 Found 282 hits of ic50 data for polymerid = 50004900,5024
Found 282 hits of ic50 data for polymerid = 50004900,5024 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179960

(1-[8-(2-acetyl-phenyl)-2-chloro-11,12-dihydro-6H-d...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1C(C)=O Show InChI InChI=1S/C25H22ClNO2/c1-16(28)23-5-3-4-6-24(23)19-9-7-18-8-10-20-14-22(26)11-12-25(20)27(17(2)29)15-21(18)13-19/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179935
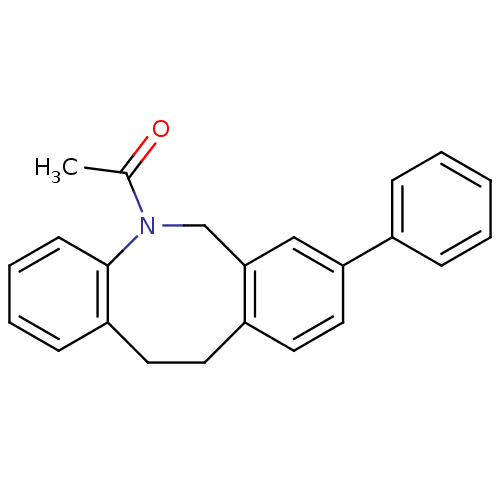
(1-(8-Phenyl-11,12-dihydro-6H-dibenzo[b,f]azocin-5-...)Show InChI InChI=1S/C23H21NO/c1-17(25)24-16-22-15-21(18-7-3-2-4-8-18)14-12-19(22)11-13-20-9-5-6-10-23(20)24/h2-10,12,14-15H,11,13,16H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179969
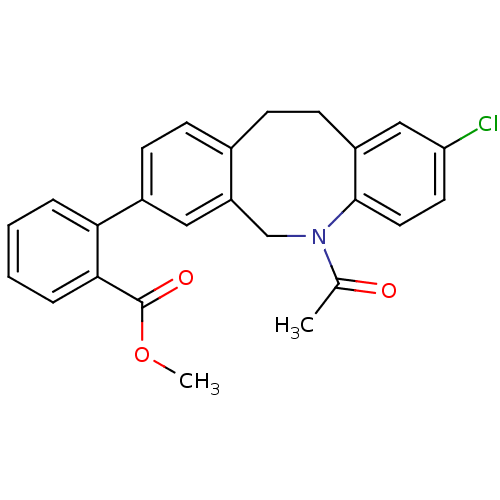
(2-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179969

(2-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179960

(1-[8-(2-acetyl-phenyl)-2-chloro-11,12-dihydro-6H-d...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1C(C)=O Show InChI InChI=1S/C25H22ClNO2/c1-16(28)23-5-3-4-6-24(23)19-9-7-18-8-10-20-14-22(26)11-12-25(20)27(17(2)29)15-21(18)13-19/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179940

(2-(5-acetyl-2-bromo-5,6,11,12-tetrahydro-dibenzo[b...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Br)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22BrNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179964
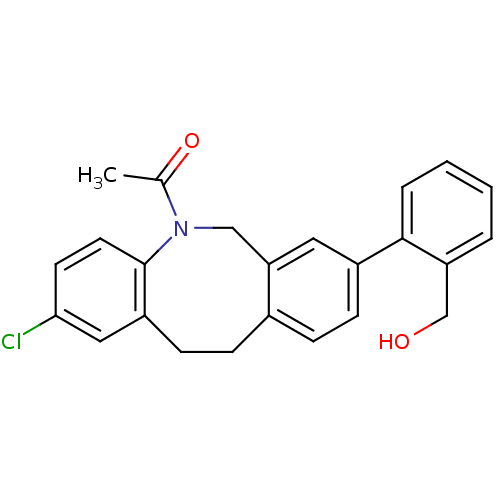
(1-[2-chloro-8-(2-hydroxymethyl-phenyl)-11,12-dihyd...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1CO Show InChI InChI=1S/C24H22ClNO2/c1-16(28)26-14-21-12-18(23-5-3-2-4-20(23)15-27)8-6-17(21)7-9-19-13-22(25)10-11-24(19)26/h2-6,8,10-13,27H,7,9,14-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381600
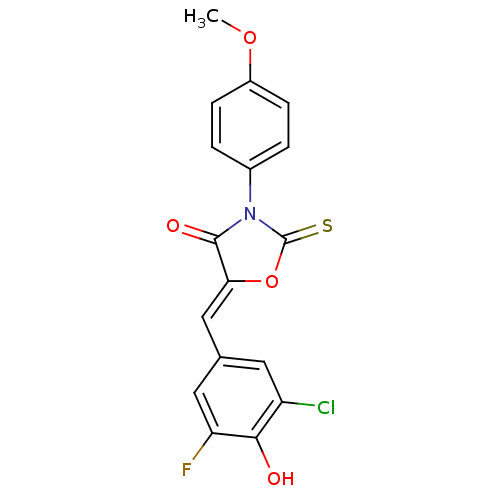
(CHEMBL2018254)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2cc(F)c(O)c(Cl)c2)C1=O Show InChI InChI=1S/C17H11ClFNO4S/c1-23-11-4-2-10(3-5-11)20-16(22)14(24-17(20)25)8-9-6-12(18)15(21)13(19)7-9/h2-8,21H,1H3/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179940

(2-(5-acetyl-2-bromo-5,6,11,12-tetrahydro-dibenzo[b...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Br)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22BrNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179967
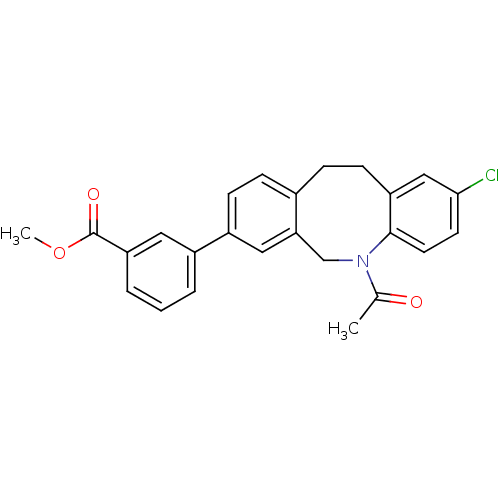
(3-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1cccc(c1)-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-22-13-19(18-4-3-5-21(12-18)25(29)30-2)8-6-17(22)7-9-20-14-23(26)10-11-24(20)27/h3-6,8,10-14H,7,9,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305335

(7-hydroxy-4-((6-methylpyridin-2-ylthio)methyl)-2H-...)Show InChI InChI=1S/C16H13NO3S/c1-10-3-2-4-15(17-10)21-9-11-7-16(19)20-14-8-12(18)5-6-13(11)14/h2-8,18H,9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381570
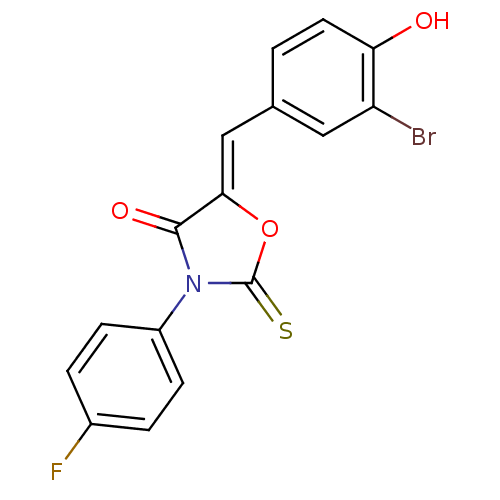
(CHEMBL2018139)Show SMILES Oc1ccc(\C=C2/OC(=S)N(C2=O)c2ccc(F)cc2)cc1Br Show InChI InChI=1S/C16H9BrFNO3S/c17-12-7-9(1-6-13(12)20)8-14-15(21)19(16(23)22-14)11-4-2-10(18)3-5-11/h1-8,20H/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381572
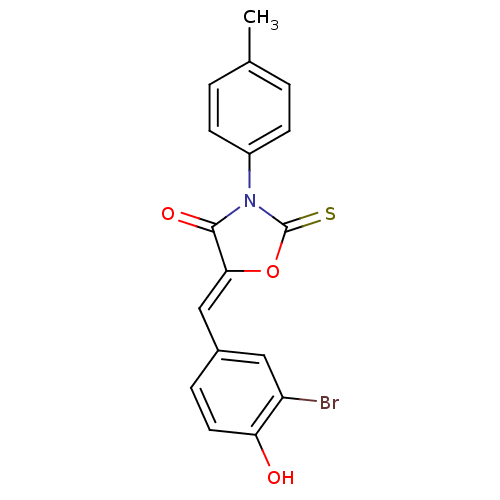
(CHEMBL2018141)Show SMILES Cc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO3S/c1-10-2-5-12(6-3-10)19-16(21)15(22-17(19)23)9-11-4-7-14(20)13(18)8-11/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381596
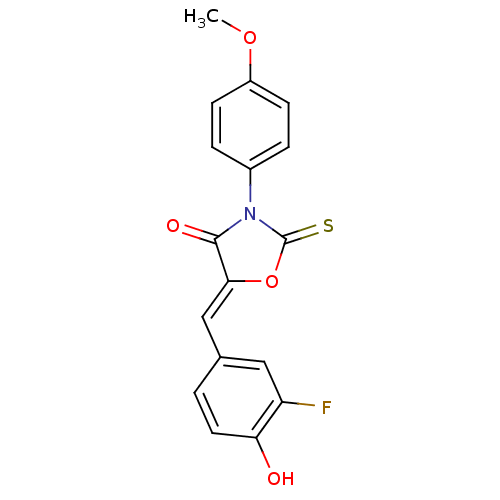
(CHEMBL2018249)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(F)c2)C1=O Show InChI InChI=1S/C17H12FNO4S/c1-22-12-5-3-11(4-6-12)19-16(21)15(23-17(19)24)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179936
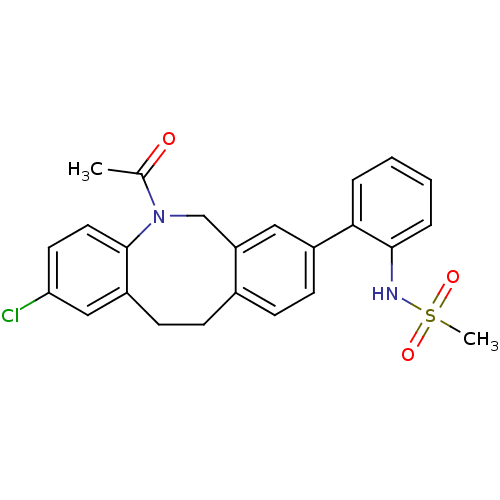
(CHEMBL382563 | N-[2-(5-acetyl-2-chloro-5,6,11,12-t...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1NS(C)(=O)=O Show InChI InChI=1S/C24H23ClN2O3S/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)26-31(2,29)30)9-7-17(20)8-10-19-14-21(25)11-12-24(19)27/h3-7,9,11-14,26H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179964

(1-[2-chloro-8-(2-hydroxymethyl-phenyl)-11,12-dihyd...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1CO Show InChI InChI=1S/C24H22ClNO2/c1-16(28)26-14-21-12-18(23-5-3-2-4-20(23)15-27)8-6-17(21)7-9-19-13-22(25)10-11-24(19)26/h2-6,8,10-13,27H,7,9,14-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179967

(3-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1cccc(c1)-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-22-13-19(18-4-3-5-21(12-18)25(29)30-2)8-6-17(22)7-9-20-14-23(26)10-11-24(20)27/h3-6,8,10-14H,7,9,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179932

(1-[2-chloro-8-(2-methoxy-phenyl)-11,12-dihydro-6H-...)Show SMILES COc1ccccc1-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C24H22ClNO2/c1-16(27)26-15-20-13-18(22-5-3-4-6-24(22)28-2)9-7-17(20)8-10-19-14-21(25)11-12-23(19)26/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381564
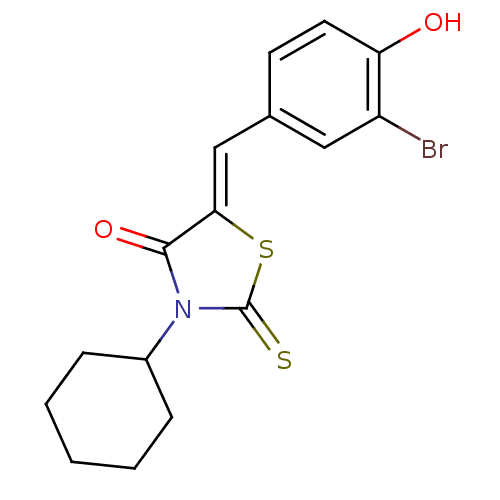
(CHEMBL2018131)Show InChI InChI=1S/C16H16BrNO2S2/c17-12-8-10(6-7-13(12)19)9-14-15(20)18(16(21)22-14)11-4-2-1-3-5-11/h6-9,11,19H,1-5H2/b14-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381573

(CHEMBL2018142)Show SMILES Oc1ccc(\C=C2/OC(=S)N(C2=O)c2ccc(cc2)C(F)(F)F)cc1Br Show InChI InChI=1S/C17H9BrF3NO3S/c18-12-7-9(1-6-13(12)23)8-14-15(24)22(16(26)25-14)11-4-2-10(3-5-11)17(19,20)21/h1-8,23H/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305325
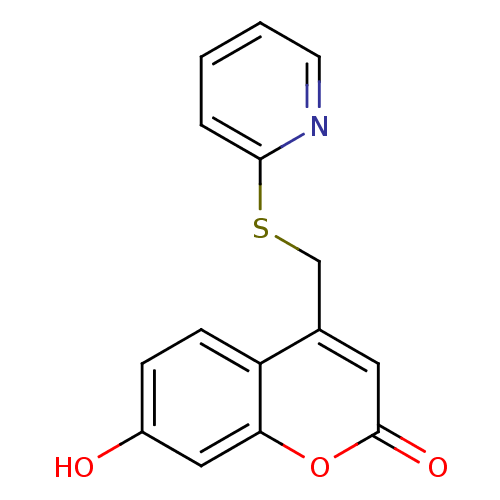
(7-hydroxy-4-((pyridin-2-ylthio)methyl)-2H-chromen-...)Show InChI InChI=1S/C15H11NO3S/c17-11-4-5-12-10(7-15(18)19-13(12)8-11)9-20-14-3-1-2-6-16-14/h1-8,17H,9H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179939
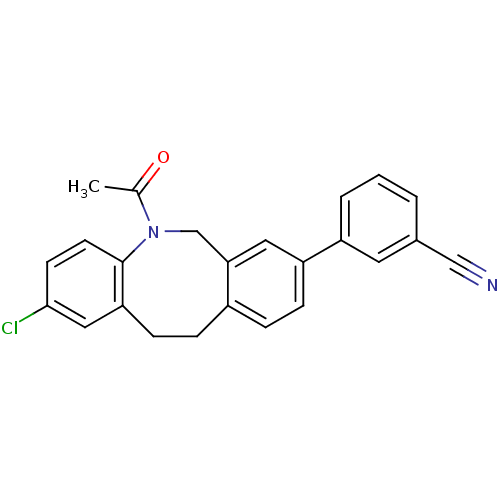
(3-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1cccc(c1)C#N Show InChI InChI=1S/C24H19ClN2O/c1-16(28)27-15-22-12-20(19-4-2-3-17(11-19)14-26)7-5-18(22)6-8-21-13-23(25)9-10-24(21)27/h2-5,7,9-13H,6,8,15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179970
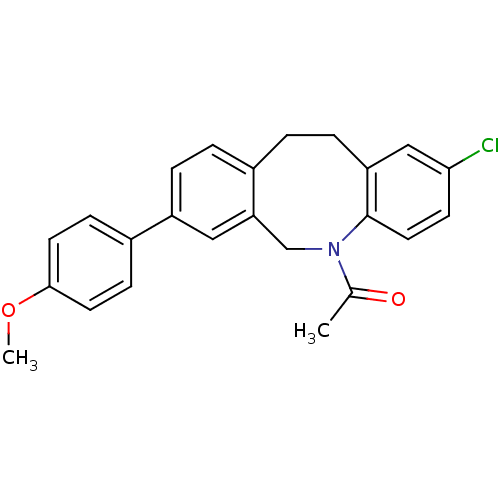
(1-[2-chloro-8-(4-methoxy-phenyl)-11,12-dihydro-6H-...)Show SMILES COc1ccc(cc1)-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C24H22ClNO2/c1-16(27)26-15-21-13-19(17-7-10-23(28-2)11-8-17)5-3-18(21)4-6-20-14-22(25)9-12-24(20)26/h3,5,7-14H,4,6,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179934

(4-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccc(cc1)C#N Show InChI InChI=1S/C24H19ClN2O/c1-16(28)27-15-22-12-20(18-4-2-17(14-26)3-5-18)8-6-19(22)7-9-21-13-23(25)10-11-24(21)27/h2-6,8,10-13H,7,9,15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381586
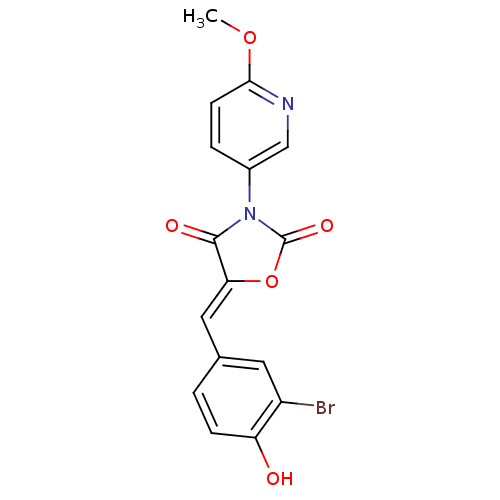
(CHEMBL2018238)Show SMILES COc1ccc(cn1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C16H11BrN2O5/c1-23-14-5-3-10(8-18-14)19-15(21)13(24-16(19)22)7-9-2-4-12(20)11(17)6-9/h2-8,20H,1H3/b13-7- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381590

(CHEMBL2018242)Show SMILES CN(C)c1ccc(cn1)N1C(=O)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H14BrN3O4/c1-20(2)15-6-4-11(9-19-15)21-16(23)14(25-17(21)24)8-10-3-5-13(22)12(18)7-10/h3-9,22H,1-2H3/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179956

(1-(2-chloro-8-phenyl-11,12-dihydro-6H-dibenzo[b,f]...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1 Show InChI InChI=1S/C23H20ClNO/c1-16(26)25-15-21-13-19(17-5-3-2-4-6-17)9-7-18(21)8-10-20-14-22(24)11-12-23(20)25/h2-7,9,11-14H,8,10,15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179935

(1-(8-Phenyl-11,12-dihydro-6H-dibenzo[b,f]azocin-5-...)Show InChI InChI=1S/C23H21NO/c1-17(25)24-16-22-15-21(18-7-3-2-4-8-18)14-12-19(22)11-13-20-9-5-6-10-23(20)24/h2-10,12,14-15H,11,13,16H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179958
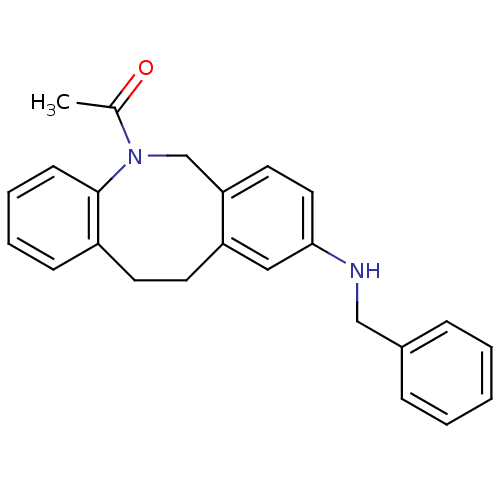
(1-(9-benzylamino-11,12-dihydro-6H-dibenzo[b,f]azoc...)Show InChI InChI=1S/C24H24N2O/c1-18(27)26-17-22-13-14-23(25-16-19-7-3-2-4-8-19)15-21(22)12-11-20-9-5-6-10-24(20)26/h2-10,13-15,25H,11-12,16-17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381597
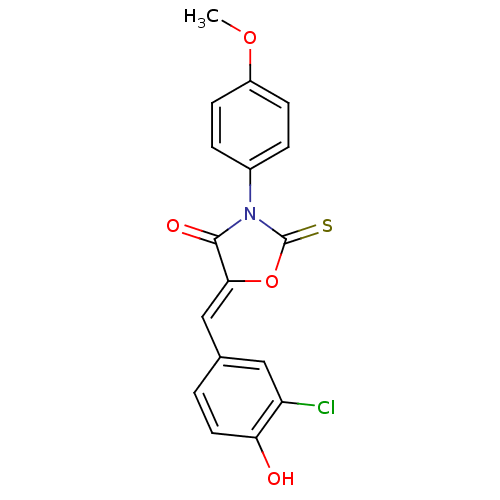
(CHEMBL2018250)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)c(Cl)c2)C1=O Show InChI InChI=1S/C17H12ClNO4S/c1-22-12-5-3-11(4-6-12)19-16(21)15(23-17(19)24)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381559

(CHEMBL2018253)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2cc(F)c(O)c(F)c2)C1=O Show InChI InChI=1S/C17H11F2NO4S/c1-23-11-4-2-10(3-5-11)20-16(22)14(24-17(20)25)8-9-6-12(18)15(21)13(19)7-9/h2-8,21H,1H3/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179932

(1-[2-chloro-8-(2-methoxy-phenyl)-11,12-dihydro-6H-...)Show SMILES COc1ccccc1-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C24H22ClNO2/c1-16(27)26-15-20-13-18(22-5-3-4-6-24(22)28-2)9-7-17(20)8-10-19-14-21(25)11-12-23(19)26/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179966
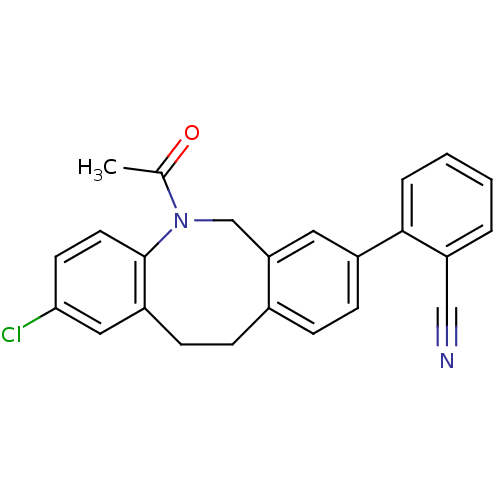
(2-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1C#N Show InChI InChI=1S/C24H19ClN2O/c1-16(28)27-15-21-12-18(23-5-3-2-4-20(23)14-26)8-6-17(21)7-9-19-13-22(25)10-11-24(19)27/h2-6,8,10-13H,7,9,15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381598
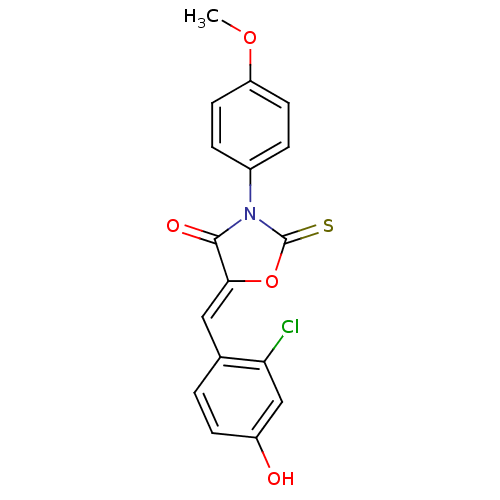
(CHEMBL2018251)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2ccc(O)cc2Cl)C1=O Show InChI InChI=1S/C17H12ClNO4S/c1-22-13-6-3-11(4-7-13)19-16(21)15(23-17(19)24)8-10-2-5-12(20)9-14(10)18/h2-9,20H,1H3/b15-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381566
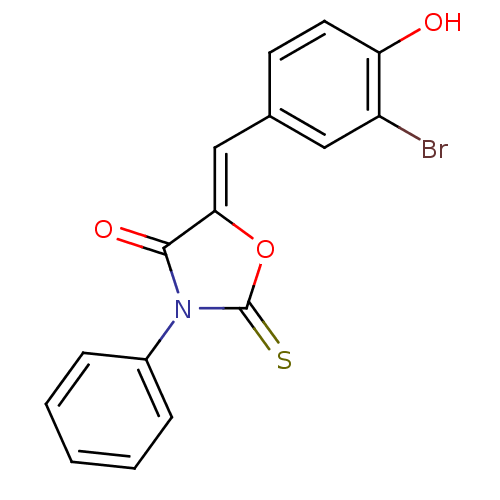
(CHEMBL2018135)Show InChI InChI=1S/C16H10BrNO3S/c17-12-8-10(6-7-13(12)19)9-14-15(20)18(16(22)21-14)11-4-2-1-3-5-11/h1-9,19H/b14-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381571
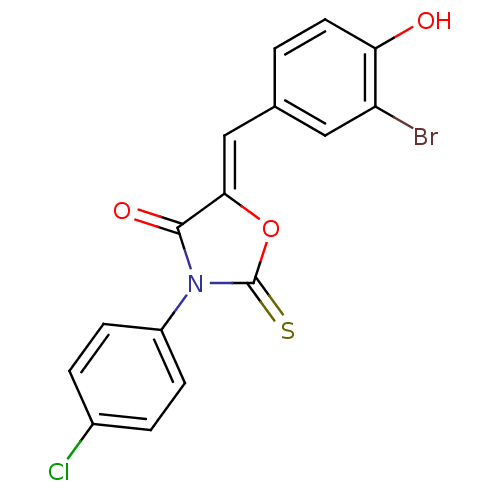
(CHEMBL2018140)Show SMILES Oc1ccc(\C=C2/OC(=S)N(C2=O)c2ccc(Cl)cc2)cc1Br Show InChI InChI=1S/C16H9BrClNO3S/c17-12-7-9(1-6-13(12)20)8-14-15(21)19(16(23)22-14)11-4-2-10(18)3-5-11/h1-8,20H/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50350421
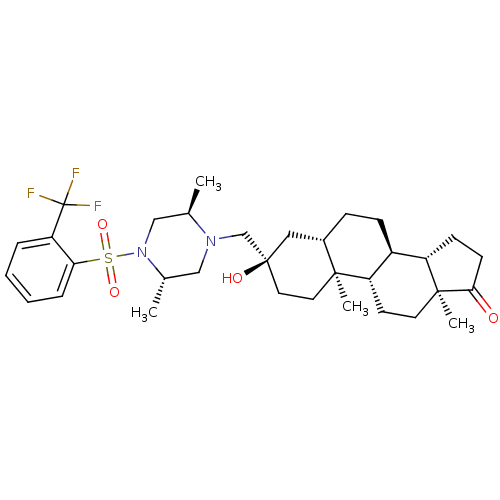
(CHEMBL1813731)Show SMILES C[C@@H]1CN([C@@H](C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21-,22+,23+,24+,25+,26+,30+,31+,32-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CHUQ (CHUL)-Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 17Beta-HSD3 expressed in intact HEK293 cells assessed as transformation of [14C]-4-androstene-3,17-dione into [14C]-testosterone in pre... |
Bioorg Med Chem 19: 4652-68 (2011)
Article DOI: 10.1016/j.bmc.2011.06.003
BindingDB Entry DOI: 10.7270/Q2222V4T |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50360767
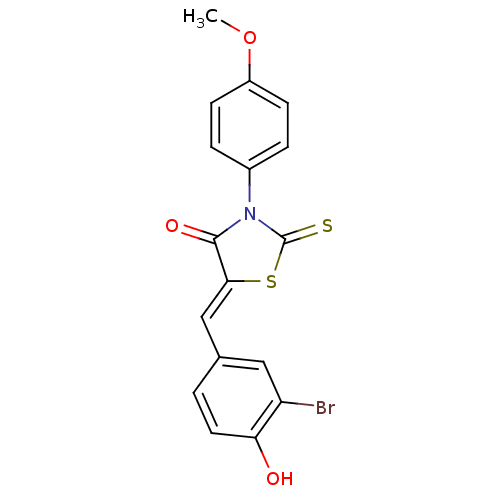
(CHEMBL1934493)Show SMILES COc1ccc(cc1)N1C(=S)S\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO3S2/c1-22-12-5-3-11(4-6-12)19-16(21)15(24-17(19)23)9-10-2-7-14(20)13(18)8-10/h2-9,20H,1H3/b15-9- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 in human testes homogenate |
Bioorg Med Chem Lett 22: 504-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.095
BindingDB Entry DOI: 10.7270/Q2BG2PFD |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50350421

(CHEMBL1813731)Show SMILES C[C@@H]1CN([C@@H](C)CN1C[C@@]1(O)CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CCC(=O)[C@@]4(C)CC[C@H]23)C1)S(=O)(=O)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C33H47F3N2O4S/c1-21-19-38(43(41,42)28-8-6-5-7-27(28)33(34,35)36)22(2)18-37(21)20-32(40)16-15-30(3)23(17-32)9-10-24-25-11-12-29(39)31(25,4)14-13-26(24)30/h5-8,21-26,40H,9-20H2,1-4H3/t21-,22+,23+,24+,25+,26+,30+,31+,32-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CHU de Qu£bec-Research Center
Curated by ChEMBL
| Assay Description
Inhibition of 17betaHSD3 (unknown origin) transfected in HEK293 cells |
Bioorg Med Chem Lett 26: 2179-83 (2016)
Article DOI: 10.1016/j.bmcl.2016.03.069
BindingDB Entry DOI: 10.7270/Q2FT8Q2J |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179933
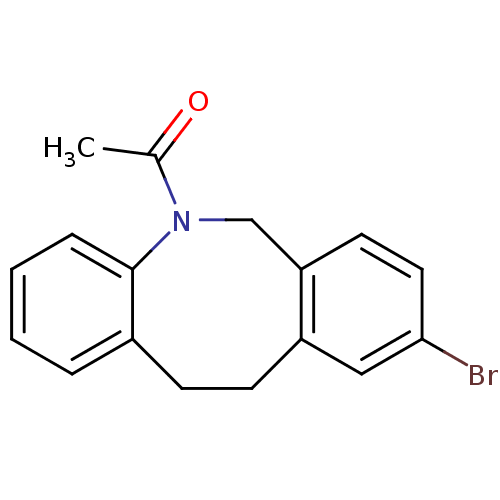
(1-(9-bromo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-y...)Show InChI InChI=1S/C17H16BrNO/c1-12(20)19-11-15-8-9-16(18)10-14(15)7-6-13-4-2-3-5-17(13)19/h2-5,8-10H,6-7,11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381567
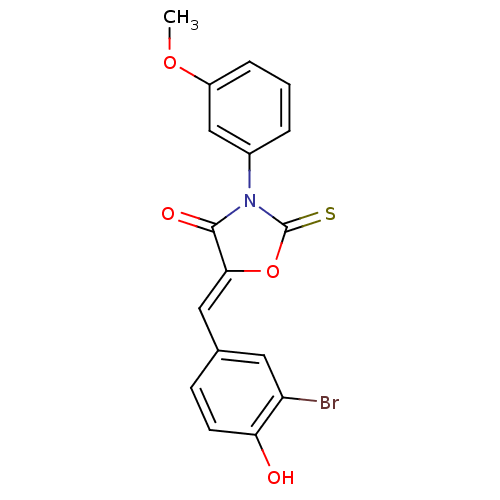
(CHEMBL2018136)Show SMILES COc1cccc(c1)N1C(=S)O\C(=C/c2ccc(O)c(Br)c2)C1=O Show InChI InChI=1S/C17H12BrNO4S/c1-22-12-4-2-3-11(9-12)19-16(21)15(23-17(19)24)8-10-5-6-14(20)13(18)7-10/h2-9,20H,1H3/b15-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179937

(1-[2-chloro-8-(3-methoxy-phenyl)-11,12-dihydro-6H-...)Show SMILES COc1cccc(c1)-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C24H22ClNO2/c1-16(27)26-15-21-12-19(18-4-3-5-23(14-18)28-2)8-6-17(21)7-9-20-13-22(25)10-11-24(20)26/h3-6,8,10-14H,7,9,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305336
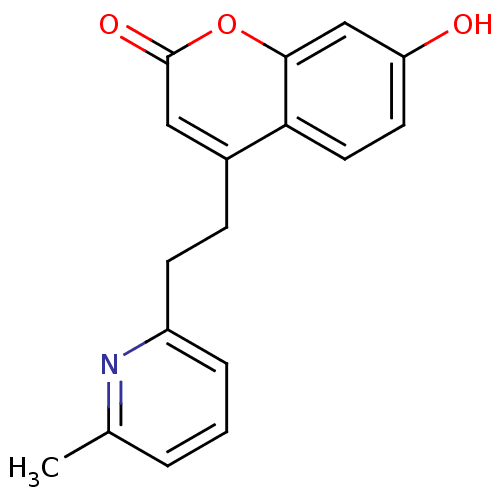
(7-hydroxy-4-(2-(6-methylpyridin-2-yl)ethyl)-2H-chr...)Show InChI InChI=1S/C17H15NO3/c1-11-3-2-4-13(18-11)6-5-12-9-17(20)21-16-10-14(19)7-8-15(12)16/h2-4,7-10,19H,5-6H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179958

(1-(9-benzylamino-11,12-dihydro-6H-dibenzo[b,f]azoc...)Show InChI InChI=1S/C24H24N2O/c1-18(27)26-17-22-13-14-23(25-16-19-7-3-2-4-8-19)15-21(22)12-11-20-9-5-6-10-24(20)26/h2-10,13-15,25H,11-12,16-17H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179944
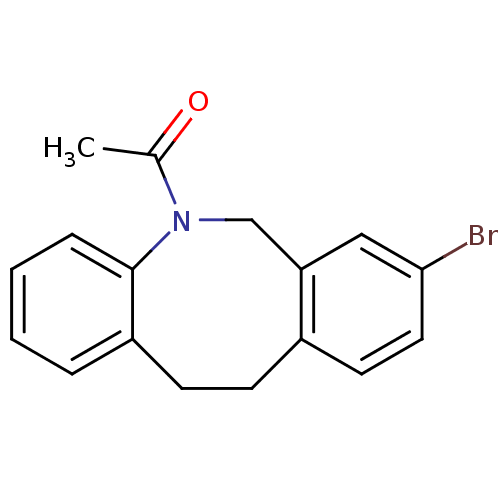
(1-(8-bromo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-y...)Show InChI InChI=1S/C17H16BrNO/c1-12(20)19-11-15-10-16(18)9-8-13(15)6-7-14-4-2-3-5-17(14)19/h2-5,8-10H,6-7,11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179936

(CHEMBL382563 | N-[2-(5-acetyl-2-chloro-5,6,11,12-t...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1NS(C)(=O)=O Show InChI InChI=1S/C24H23ClN2O3S/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)26-31(2,29)30)9-7-17(20)8-10-19-14-21(25)11-12-24(19)27/h3-7,9,11-14,26H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50305330
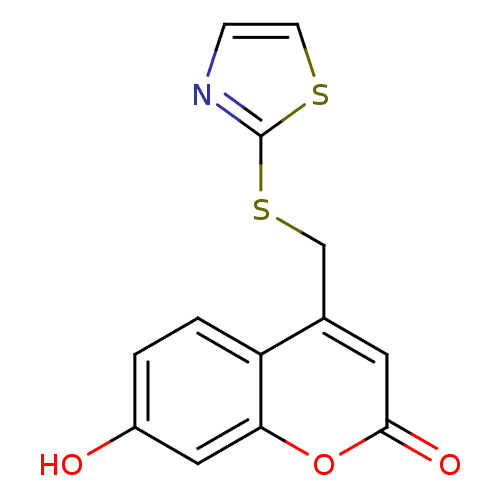
(7-hydroxy-4-((thiazol-2-ylthio)methyl)-2H-chromen-...)Show InChI InChI=1S/C13H9NO3S2/c15-9-1-2-10-8(5-12(16)17-11(10)6-9)7-19-13-14-3-4-18-13/h1-6,15H,7H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human 17beta-HSD3 expressed in HeLa cells |
Bioorg Med Chem Lett 20: 272-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.111
BindingDB Entry DOI: 10.7270/Q2SF2W8B |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179976

(1-(2,3-dichloro-6,11-dihydro-5-thia-12-aza-dibenzo...)Show InChI InChI=1S/C16H13Cl2NOS/c1-10(20)19-8-11-4-2-3-5-12(11)9-21-16-7-14(18)13(17)6-15(16)19/h2-7H,8-9H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381599

(CHEMBL2018252)Show SMILES COc1ccc(cc1)N1C(=S)O\C(=C/c2cc(Cl)c(O)c(Cl)c2)C1=O Show InChI InChI=1S/C17H11Cl2NO4S/c1-23-11-4-2-10(3-5-11)20-16(22)14(24-17(20)25)8-9-6-12(18)15(21)13(19)7-9/h2-8,21H,1H3/b14-8- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50381569
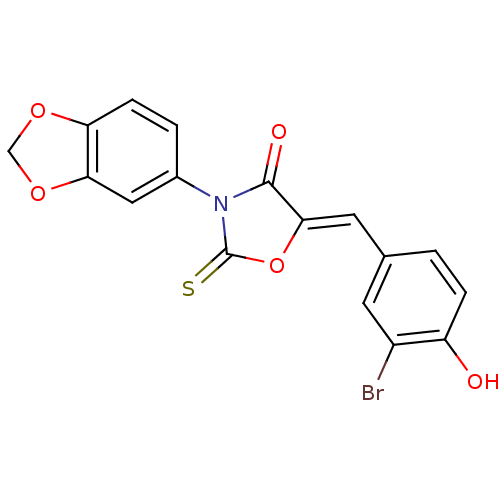
(CHEMBL2018138)Show SMILES Oc1ccc(\C=C2/OC(=S)N(C2=O)c2ccc3OCOc3c2)cc1Br Show InChI InChI=1S/C17H10BrNO5S/c18-11-5-9(1-3-12(11)20)6-15-16(21)19(17(25)24-15)10-2-4-13-14(7-10)23-8-22-13/h1-7,20H,8H2/b15-6- | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Chemical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant 17-beta-HSD3 expressed in human HeLa cells using androstenedione as substrate preincubated for 30 mins prior substrat... |
Bioorg Med Chem 20: 3242-54 (2012)
Article DOI: 10.1016/j.bmc.2012.03.052
BindingDB Entry DOI: 10.7270/Q2RF5W2G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data