 Found 14 hits of ic50 data for polymerid = 50006174
Found 14 hits of ic50 data for polymerid = 50006174 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50396027
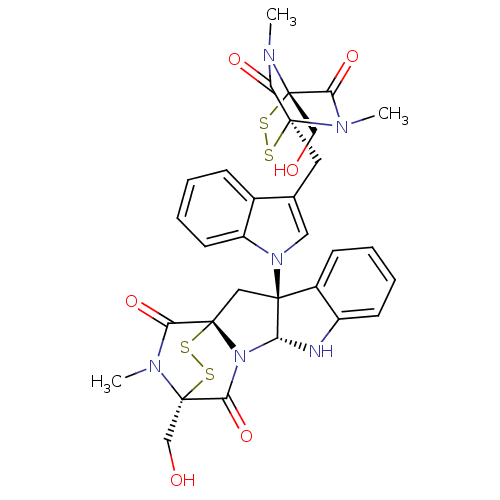
(CHEMBL499593)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)n1cc(C[C@]23SS[C@](CO)(N(C)C2=O)C(=O)N3C)c2ccccc12 |r,TLB:17:16:1.2:21.22,7:15:1.2:21.22| Show InChI InChI=1S/C31H30N6O6S4/c1-33-25(42)30(15-38)34(2)23(40)28(33,44-46-30)12-17-13-36(21-11-7-4-8-18(17)21)27-14-29-24(41)35(3)31(16-39,47-45-29)26(43)37(29)22(27)32-20-10-6-5-9-19(20)27/h4-11,13,22,32,38-39H,12,14-16H2,1-3H3/t22-,27+,28+,29+,30+,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50134315
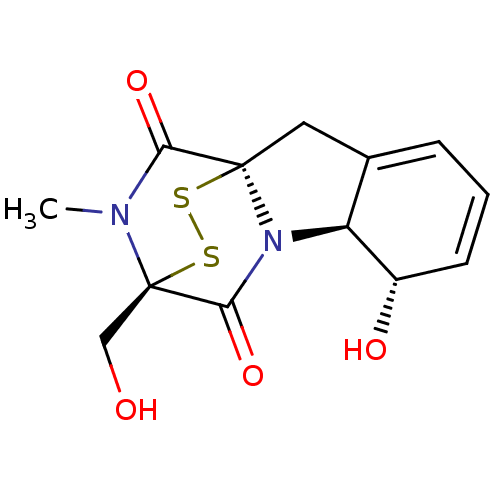
(7-hydroxy-11-hydroxymethyl-12-methyl-14,15-dithia-...)Show SMILES CN1C(=O)[C@]23CC4=CC=C[C@H](O)[C@H]4N2C(=O)[C@@]1(CO)SS3 |r,c:8,t:6,THB:12:13:1.2:19.20,15:14:1.2:19.20| Show InChI InChI=1S/C13H14N2O4S2/c1-14-10(18)12-5-7-3-2-4-8(17)9(7)15(12)11(19)13(14,6-16)21-20-12/h2-4,8-9,16-17H,5-6H2,1H3/t8-,9-,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50396026
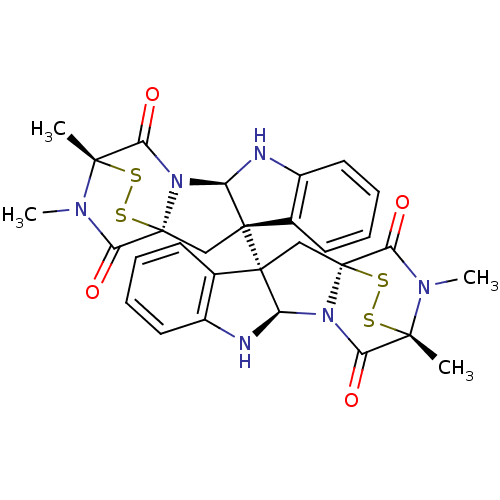
(CHEMBL2172426)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(C)SS3)[C@]12C[C@]34SS[C@](C)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,TLB:17:16:1.2:20.21,7:15:1.2:20.21,THB:34:33:29.31:26.25,36:35:29.31:26.25| Show InChI InChI=1S/C30H28N6O4S4/c1-25-21(37)35-19-27(15-9-5-7-11-17(15)31-19,13-29(35,43-41-25)23(39)33(25)3)28-14-30-24(40)34(4)26(2,42-44-30)22(38)36(30)20(28)32-18-12-8-6-10-16(18)28/h5-12,19-20,31-32H,13-14H2,1-4H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50315537
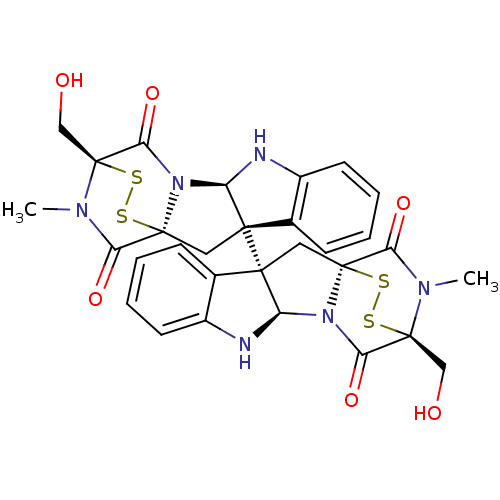
(CHEMBL1089316 | chaetocin)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)[C@]12C[C@]34SS[C@](CO)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,THB:36:35:31.33:26.27,7:15:1.2:22.21,38:37:31.33:26.27,17:16:1.2:22.21| Show InChI InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London
Curated by ChEMBL
| Assay Description
Inhibition of human Histone-lysine N-methyltransferase SUV39H1 |
J Med Chem 56: 8616-25 (2013)
Article DOI: 10.1021/jm401063r
BindingDB Entry DOI: 10.7270/Q2NS0WDK |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50315537

(CHEMBL1089316 | chaetocin)Show SMILES CN1C(=O)[C@@]23C[C@]4([C@H](Nc5ccccc45)N2C(=O)[C@]1(CO)SS3)[C@]12C[C@]34SS[C@](CO)(N(C)C3=O)C(=O)N4[C@H]1Nc1ccccc21 |r,THB:36:35:31.33:26.27,7:15:1.2:22.21,38:37:31.33:26.27,17:16:1.2:22.21| Show InChI InChI=1S/C30H28N6O6S4/c1-33-21(39)27-11-25(15-7-3-5-9-17(15)31-19(25)35(27)23(41)29(33,13-37)45-43-27)26-12-28-22(40)34(2)30(14-38,46-44-28)24(42)36(28)20(26)32-18-10-6-4-8-16(18)26/h3-10,19-20,31-32,37-38H,11-14H2,1-2H3/t19-,20-,25+,26+,27+,28+,29+,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50009672
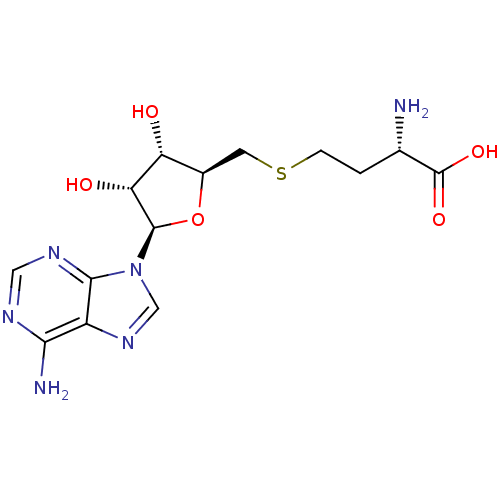
(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 (unknown origin) by HMT assay |
Bioorg Med Chem Lett 25: 1532-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.017
BindingDB Entry DOI: 10.7270/Q2TM7CSS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50063670
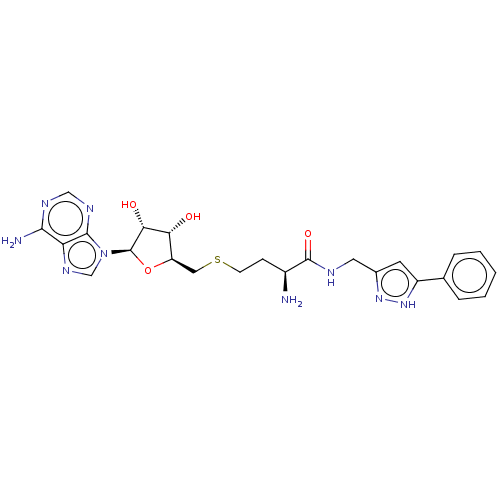
(CHEMBL3397332)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(=O)NCc1cc([nH]n1)-c1ccccc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 (unknown origin) by HMT assay |
Bioorg Med Chem Lett 25: 1532-7 (2015)
Article DOI: 10.1016/j.bmcl.2015.02.017
BindingDB Entry DOI: 10.7270/Q2TM7CSS |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50396025

(CHEMBL1089088)Show SMILES CS[C@@]12CC3=CC=C[C@H](OC(C)=O)[C@H]3N1C(=O)[C@@](CO)(SC)N(C)C2=O |r,c:6,t:4| Show InChI InChI=1S/C17H22N2O5S2/c1-10(21)24-12-7-5-6-11-8-16(25-3)14(22)18(2)17(9-20,26-4)15(23)19(16)13(11)12/h5-7,12-13,20H,8-9H2,1-4H3/t12-,13-,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50400781
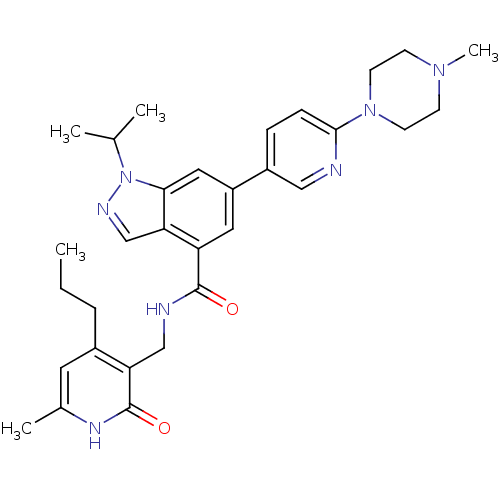
(CHEMBL2204997)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-22-14-21(4)35-31(40)26(22)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)23-8-9-29(32-17-23)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50396017
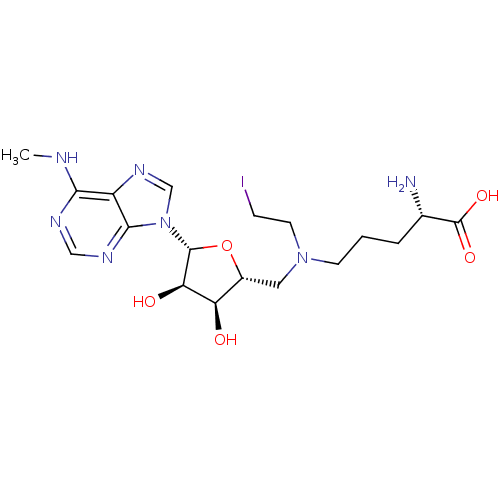
(CHEMBL2172427)Show SMILES CNc1ncnc2n(cnc12)[C@@H]1O[C@H](CN(CCI)CCC[C@H](N)C(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H28IN7O5/c1-21-15-12-16(23-8-22-15)26(9-24-12)17-14(28)13(27)11(31-17)7-25(6-4-19)5-2-3-10(20)18(29)30/h8-11,13-14,17,27-28H,2-7,20H2,1H3,(H,29,30)(H,21,22,23)/t10-,11+,13+,14+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 using [3H]-SAM as substrate preincubated for 10 mins before substrate addition measured after 30 mins by scintillation counter |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50396016
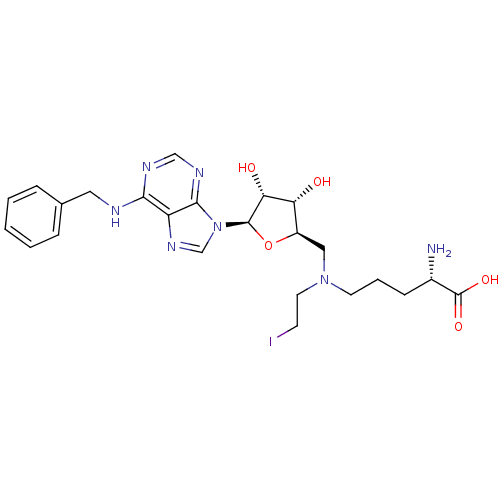
(CHEMBL2169918)Show SMILES N[C@@H](CCCN(CCI)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCc3ccccc3)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H32IN7O5/c25-8-10-31(9-4-7-16(26)24(35)36)12-17-19(33)20(34)23(37-17)32-14-30-18-21(28-13-29-22(18)32)27-11-15-5-2-1-3-6-15/h1-3,5-6,13-14,16-17,19-20,23,33-34H,4,7-12,26H2,(H,35,36)(H,27,28,29)/t16-,17+,19+,20+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 using [3H]-SAM as substrate preincubated for 10 mins before substrate addition measured after 30 mins by scintillation counter |
Eur J Med Chem 56: 179-194 (2012)
Article DOI: 10.1016/j.ejmech.2012.08.010
BindingDB Entry DOI: 10.7270/Q2TQ62NX |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50400778

(CHEMBL2204995)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-23-14-21(4)35-31(40)26(23)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)22-8-9-32-29(17-22)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50400779
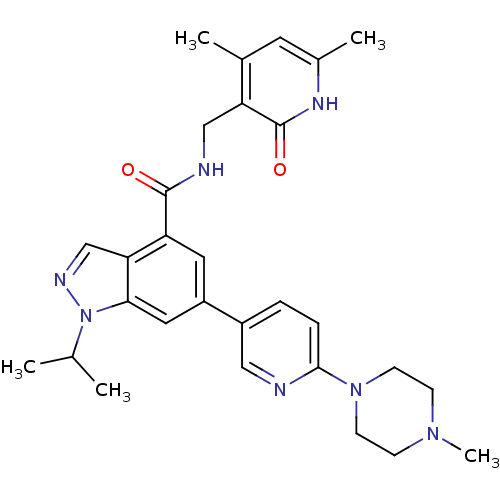
(CHEMBL2204998)Show SMILES CC(C)n1ncc2c(cc(cc12)-c1ccc(nc1)N1CCN(C)CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C29H35N7O2/c1-18(2)36-26-14-22(21-6-7-27(30-15-21)35-10-8-34(5)9-11-35)13-23(25(26)17-32-36)28(37)31-16-24-19(3)12-20(4)33-29(24)38/h6-7,12-15,17-18H,8-11,16H2,1-5H3,(H,31,37)(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50031317
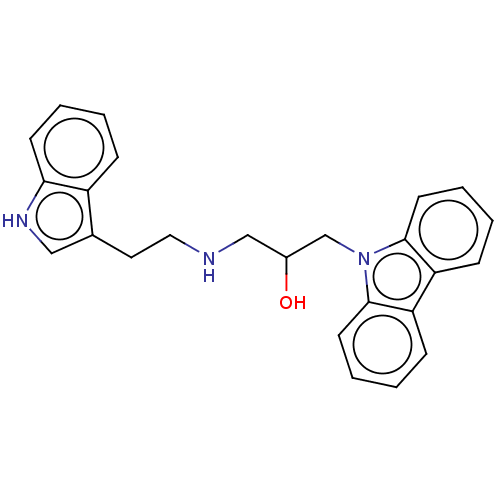
(CHEMBL3358015)Show SMILES OC(CNCCc1c[nH]c2ccccc12)Cn1c2ccccc2c2ccccc12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 (unknown origin) incubated for 15 mins before addition of biotinylated peptide H3 (1 to 21) and SAM by methylation assay |
J Med Chem 57: 9028-41 (2014)
Article DOI: 10.1021/jm501134e
BindingDB Entry DOI: 10.7270/Q2JD4ZD6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data