 Found 72 hits of ic50 data for polymerid = 50006195
Found 72 hits of ic50 data for polymerid = 50006195 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249568
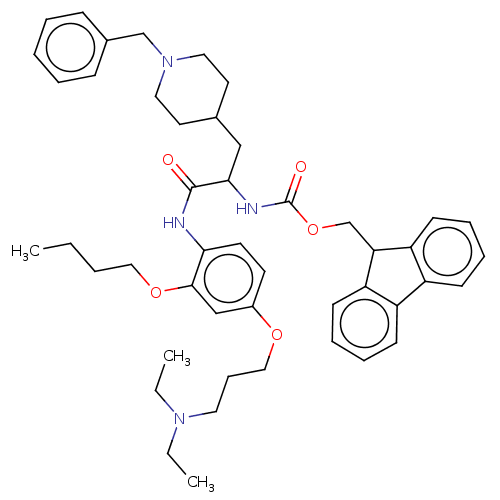
(CHEMBL4068269)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)C(CC1CCN(Cc2ccccc2)CC1)NC(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C47H60N4O5/c1-4-7-29-55-45-32-37(54-30-15-26-50(5-2)6-3)22-23-43(45)48-46(52)44(31-35-24-27-51(28-25-35)33-36-16-9-8-10-17-36)49-47(53)56-34-42-40-20-13-11-18-38(40)39-19-12-14-21-41(39)42/h8-14,16-23,32,35,42,44H,4-7,15,24-31,33-34H2,1-3H3,(H,48,52)(H,49,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249569
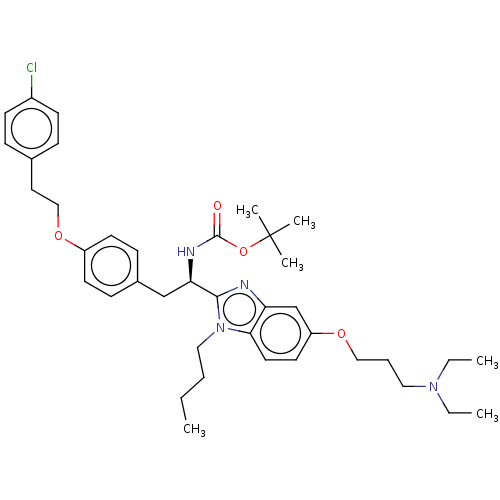
(CHEMBL4063487)Show SMILES CCCCn1c(nc2cc(OCCCN(CC)CC)ccc12)[C@@H](Cc1ccc(OCCc2ccc(Cl)cc2)cc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H53ClN4O4/c1-7-10-24-44-36-21-20-33(46-25-11-23-43(8-2)9-3)28-34(36)41-37(44)35(42-38(45)48-39(4,5)6)27-30-14-18-32(19-15-30)47-26-22-29-12-16-31(40)17-13-29/h12-21,28,35H,7-11,22-27H2,1-6H3,(H,42,45)/t35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of S110B binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249569

(CHEMBL4063487)Show SMILES CCCCn1c(nc2cc(OCCCN(CC)CC)ccc12)[C@@H](Cc1ccc(OCCc2ccc(Cl)cc2)cc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H53ClN4O4/c1-7-10-24-44-36-21-20-33(46-25-11-23-43(8-2)9-3)28-34(36)41-37(44)35(42-38(45)48-39(4,5)6)27-30-14-18-32(19-15-30)47-26-22-29-12-16-31(40)17-13-29/h12-21,28,35H,7-11,22-27H2,1-6H3,(H,42,45)/t35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249569

(CHEMBL4063487)Show SMILES CCCCn1c(nc2cc(OCCCN(CC)CC)ccc12)[C@@H](Cc1ccc(OCCc2ccc(Cl)cc2)cc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H53ClN4O4/c1-7-10-24-44-36-21-20-33(46-25-11-23-43(8-2)9-3)28-34(36)41-37(44)35(42-38(45)48-39(4,5)6)27-30-14-18-32(19-15-30)47-26-22-29-12-16-31(40)17-13-29/h12-21,28,35H,7-11,22-27H2,1-6H3,(H,42,45)/t35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249568

(CHEMBL4068269)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)C(CC1CCN(Cc2ccccc2)CC1)NC(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C47H60N4O5/c1-4-7-29-55-45-32-37(54-30-15-26-50(5-2)6-3)22-23-43(45)48-46(52)44(31-35-24-27-51(28-25-35)33-36-16-9-8-10-17-36)49-47(53)56-34-42-40-20-13-11-18-38(40)39-19-12-14-21-41(39)42/h8-14,16-23,32,35,42,44H,4-7,15,24-31,33-34H2,1-3H3,(H,48,52)(H,49,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249568

(CHEMBL4068269)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)C(CC1CCN(Cc2ccccc2)CC1)NC(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C47H60N4O5/c1-4-7-29-55-45-32-37(54-30-15-26-50(5-2)6-3)22-23-43(45)48-46(52)44(31-35-24-27-51(28-25-35)33-36-16-9-8-10-17-36)49-47(53)56-34-42-40-20-13-11-18-38(40)39-19-12-14-21-41(39)42/h8-14,16-23,32,35,42,44H,4-7,15,24-31,33-34H2,1-3H3,(H,48,52)(H,49,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of S110B binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402568
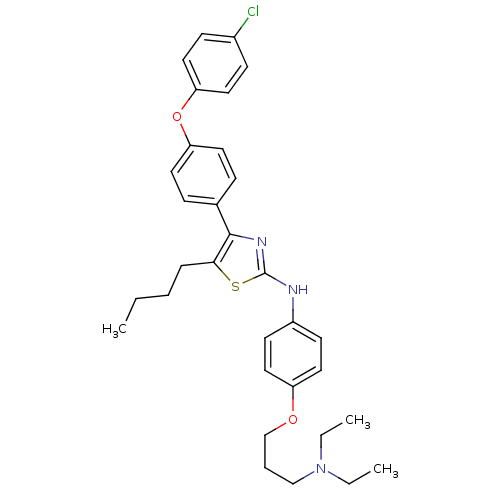
(CHEMBL2205566)Show SMILES CCCCc1sc(Nc2ccc(OCCCN(CC)CC)cc2)nc1-c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C32H38ClN3O2S/c1-4-7-9-30-31(24-10-16-28(17-11-24)38-29-18-12-25(33)13-19-29)35-32(39-30)34-26-14-20-27(21-15-26)37-23-8-22-36(5-2)6-3/h10-21H,4-9,22-23H2,1-3H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402572
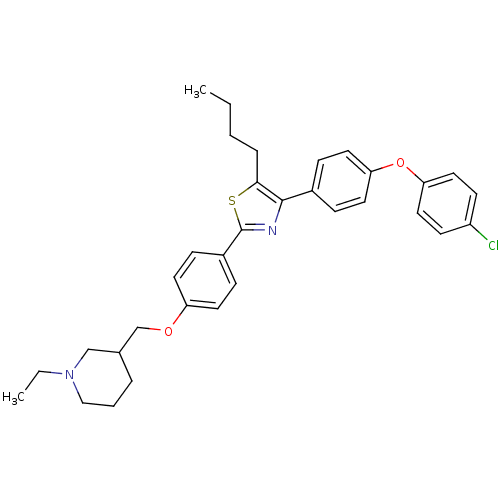
(CHEMBL2205559)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCC2CCCN(CC)C2)cc1 Show InChI InChI=1S/C33H37ClN2O2S/c1-3-5-8-31-32(25-9-17-29(18-10-25)38-30-19-13-27(34)14-20-30)35-33(39-31)26-11-15-28(16-12-26)37-23-24-7-6-21-36(4-2)22-24/h9-20,24H,3-8,21-23H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402565

(CHEMBL2205572)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCN2CCCC2)cc1 Show InChI InChI=1S/C31H33ClN2O2S/c1-2-3-6-29-30(23-7-15-27(16-8-23)36-28-17-11-25(32)12-18-28)33-31(37-29)24-9-13-26(14-10-24)35-22-21-34-19-4-5-20-34/h7-18H,2-6,19-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402564
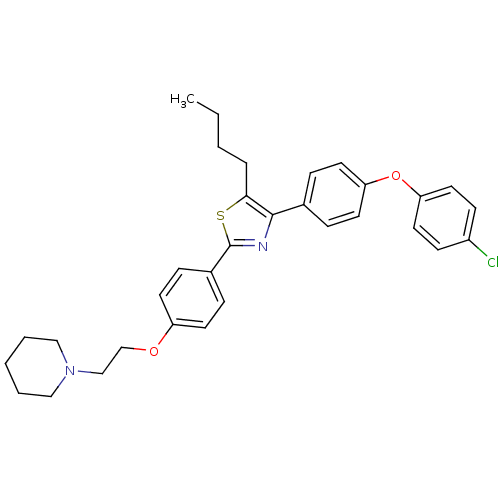
(CHEMBL2205549)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C32H35ClN2O2S/c1-2-3-7-30-31(24-8-16-28(17-9-24)37-29-18-12-26(33)13-19-29)34-32(38-30)25-10-14-27(15-11-25)36-23-22-35-20-5-4-6-21-35/h8-19H,2-7,20-23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402575
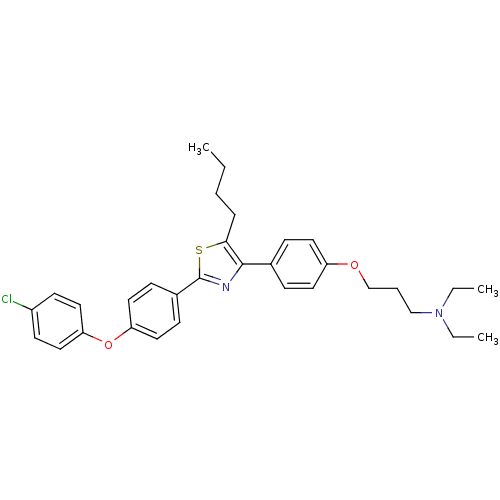
(CHEMBL2205556)Show SMILES CCCCc1sc(nc1-c1ccc(OCCCN(CC)CC)cc1)-c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C32H37ClN2O2S/c1-4-7-9-30-31(24-10-16-27(17-11-24)36-23-8-22-35(5-2)6-3)34-32(38-30)25-12-18-28(19-13-25)37-29-20-14-26(33)15-21-29/h10-21H,4-9,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402570
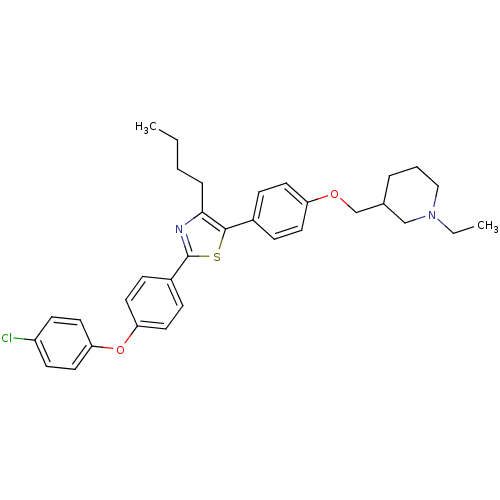
(CHEMBL2205561)Show SMILES CCCCc1nc(sc1-c1ccc(OCC2CCCN(CC)C2)cc1)-c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C33H37ClN2O2S/c1-3-5-8-31-32(25-9-15-28(16-10-25)37-23-24-7-6-21-36(4-2)22-24)39-33(35-31)26-11-17-29(18-12-26)38-30-19-13-27(34)14-20-30/h9-20,24H,3-8,21-23H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402563
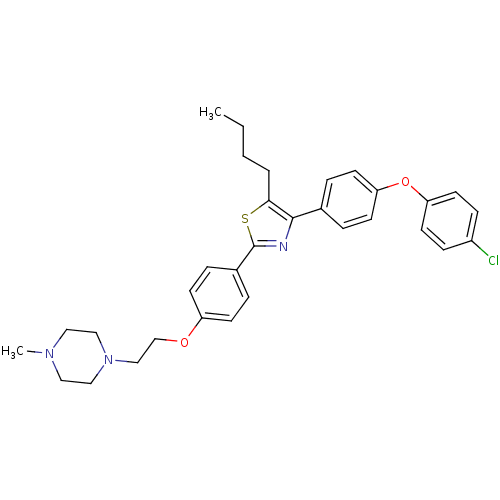
(CHEMBL2205550)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCN2CCN(C)CC2)cc1 Show InChI InChI=1S/C32H36ClN3O2S/c1-3-4-5-30-31(24-6-14-28(15-7-24)38-29-16-10-26(33)11-17-29)34-32(39-30)25-8-12-27(13-9-25)37-23-22-36-20-18-35(2)19-21-36/h6-17H,3-5,18-23H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402566

(CHEMBL2205569)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCCN2CCN(C)CC2)cc1 Show InChI InChI=1S/C33H38ClN3O2S/c1-3-4-6-31-32(25-7-15-29(16-8-25)39-30-17-11-27(34)12-18-30)35-33(40-31)26-9-13-28(14-10-26)38-24-5-19-37-22-20-36(2)21-23-37/h7-18H,3-6,19-24H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402561
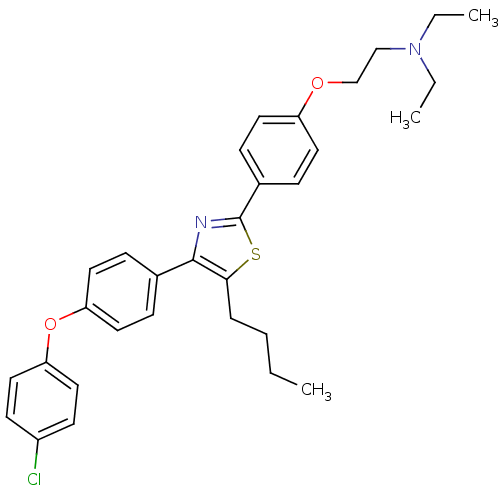
(CHEMBL2205553)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCN(CC)CC)cc1 Show InChI InChI=1S/C31H35ClN2O2S/c1-4-7-8-29-30(23-9-17-27(18-10-23)36-28-19-13-25(32)14-20-28)33-31(37-29)24-11-15-26(16-12-24)35-22-21-34(5-2)6-3/h9-20H,4-8,21-22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402573
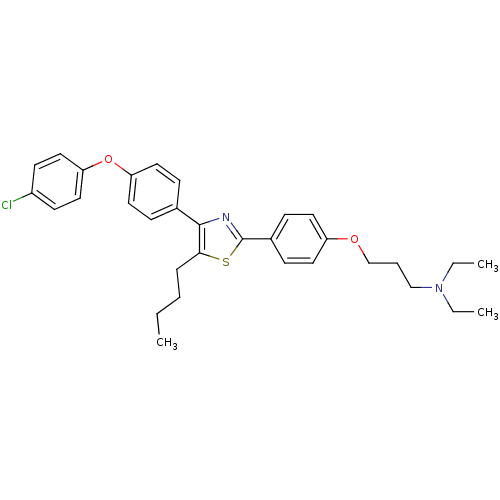
(CHEMBL2205558)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCCN(CC)CC)cc1 Show InChI InChI=1S/C32H37ClN2O2S/c1-4-7-9-30-31(24-10-18-28(19-11-24)37-29-20-14-26(33)15-21-29)34-32(38-30)25-12-16-27(17-13-25)36-23-8-22-35(5-2)6-3/h10-21H,4-9,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249578
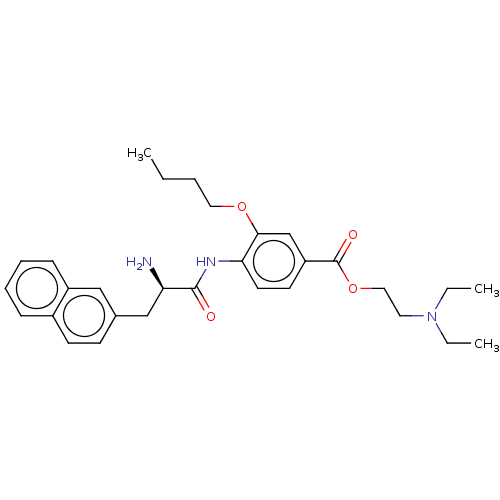
(CHEMBL4095634)Show SMILES CCCCOc1cc(ccc1NC(=O)[C@H](N)Cc1ccc2ccccc2c1)C(=O)OCCN(CC)CC |r| Show InChI InChI=1S/C30H39N3O4/c1-4-7-17-36-28-21-25(30(35)37-18-16-33(5-2)6-3)14-15-27(28)32-29(34)26(31)20-22-12-13-23-10-8-9-11-24(23)19-22/h8-15,19,21,26H,4-7,16-18,20,31H2,1-3H3,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of S110B binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402567

(CHEMBL2205568)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCCN2CCCCC2)cc1 Show InChI InChI=1S/C33H37ClN2O2S/c1-2-3-8-31-32(25-9-17-29(18-10-25)38-30-19-13-27(34)14-20-30)35-33(39-31)26-11-15-28(16-12-26)37-24-7-23-36-21-5-4-6-22-36/h9-20H,2-8,21-24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50005634
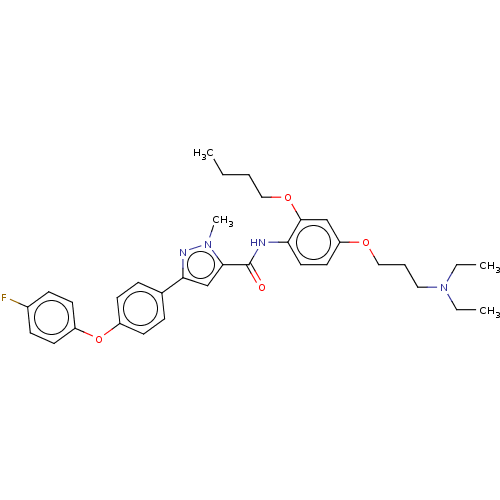
(CHEMBL3235375)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)c1cc(nn1C)-c1ccc(Oc2ccc(F)cc2)cc1 Show InChI InChI=1S/C34H41FN4O4/c1-5-8-21-42-33-23-29(41-22-9-20-39(6-2)7-3)18-19-30(33)36-34(40)32-24-31(37-38(32)4)25-10-14-27(15-11-25)43-28-16-12-26(35)13-17-28/h10-19,23-24H,5-9,20-22H2,1-4H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to biotin-labeled human RAGE domain V after 60 mins by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50005633
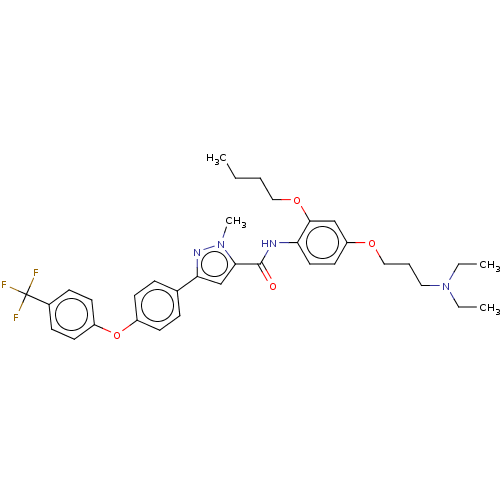
(CHEMBL3235374)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)c1cc(nn1C)-c1ccc(Oc2ccc(cc2)C(F)(F)F)cc1 Show InChI InChI=1S/C35H41F3N4O4/c1-5-8-21-45-33-23-29(44-22-9-20-42(6-2)7-3)18-19-30(33)39-34(43)32-24-31(40-41(32)4)25-10-14-27(15-11-25)46-28-16-12-26(13-17-28)35(36,37)38/h10-19,23-24H,5-9,20-22H2,1-4H3,(H,39,43) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human RAGE assessed as bound amyloid beta level after 60 mins by ELISA |
Eur J Med Chem 79: 128-42 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.072
BindingDB Entry DOI: 10.7270/Q2C82BTF |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50005634

(CHEMBL3235375)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)c1cc(nn1C)-c1ccc(Oc2ccc(F)cc2)cc1 Show InChI InChI=1S/C34H41FN4O4/c1-5-8-21-42-33-23-29(41-22-9-20-39(6-2)7-3)18-19-30(33)36-34(40)32-24-31(37-38(32)4)25-10-14-27(15-11-25)43-28-16-12-26(35)13-17-28/h10-19,23-24H,5-9,20-22H2,1-4H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human RAGE assessed as bound amyloid beta level after 60 mins by ELISA |
Eur J Med Chem 79: 128-42 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.072
BindingDB Entry DOI: 10.7270/Q2C82BTF |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402574
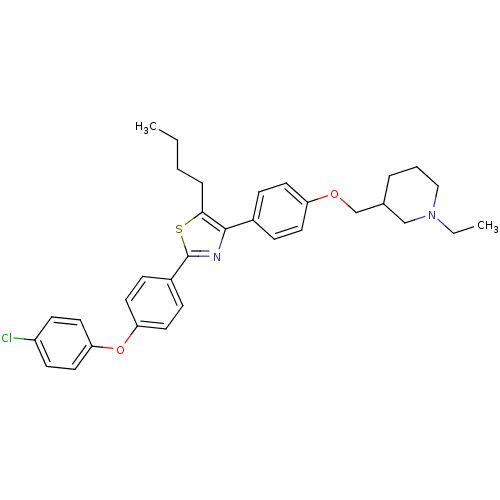
(CHEMBL2205557)Show SMILES CCCCc1sc(nc1-c1ccc(OCC2CCCN(CC)C2)cc1)-c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C33H37ClN2O2S/c1-3-5-8-31-32(25-9-15-28(16-10-25)37-23-24-7-6-21-36(4-2)22-24)35-33(39-31)26-11-17-29(18-12-26)38-30-19-13-27(34)14-20-30/h9-20,24H,3-8,21-23H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402571
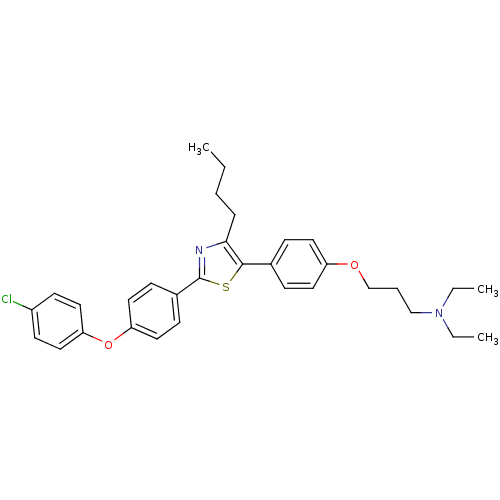
(CHEMBL2205560)Show SMILES CCCCc1nc(sc1-c1ccc(OCCCN(CC)CC)cc1)-c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C32H37ClN2O2S/c1-4-7-9-30-31(24-10-16-27(17-11-24)36-23-8-22-35(5-2)6-3)38-32(34-30)25-12-18-28(19-13-25)37-29-20-14-26(33)15-21-29/h10-21H,4-9,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249578

(CHEMBL4095634)Show SMILES CCCCOc1cc(ccc1NC(=O)[C@H](N)Cc1ccc2ccccc2c1)C(=O)OCCN(CC)CC |r| Show InChI InChI=1S/C30H39N3O4/c1-4-7-17-36-28-21-25(30(35)37-18-16-33(5-2)6-3)14-15-27(28)32-29(34)26(31)20-22-12-13-23-10-8-9-11-24(23)19-22/h8-15,19,21,26H,4-7,16-18,20,31H2,1-3H3,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50005629

(CHEMBL3235049)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)c1cc(nn1C)-c1ccc(OC2CCCCC2)cc1 Show InChI InChI=1S/C34H48N4O4/c1-5-8-22-41-33-24-29(40-23-12-21-38(6-2)7-3)19-20-30(33)35-34(39)32-25-31(36-37(32)4)26-15-17-28(18-16-26)42-27-13-10-9-11-14-27/h15-20,24-25,27H,5-14,21-23H2,1-4H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human RAGE assessed as bound amyloid beta level after 60 mins by ELISA |
Eur J Med Chem 79: 128-42 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.072
BindingDB Entry DOI: 10.7270/Q2C82BTF |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402562
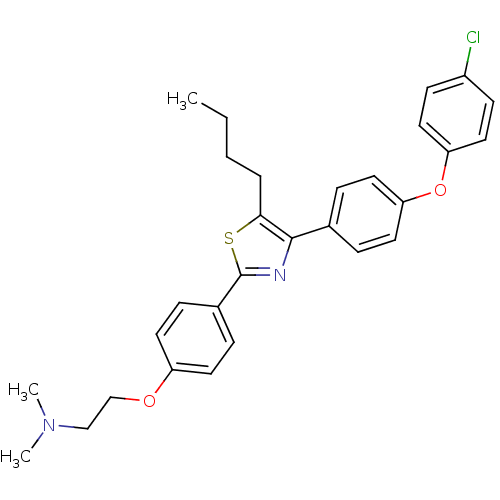
(CHEMBL2205552)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCN(C)C)cc1 Show InChI InChI=1S/C29H31ClN2O2S/c1-4-5-6-27-28(21-7-15-25(16-8-21)34-26-17-11-23(30)12-18-26)31-29(35-27)22-9-13-24(14-10-22)33-20-19-32(2)3/h7-18H,4-6,19-20H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249578

(CHEMBL4095634)Show SMILES CCCCOc1cc(ccc1NC(=O)[C@H](N)Cc1ccc2ccccc2c1)C(=O)OCCN(CC)CC |r| Show InChI InChI=1S/C30H39N3O4/c1-4-7-17-36-28-21-25(30(35)37-18-16-33(5-2)6-3)14-15-27(28)32-29(34)26(31)20-22-12-13-23-10-8-9-11-24(23)19-22/h8-15,19,21,26H,4-7,16-18,20,31H2,1-3H3,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605302

(CHEMBL5191418)Show SMILES CCc1nc(cn1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605310
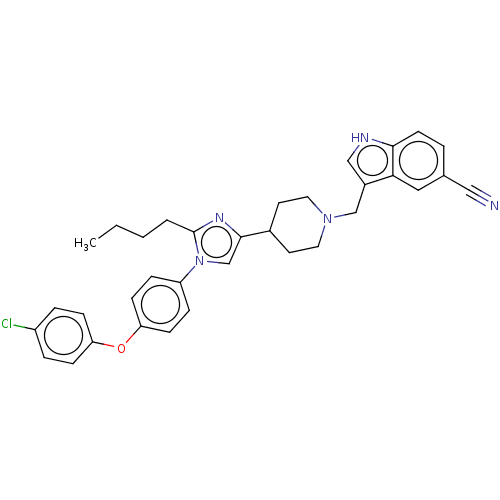
(CHEMBL5194592)Show SMILES CCCCc1nc(cn1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(Cc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605284
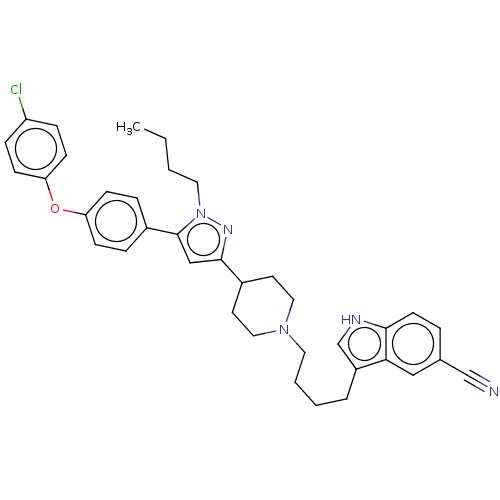
(CHEMBL5170867)Show SMILES CCCCn1nc(cc1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50397837
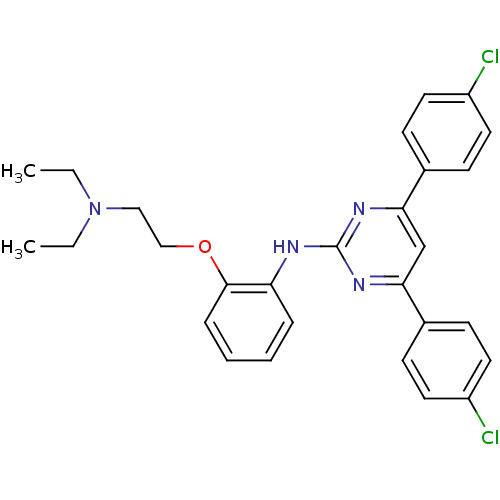
(CHEMBL2179074)Show SMILES CCN(CC)CCOc1ccccc1Nc1nc(cc(n1)-c1ccc(Cl)cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H28Cl2N4O/c1-3-34(4-2)17-18-35-27-8-6-5-7-24(27)31-28-32-25(20-9-13-22(29)14-10-20)19-26(33-28)21-11-15-23(30)16-12-21/h5-16,19H,3-4,17-18H2,1-2H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant biotinylated receptor for advanced glycation end product expressed in Escherichia coli DE3 assessed as inhibition of ... |
J Med Chem 55: 9120-35 (2012)
Article DOI: 10.1021/jm300172z
BindingDB Entry DOI: 10.7270/Q289170G |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50005635

(CHEMBL3235378)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)c1cc(nn1C)-c1ccc(Oc2ccc(Cl)c(OC)c2)cc1 Show InChI InChI=1S/C35H43ClN4O5/c1-6-9-20-44-34-22-27(43-21-10-19-40(7-2)8-3)16-18-30(34)37-35(41)32-24-31(38-39(32)4)25-11-13-26(14-12-25)45-28-15-17-29(36)33(23-28)42-5/h11-18,22-24H,6-10,19-21H2,1-5H3,(H,37,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human RAGE assessed as bound amyloid beta level after 60 mins by ELISA |
Eur J Med Chem 79: 128-42 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.072
BindingDB Entry DOI: 10.7270/Q2C82BTF |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50005630
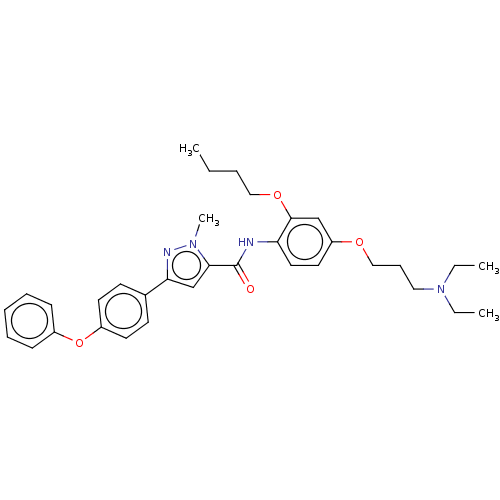
(CHEMBL3235050)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)c1cc(nn1C)-c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C34H42N4O4/c1-5-8-22-41-33-24-29(40-23-12-21-38(6-2)7-3)19-20-30(33)35-34(39)32-25-31(36-37(32)4)26-15-17-28(18-16-26)42-27-13-10-9-11-14-27/h9-11,13-20,24-25H,5-8,12,21-23H2,1-4H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human RAGE assessed as bound amyloid beta level after 60 mins by ELISA |
Eur J Med Chem 79: 128-42 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.072
BindingDB Entry DOI: 10.7270/Q2C82BTF |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50402569
(CHEMBL2205565)Show SMILES CCCCc1sc(NC(=O)c2ccc(OCCCN(CC)CC)cc2)nc1-c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C33H38ClN3O3S/c1-4-7-9-30-31(24-10-18-28(19-11-24)40-29-20-14-26(34)15-21-29)35-33(41-30)36-32(38)25-12-16-27(17-13-25)39-23-8-22-37(5-2)6-3/h10-21H,4-9,22-23H2,1-3H3,(H,35,36,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Antagonist activity at human biotinylated RAGE assessed as inhibition of amyloid beta binding after 60 mins by ELISA |
Bioorg Med Chem Lett 22: 7555-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.022
BindingDB Entry DOI: 10.7270/Q2XG9S9S |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605313

(CHEMBL5180815)Show SMILES CCCCCc1nc(cn1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2cn(C)c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50005632

(CHEMBL3235052)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)c1cc(nn1C)-c1ccc(Oc2ccc(OC)cc2)cc1 Show InChI InChI=1S/C35H44N4O5/c1-6-9-22-43-34-24-30(42-23-10-21-39(7-2)8-3)19-20-31(34)36-35(40)33-25-32(37-38(33)4)26-11-13-28(14-12-26)44-29-17-15-27(41-5)16-18-29/h11-20,24-25H,6-10,21-23H2,1-5H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human RAGE assessed as bound amyloid beta level after 60 mins by ELISA |
Eur J Med Chem 79: 128-42 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.072
BindingDB Entry DOI: 10.7270/Q2C82BTF |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605294

(CHEMBL5203206)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2cn(C)c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605307

(CHEMBL5175011)Show SMILES Clc1ccc(Oc2ccc(cc2)-n2cc(nc2C2CCC2)C2CCN(CCCCc3c[nH]c4ccc(cc34)C#N)CC2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50397836

(CHEMBL2179073)Show SMILES CCN(CC)CCOc1cccc(Nc2nc(cc(n2)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C28H28Cl2N4O/c1-3-34(4-2)16-17-35-25-7-5-6-24(18-25)31-28-32-26(20-8-12-22(29)13-9-20)19-27(33-28)21-10-14-23(30)15-11-21/h5-15,18-19H,3-4,16-17H2,1-2H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605303

(CHEMBL5208902)Show SMILES CCCc1nc(cn1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605283

(CHEMBL5188606)Show SMILES CCCCc1nc(cn1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249580
(Azeliragon | PF-04494700 | TTP448 | US11524942, Co...)Show SMILES CCCCc1nc(cn1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCCN(CC)CC)cc1 Show InChI InChI=1S/C32H38ClN3O2/c1-4-7-9-32-34-31(25-10-16-28(17-11-25)37-23-8-22-35(5-2)6-3)24-36(32)27-14-20-30(21-15-27)38-29-18-12-26(33)13-19-29/h10-21,24H,4-9,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605286

(CHEMBL5192104)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605291

(CHEMBL5170035)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(Cc2c[nH]c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50397836

(CHEMBL2179073)Show SMILES CCN(CC)CCOc1cccc(Nc2nc(cc(n2)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C28H28Cl2N4O/c1-3-34(4-2)16-17-35-25-7-5-6-24(18-25)31-28-32-26(20-8-12-22(29)13-9-20)19-27(33-28)21-10-14-23(30)15-11-21/h5-15,18-19H,3-4,16-17H2,1-2H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to biotin-labeled human RAGE domain V after 60 mins by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50397836

(CHEMBL2179073)Show SMILES CCN(CC)CCOc1cccc(Nc2nc(cc(n2)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C28H28Cl2N4O/c1-3-34(4-2)16-17-35-25-7-5-6-24(18-25)31-28-32-26(20-8-12-22(29)13-9-20)19-27(33-28)21-10-14-23(30)15-11-21/h5-15,18-19H,3-4,16-17H2,1-2H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant biotinylated receptor for advanced glycation end product expressed in Escherichia coli DE3 assessed as inhibition of ... |
J Med Chem 55: 9120-35 (2012)
Article DOI: 10.1021/jm300172z
BindingDB Entry DOI: 10.7270/Q289170G |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50005631
(CHEMBL3235051)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)c1cc(nn1C)-c1ccc(Oc2ccccn2)cc1 Show InChI InChI=1S/C33H41N5O4/c1-5-8-21-41-31-23-27(40-22-11-20-38(6-2)7-3)17-18-28(31)35-33(39)30-24-29(36-37(30)4)25-13-15-26(16-14-25)42-32-12-9-10-19-34-32/h9-10,12-19,23-24H,5-8,11,20-22H2,1-4H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Woosuk University
Curated by ChEMBL
| Assay Description
Inhibition of biotinylated human RAGE assessed as bound amyloid beta level after 60 mins by ELISA |
Eur J Med Chem 79: 128-42 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.072
BindingDB Entry DOI: 10.7270/Q2C82BTF |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605316
(CHEMBL5178292)Show SMILES CCCCCc1nc(cn1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2cn(C(C)C)c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605297

(CHEMBL5172417)Show SMILES CCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2cn(C)c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50605298
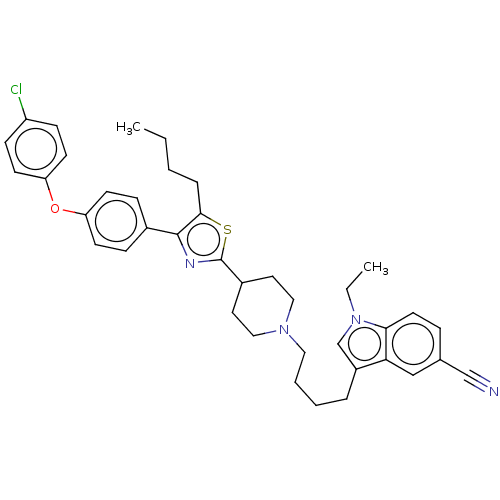
(CHEMBL5174761)Show SMILES CCCCc1sc(nc1-c1ccc(Oc2ccc(Cl)cc2)cc1)C1CCN(CCCCc2cn(CC)c3ccc(cc23)C#N)CC1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114347
BindingDB Entry DOI: 10.7270/Q2PG1WTJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data