

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine synthase (Homo sapiens (Human)) | BDBM66082 ((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using methyltetrahydrofolate as substrate incubated for 10 mins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392652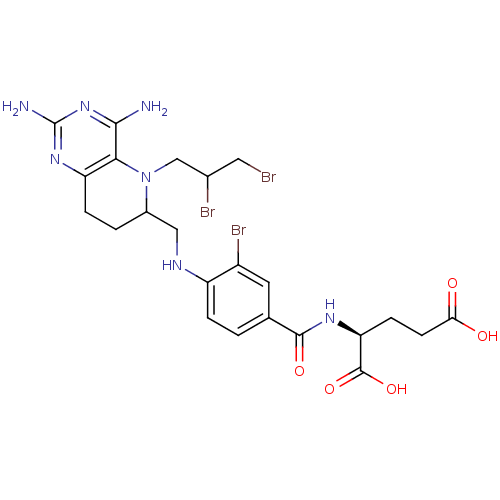 (CHEMBL2153714) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392649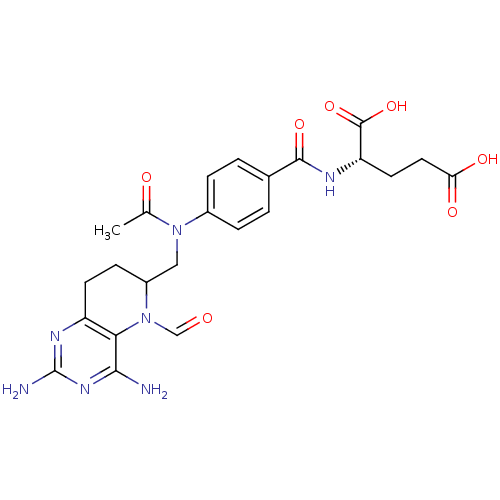 (CHEMBL2153712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564145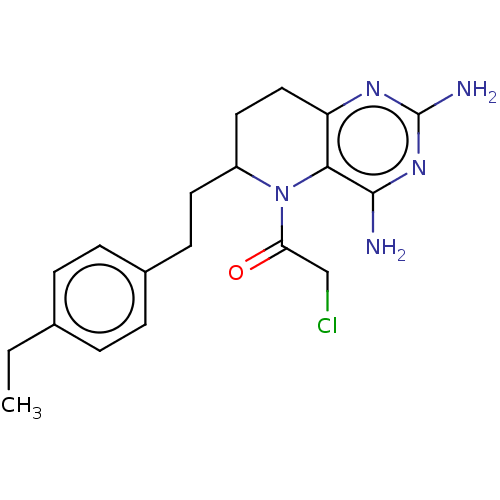 (CHEMBL4778495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using 63 uM of methyltetrahydrofolate as substrate incubated fo... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564148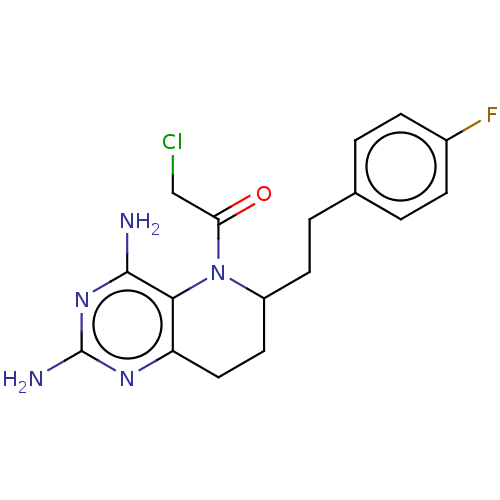 (CHEMBL4789573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using methyltetrahydrofolate as substrate incubated for 10 mins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564143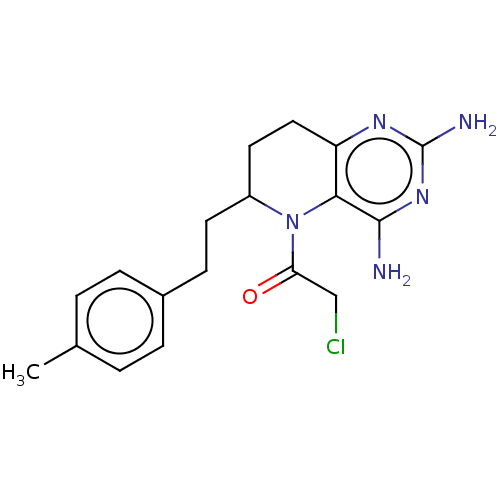 (CHEMBL4787377) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using methyltetrahydrofolate as substrate incubated for 10 mins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564145 (CHEMBL4778495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using 126 uM of methyltetrahydrofolate as substrate incubated f... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392656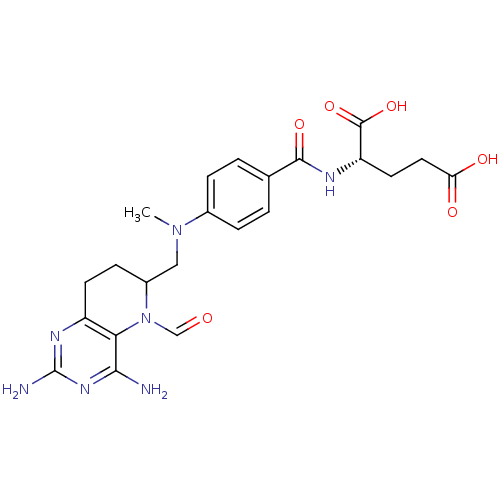 (CHEMBL2153711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564145 (CHEMBL4778495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using 252 uM of methyltetrahydrofolate as substrate incubated f... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564145 (CHEMBL4778495) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using methyltetrahydrofolate as substrate incubated for 10 mins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564147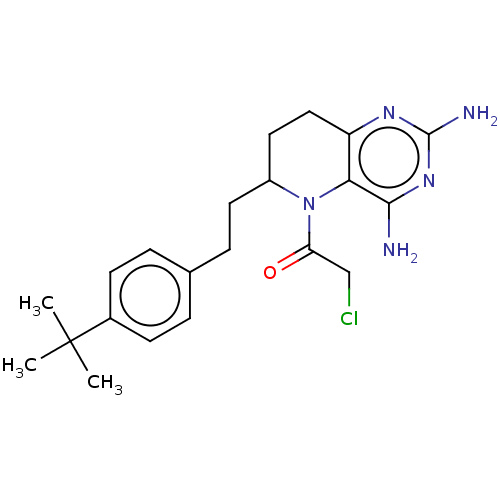 (CHEMBL4776344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using methyltetrahydrofolate as substrate incubated for 10 mins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564149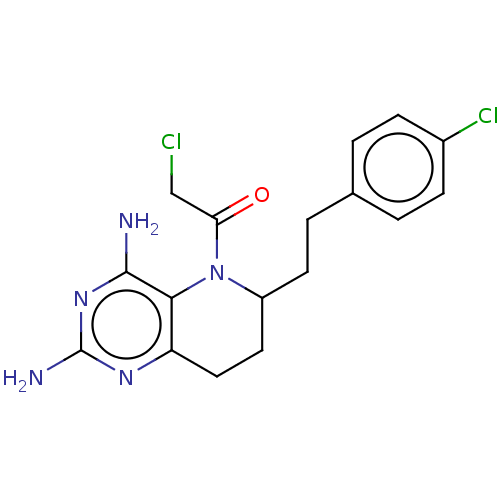 (CHEMBL4796741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.49E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using methyltetrahydrofolate as substrate incubated for 10 mins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392661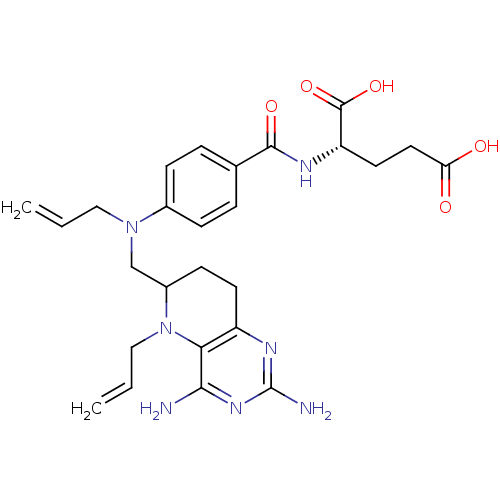 (CHEMBL2153703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392651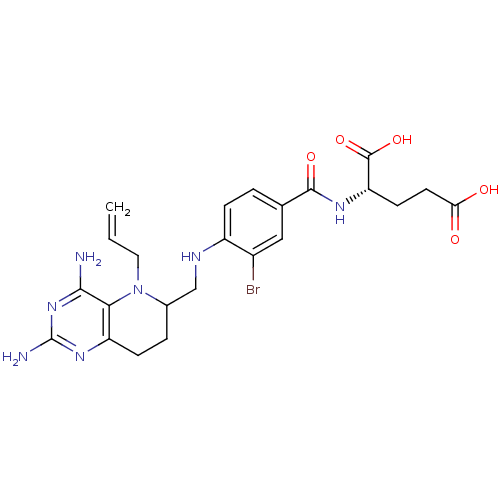 (CHEMBL2153713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392660 (CHEMBL2153706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564146 (CHEMBL4777807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using methyltetrahydrofolate as substrate incubated for 10 mins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564144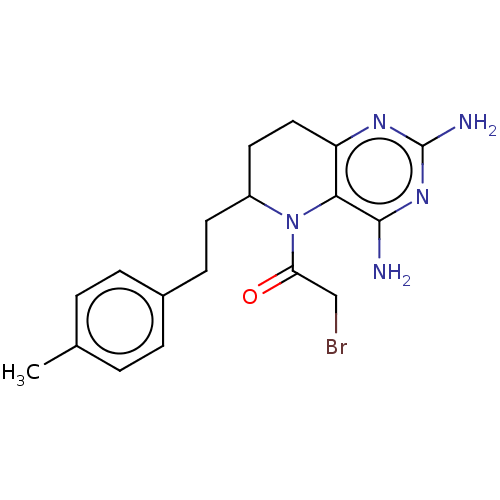 (CHEMBL4795611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using methyltetrahydrofolate as substrate incubated for 10 mins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50564150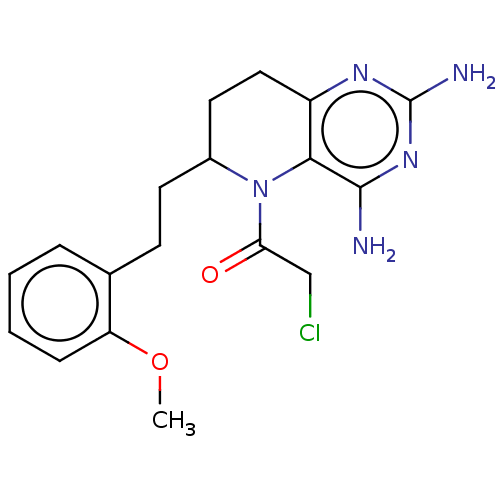 (CHEMBL4791051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human Cobalamin-dependent methionine synthase isolated from HL-60 cells using methyltetrahydrofolate as substrate incubated for 10 mins... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112113 BindingDB Entry DOI: 10.7270/Q2DJ5KCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392655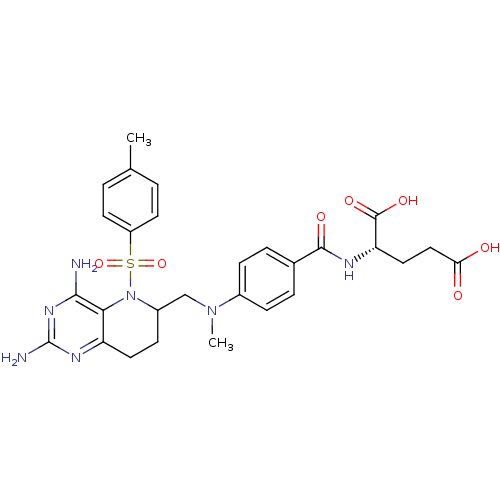 (CHEMBL2153710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392650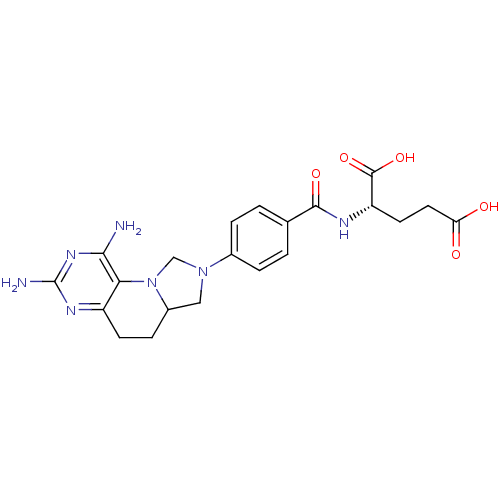 (CHEMBL2153704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392659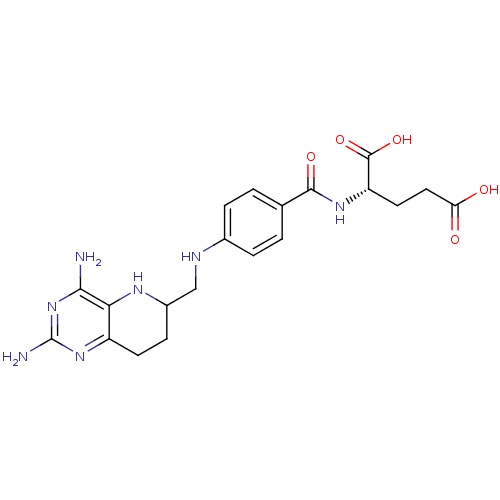 (CHEMBL2153708) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392653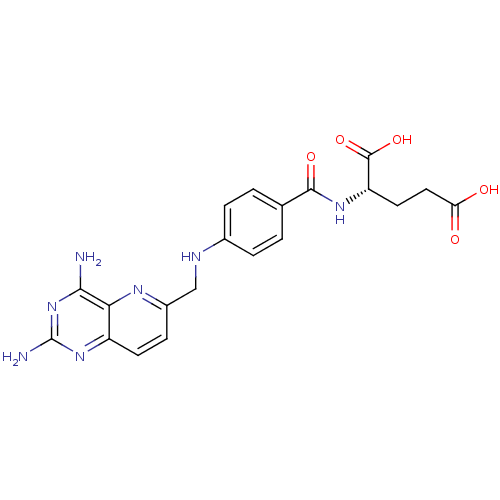 (CHEMBL2153707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392658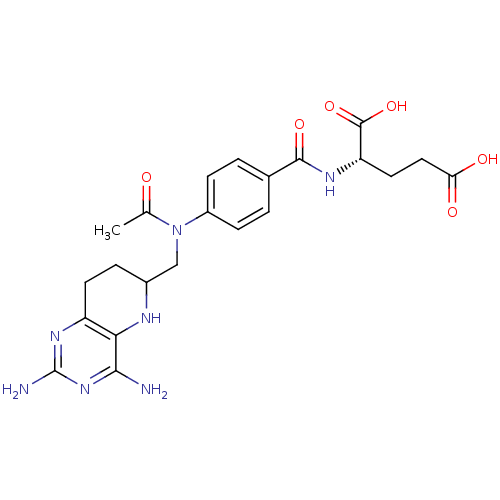 (CHEMBL2151066) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392657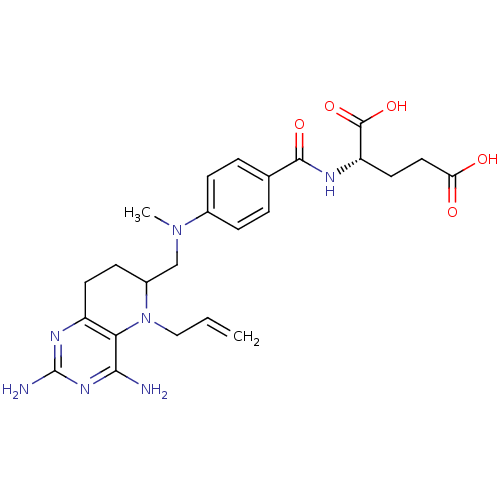 (CHEMBL2153705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine synthase (Homo sapiens (Human)) | BDBM50392654 (CHEMBL2153709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Curated by ChEMBL | Assay Description Inhibition of methionine synthase in human HL60 cells using L-homocysteine as substrate incubated for 3 hrs prior to substrate addition measured afte... | Eur J Med Chem 58: 228-36 (2012) Article DOI: 10.1016/j.ejmech.2012.09.027 BindingDB Entry DOI: 10.7270/Q2RX9D5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||