28K7783 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3603

(4-N-(3-bromophenyl)-6-N-methylpyrido[3,4-d]pyrimid...)Show InChI InChI=1S/C14H12BrN5/c1-16-13-6-11-12(7-17-13)18-8-19-14(11)20-10-4-2-3-9(15)5-10/h2-8H,1H3,(H,16,17)(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3570

(8-[(3-Bromophenyl)amino]-1H-imidazo[4,5-g]quinazol...)Show InChI InChI=1S/C15H10BrN5/c16-9-2-1-3-10(4-9)21-15-11-5-13-14(19-7-18-13)6-12(11)17-8-20-15/h1-8H,(H,18,19)(H,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 918-28 (1996)
Article DOI: 10.1021/jm950692f
BindingDB Entry DOI: 10.7270/Q2222RZB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3603

(4-N-(3-bromophenyl)-6-N-methylpyrido[3,4-d]pyrimid...)Show InChI InChI=1S/C14H12BrN5/c1-16-13-6-11-12(7-17-13)18-8-19-14(11)20-10-4-2-3-9(15)5-10/h2-8H,1H3,(H,16,17)(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 41: 742-51 (1998)
Article DOI: 10.1021/jm970641d
BindingDB Entry DOI: 10.7270/Q2DB800T |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3572
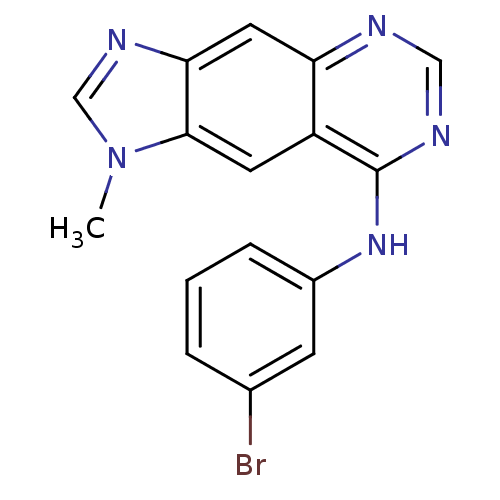
(8-[(3-Bromophenyl)amino]-1-methyl-1H-imidazo[4,5-g...)Show InChI InChI=1S/C16H12BrN5/c1-22-9-20-14-7-13-12(6-15(14)22)16(19-8-18-13)21-11-4-2-3-10(17)5-11/h2-9H,1H3,(H,18,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 918-28 (1996)
Article DOI: 10.1021/jm950692f
BindingDB Entry DOI: 10.7270/Q2222RZB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823
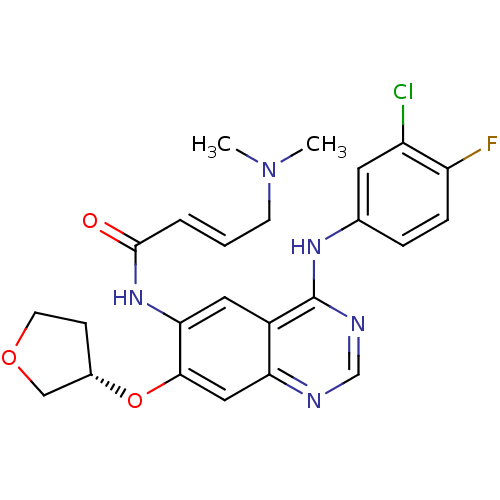
((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged EGFR (669 to 1210 residues) expressed in baculovirus infected Sf9 insect cells using TK as substrate preinc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00870
BindingDB Entry DOI: 10.7270/Q2VQ368T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50322823

((S)-N-(4-(3-chloro-4-fluorophenylamino)-7-(tetrahy...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cc1O[C@H]1CCOC1 |r| Show InChI InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged EGFR L858R mutant (669 to 1210 residues) expressed in baculovirus infected Sf9 insect cells using TK as sub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00870
BindingDB Entry DOI: 10.7270/Q2VQ368T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM504408

(US11040984, Compound 6)Show SMILES COc1cc2ncnc(Nc3ccc(Cl)c(Cl)c3F)c2cc1N1CC2(CCN(C)CC2)OC1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FN199B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM504412
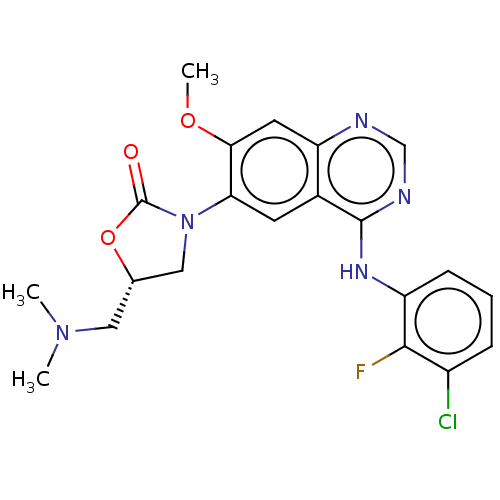
(US11040984, Compound 18)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1N1C[C@H](CN(C)C)OC1=O |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FN199B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM504411
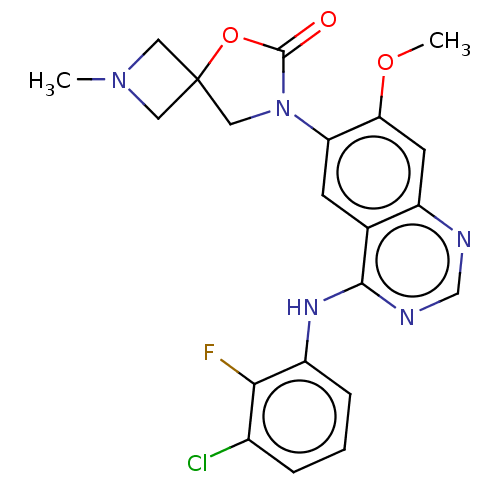
(US11040984, Compound 13)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1N1CC2(CN(C)C2)OC1=O | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FN199B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM504410
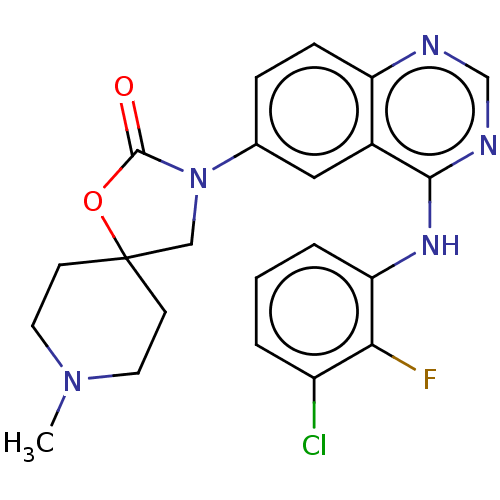
(US11040984, Compound 12)Show SMILES CN1CCC2(CN(C(=O)O2)c2ccc3ncnc(Nc4cccc(Cl)c4F)c3c2)CC1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FN199B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50161957
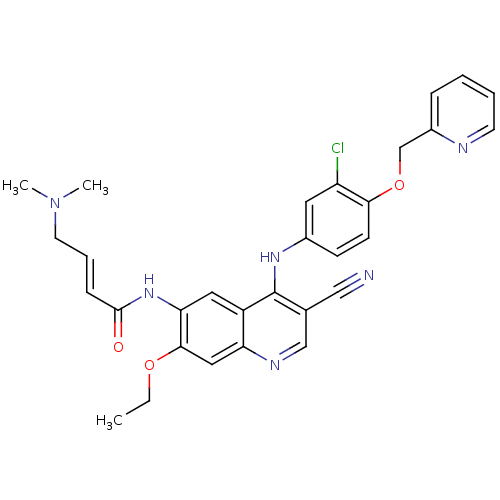
(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged EGFR (669 to 1210 residues) expressed in baculovirus infected Sf9 insect cells using TK as substrate preinc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00870
BindingDB Entry DOI: 10.7270/Q2VQ368T |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50505838
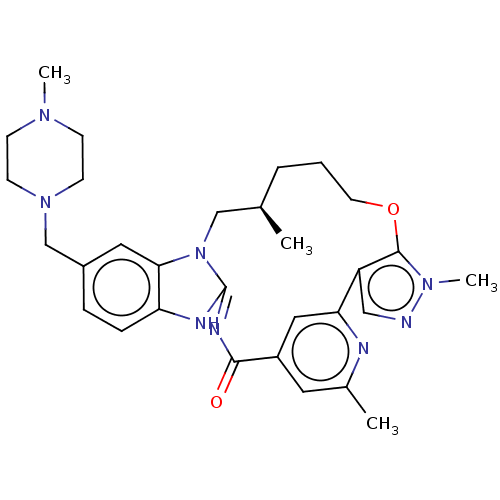
(CHEMBL4537790)Show SMILES C[C@@H]1CCCOc2c(cnn2C)-c2cc(cc(C)n2)C(=O)\N=C2/Nc3ccc(CN4CCN(C)CC4)cc3N2C1 |r,c:23| Show InChI InChI=1S/C30H38N8O2/c1-20-6-5-13-40-29-24(17-31-36(29)4)26-16-23(14-21(2)32-26)28(39)34-30-33-25-8-7-22(15-27(25)38(30)18-20)19-37-11-9-35(3)10-12-37/h7-8,14-17,20H,5-6,9-13,18-19H2,1-4H3,(H,33,34,39)/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01041
BindingDB Entry DOI: 10.7270/Q2B280CC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50161957

(4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(OCc4ccccn4)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H29ClN6O3/c1-4-39-28-16-25-23(15-26(28)36-29(38)9-7-13-37(2)3)30(20(17-32)18-34-25)35-21-10-11-27(24(31)14-21)40-19-22-8-5-6-12-33-22/h5-12,14-16,18H,4,13,19H2,1-3H3,(H,34,35)(H,36,38)/b9-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged EGFR L858R mutant (669 to 1210 residues) expressed in baculovirus infected Sf9 insect cells using TK as sub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00870
BindingDB Entry DOI: 10.7270/Q2VQ368T |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged EGFR L858R/T790M mutant (669 to 1210 residues) expressed in baculovirus infected Sf9 insect cells using TK ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00870
BindingDB Entry DOI: 10.7270/Q2VQ368T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM504415
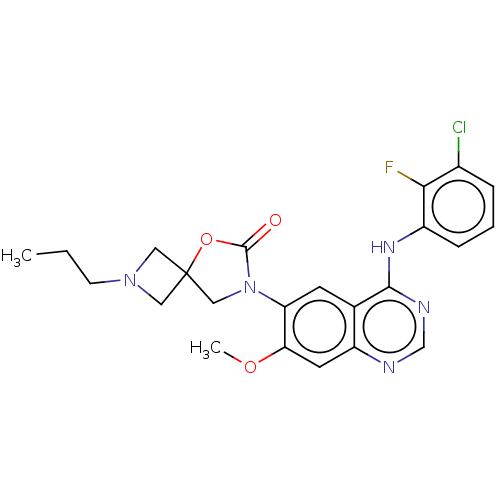
(US11040984, Compound 30)Show SMILES CCCN1CC2(C1)CN(C(=O)O2)c1cc2c(Nc3cccc(Cl)c3F)ncnc2cc1OC | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FN199B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM504414
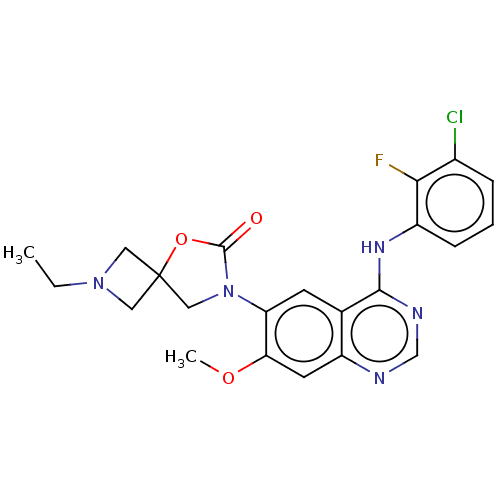
(US11040984, Compound 25)Show SMILES CCN1CC2(C1)CN(C(=O)O2)c1cc2c(Nc3cccc(Cl)c3F)ncnc2cc1OC | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FN199B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032
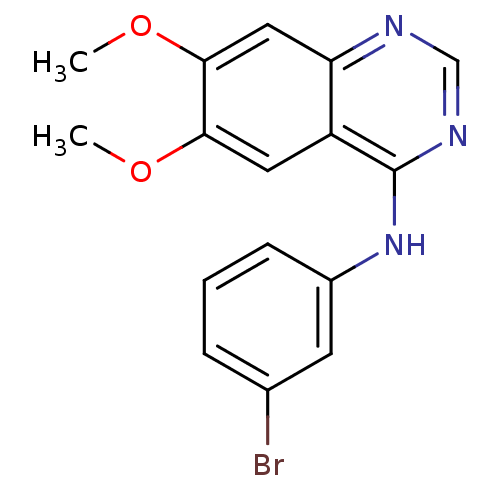
(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research
| Assay Description
Concentration required to inhibit the phosphorylation of a 14-residue fragment of phospholipase C gamma 1 by Epidermal growth factor receptor from A4... |
J Med Chem 39: 267-76 (1996)
Article DOI: 10.1021/jm9503613
BindingDB Entry DOI: 10.7270/Q25T3HPR |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032

(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Proc Natl Acad Sci USA 104: 20523-8 (2007)
Checked by Author
Article DOI: 10.1073/pnas.0708800104
BindingDB Entry DOI: 10.7270/Q2DB82RH |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3574
(8-[(3-Bromophenyl)amino]-3-methyl-3H-imidazo[4,5-g...)Show InChI InChI=1S/C16H12BrN5/c1-22-9-20-14-6-12-13(7-15(14)22)18-8-19-16(12)21-11-4-2-3-10(17)5-11/h2-9H,1H3,(H,18,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland
| Assay Description
IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... |
J Med Chem 39: 918-28 (1996)
Article DOI: 10.1021/jm950692f
BindingDB Entry DOI: 10.7270/Q2222RZB |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032

(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Beijing University of Technology
Curated by ChEMBL
| Assay Description
Competitive binding affinity to EGFR (unknown origin) ATP binding site |
Bioorg Med Chem 24: 2871-2881 (2016)
Article DOI: 10.1016/j.bmc.2016.01.003
BindingDB Entry DOI: 10.7270/Q2NP269P |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032

(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Eur J Med Chem 44: 448-52 (2008)
Article DOI: 10.1016/j.ejmech.2008.01.009
BindingDB Entry DOI: 10.7270/Q2862G7V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032

(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Jazan University
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor kinase (unknown origin) using [33P]-ATP after 20 to 30 mins by radiometric assay |
Eur J Med Chem 102: 115-31 (2015)
Article DOI: 10.1016/j.ejmech.2015.07.030
BindingDB Entry DOI: 10.7270/Q2JQ12TS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032

(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
| Assay Description
Inhibitory potency against isolated epidermal growth factor receptor (EGFR) from human A431 carcinoma cells |
J Med Chem 39: 1823-35 (1996)
Article DOI: 10.1021/jm9508651
BindingDB Entry DOI: 10.7270/Q2X928GF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3032

(CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...)Show InChI InChI=1S/C16H14BrN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a |
Arromax Pharmatech Co. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Eur J Med Chem 170: 55-72 (2019)
Article DOI: 10.1016/j.ejmech.2019.03.004
BindingDB Entry DOI: 10.7270/Q2474F5D |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50326519
((E)-N-(4-(3-chloro-4-fluorophenylamino)-3-cyano-7-...)Show SMILES CCOc1cc2ncc(C#N)c(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CNCC#C Show InChI InChI=1S/C25H21ClFN5O2/c1-3-9-29-10-5-6-24(33)32-22-12-18-21(13-23(22)34-4-2)30-15-16(14-28)25(18)31-17-7-8-20(27)19(26)11-17/h1,5-8,11-13,15,29H,4,9-10H2,2H3,(H,30,31)(H,32,33)/b6-5+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem 18: 6634-45 (2010)
Article DOI: 10.1016/j.bmc.2010.08.004
BindingDB Entry DOI: 10.7270/Q25H7GG2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50117657
(CHEMBL3613702)Show SMILES Fc1cc(Nc2ncnc3ccc(NC(=O)C=C)cc23)cc(Cl)c1Cl Show InChI InChI=1S/C17H11Cl2FN4O/c1-2-15(25)23-9-3-4-14-11(5-9)17(22-8-21-14)24-10-6-12(18)16(19)13(20)7-10/h2-8H,1H2,(H,23,25)(H,21,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
Jazan University
Curated by ChEMBL
| Assay Description
Inhibition of epidermal growth factor receptor kinase (unknown origin) using [33P]-ATP after 20 to 30 mins by radiometric assay |
Eur J Med Chem 102: 115-31 (2015)
Article DOI: 10.1016/j.ejmech.2015.07.030
BindingDB Entry DOI: 10.7270/Q2JQ12TS |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM504413
(US11040984, Compound 23)Show SMILES COC[C@@H]1CN(C(=O)O1)c1cc2c(Nc3cccc(Cl)c3F)ncnc2cc1OC |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Buffer formulation: a buffer consisted of 50 mM HEPES (pH 7.5), 0.01% BSA, 5 mM MgCl2, 0.1 mM Orthovanadate. After the buffer was formulated, an enzy... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2FN199B |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50383274
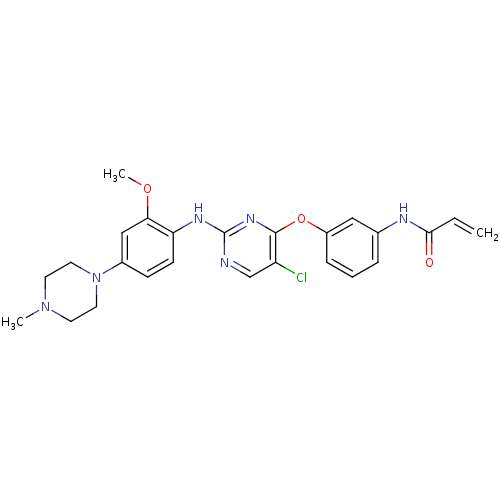
(CHEMBL1229592 | US10167264, WZ4002 | US9670213, WZ...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Oc2cccc(NC(=O)C=C)c2)n1)N1CCN(C)CC1 Show InChI InChI=1S/C25H27ClN6O3/c1-4-23(33)28-17-6-5-7-19(14-17)35-24-20(26)16-27-25(30-24)29-21-9-8-18(15-22(21)34-3)32-12-10-31(2)11-13-32/h4-9,14-16H,1,10-13H2,2-3H3,(H,28,33)(H,27,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal GST tagged EGFR L858R/T790M double mutant (669 to 1210 residues) expressed in insect expression system usi... |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50171516
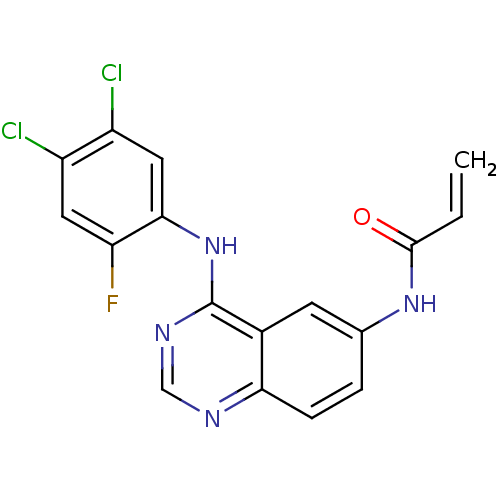
(CHEMBL363815 | N-[4-(4,5-Dichloro-2-fluoro-phenyla...)Show SMILES Fc1cc(Cl)c(Cl)cc1Nc1ncnc2ccc(NC(=O)C=C)cc12 Show InChI InChI=1S/C17H11Cl2FN4O/c1-2-16(25)23-9-3-4-14-10(5-9)17(22-8-21-14)24-15-7-12(19)11(18)6-13(15)20/h2-8H,1H2,(H,23,25)(H,21,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Hadassah Hebrew University
Curated by ChEMBL
| Assay Description
Inhibition of EGF Receptor autophosphorylation in A431 cell lysate |
J Med Chem 48: 5337-48 (2005)
Article DOI: 10.1021/jm0580196
BindingDB Entry DOI: 10.7270/Q28S4PG3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530623
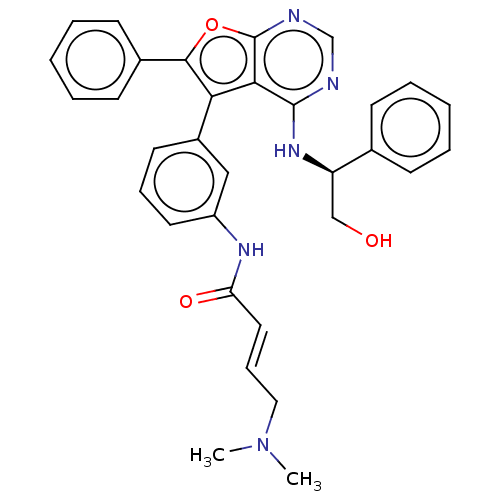
(CHEMBL4521381)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)-c1c(oc2ncnc(N[C@H](CO)c3ccccc3)c12)-c1ccccc1 |r| Show InChI InChI=1S/C32H31N5O3/c1-37(2)18-10-17-27(39)35-25-16-9-15-24(19-25)28-29-31(36-26(20-38)22-11-5-3-6-12-22)33-21-34-32(29)40-30(28)23-13-7-4-8-14-23/h3-17,19,21,26,38H,18,20H2,1-2H3,(H,35,39)(H,33,34,36)/b17-10+/t26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50530623

(CHEMBL4521381)Show SMILES CN(C)C\C=C\C(=O)Nc1cccc(c1)-c1c(oc2ncnc(N[C@H](CO)c3ccccc3)c12)-c1ccccc1 |r| Show InChI InChI=1S/C32H31N5O3/c1-37(2)18-10-17-27(39)35-25-16-9-15-24(19-25)28-29-31(36-26(20-38)22-11-5-3-6-12-22)33-21-34-32(29)40-30(28)23-13-7-4-8-14-23/h3-17,19,21,26,38H,18,20H2,1-2H3,(H,35,39)(H,33,34,36)/b17-10+/t26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged human EGFR A763_Y764insFHEA mutant using poly(Glu, Tyr) 4:1 substrate incubated for 120 mins by kinase-Glo plus luminescent ... |
J Med Chem 62: 10108-10123 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00722
BindingDB Entry DOI: 10.7270/Q21V5JFW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | CHEMBL5271803
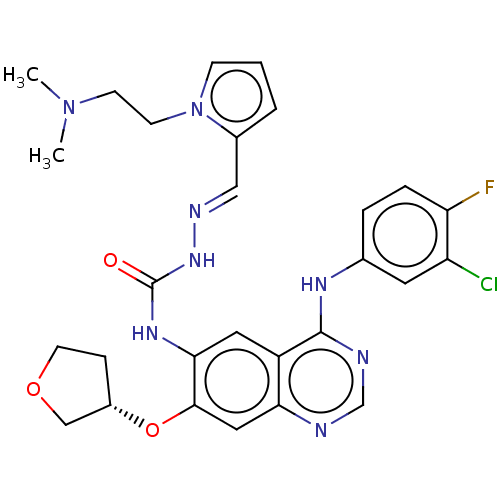
Show SMILES OC(=O)\C=C\C(O)=O.COc1ccccc1C(=O)NC(=O)N[C@H]1CN2CCC1CC2 |r,wD:22.21,(10.8,-5.36,;9.3,-5.62,;8.31,-4.43,;8.53,-6.95,;6.99,-6.95,;6.22,-8.27,;6.99,-9.61,;4.69,-8.27,;-8.23,-3.56,;-6.91,-4.3,;-6.89,-5.84,;-8.22,-6.61,;-8.22,-8.17,;-6.89,-8.94,;-5.56,-8.17,;-5.56,-6.61,;-4.23,-5.84,;-4.25,-4.3,;-2.9,-6.6,;-1.57,-5.83,;-1.57,-4.29,;-.22,-6.58,;1.1,-5.81,;2.48,-6.61,;3.89,-5.81,;3.89,-4.17,;2.48,-3.37,;1.1,-4.17,;2.8,-4.59,;1.64,-5.51,)| Show InChI InChI=1S/C16H21N3O3.C4H4O4/c1-22-14-5-3-2-4-12(14)15(20)18-16(21)17-13-10-19-8-6-11(13)7-9-19;5-3(6)1-2-4(7)8/h2-5,11,13H,6-10H2,1H3,(H2,17,18,20,21);1-2H,(H,5,6)(H,7,8)/b;2-1+/t13-;/m0./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50592748

(CHEMBL5193123)Show SMILES COc1cc(N2CCC(CC2)N2CCCN(C)CC2)c(C)cc1Nc1ncc(Br)c(Nc2ccc3OCCOc3c2P(C)(C)=O)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116907
BindingDB Entry DOI: 10.7270/Q2J96BC1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro thromboxane A2 receptor antagonism through inhibition of U-46619-induced contraction of rat isolated thoracic aortic strip |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50592743
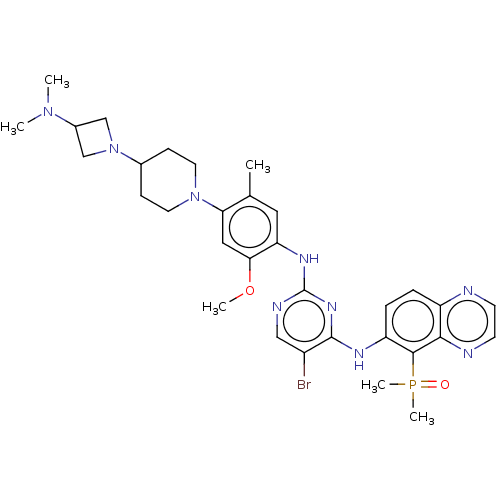
(CHEMBL5186680)Show SMILES COc1cc(N2CCC(CC2)N2CC(C2)N(C)C)c(C)cc1Nc1ncc(Br)c(Nc2ccc3nccnc3c2P(C)(C)=O)n1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116907
BindingDB Entry DOI: 10.7270/Q2J96BC1 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5445

(CHEMBL554 | GW572016 | LAPATINIB DITOSYLATE | Lapa...)Show SMILES CS(=O)(=O)CCNCc1ccc(o1)-c1ccc2ncnc(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)c2c1 Show InChI InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged EGFR (669 to 1210 residues) expressed in baculovirus infected Sf9 insect cells using TK as substrate preinc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00870
BindingDB Entry DOI: 10.7270/Q2VQ368T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human N-terminal GST-tagged EGFR L858R mutant (669 to 1210 residues) expressed in baculovirus infected Sf9 insect cells using TK as sub... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00870
BindingDB Entry DOI: 10.7270/Q2VQ368T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
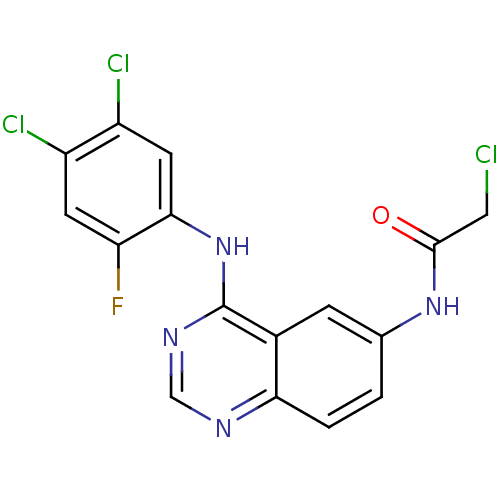
(Homo sapiens (Human)) | BDBM50171514
(2-Chloro-N-[4-(4,5-dichloro-2-fluoro-phenylamino)-...)Show SMILES Fc1cc(Cl)c(Cl)cc1Nc1ncnc2ccc(NC(=O)CCl)cc12 Show InChI InChI=1S/C16H10Cl3FN4O/c17-6-15(25)23-8-1-2-13-9(3-8)16(22-7-21-13)24-14-5-11(19)10(18)4-12(14)20/h1-5,7H,6H2,(H,23,25)(H,21,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Hadassah Hebrew University
Curated by ChEMBL
| Assay Description
Inhibition of EGF Receptor autophosphorylation in A431 cell lysate |
J Med Chem 48: 5337-48 (2005)
Article DOI: 10.1021/jm0580196
BindingDB Entry DOI: 10.7270/Q28S4PG3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
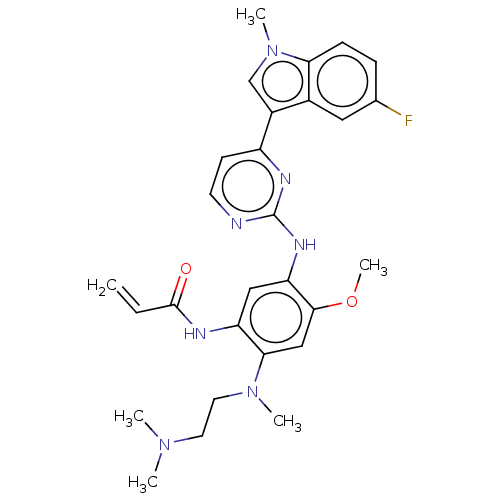
(Homo sapiens (Human)) | BDBM50260360
(CHEMBL4103912)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccc(F)cc12 Show InChI InChI=1S/C28H32FN7O2/c1-7-27(37)31-22-15-23(26(38-6)16-25(22)35(4)13-12-34(2)3)33-28-30-11-10-21(32-28)20-17-36(5)24-9-8-18(29)14-19(20)24/h7-11,14-17H,1,12-13H2,2-6H3,(H,31,37)(H,30,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangxi Science & Technology Normal University
Curated by ChEMBL
| Assay Description
Inhibition of EGFR T790M/L858R double mutant (unknown origin) using substrate-biotin by ELISA-based mobility shift assay |
Eur J Med Chem 163: 367-380 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.069
BindingDB Entry DOI: 10.7270/Q2BP0677 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data