

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50606909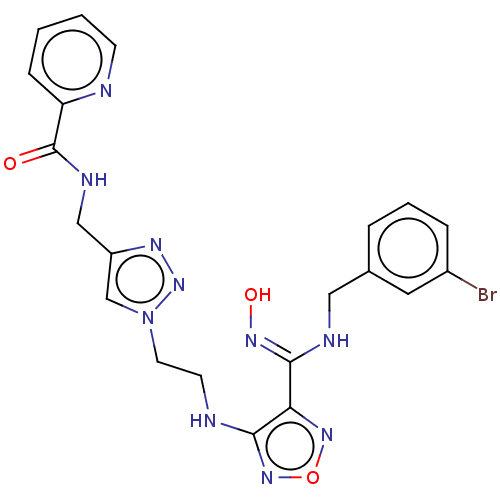 (CHEMBL5219888) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00263 BindingDB Entry DOI: 10.7270/Q2280CQZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50606907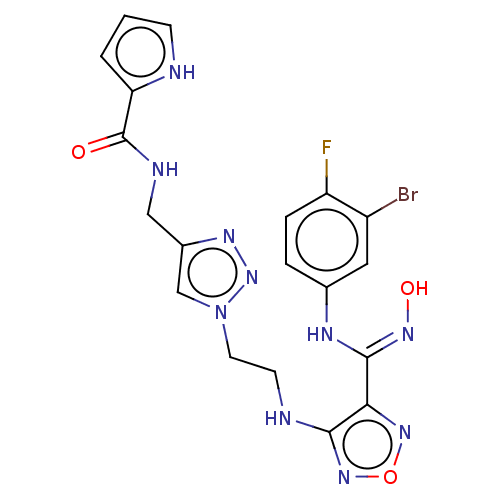 (CHEMBL5220224) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00263 BindingDB Entry DOI: 10.7270/Q2280CQZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587578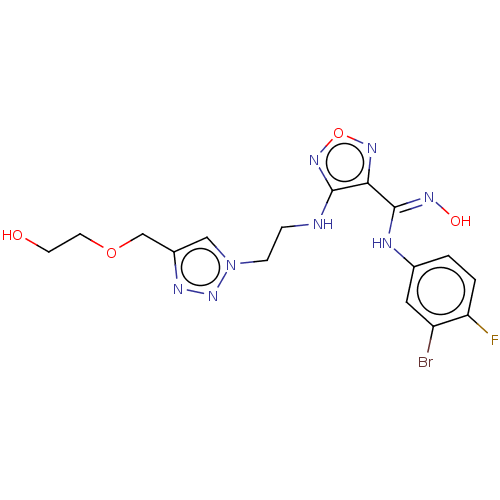 (CHEMBL5091825) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00263 BindingDB Entry DOI: 10.7270/Q2280CQZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587578 (CHEMBL5091825) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50528763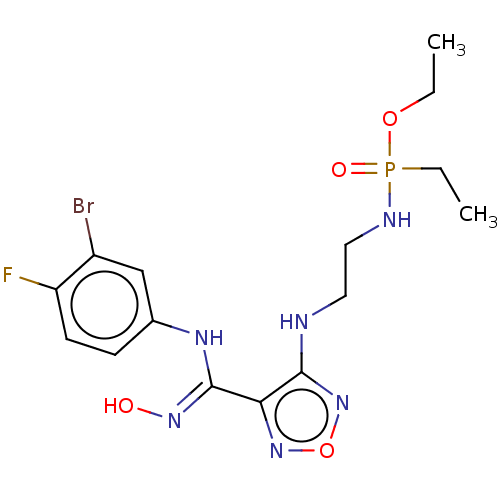 (CHEMBL4472697) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human IDO2 expressed in Escherichia coli expression system | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50562506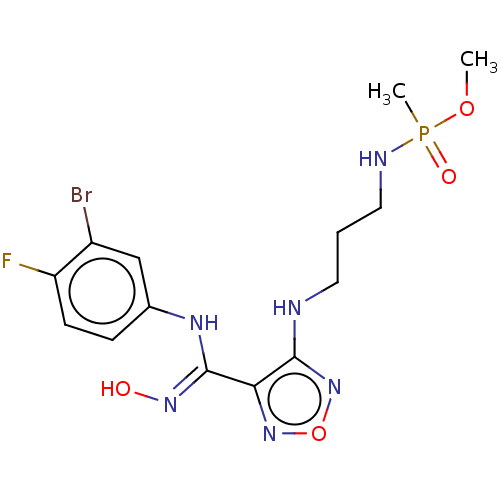 (CHEMBL4791168) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of N-terminal 6His-tagged recombinant human IDO2 (15 to 420 residues) expressed in Escherichia coli using L-Trp as substrate by UV absorba... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00925 BindingDB Entry DOI: 10.7270/Q28D010N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587572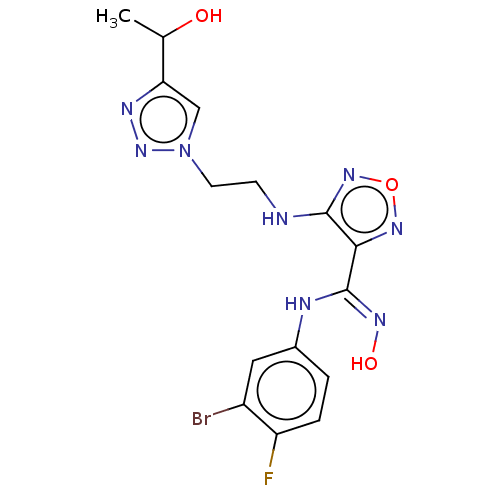 (CHEMBL5085580) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50606906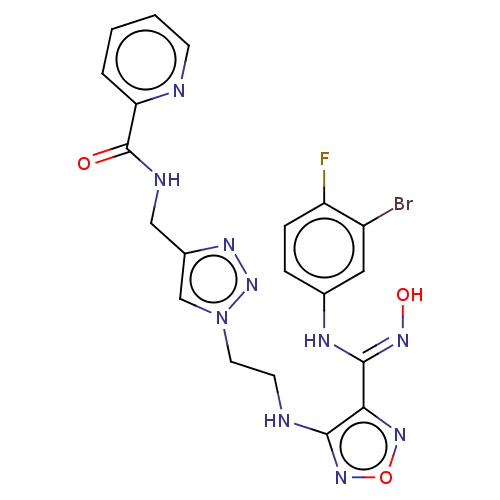 (CHEMBL5221035) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 387 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00263 BindingDB Entry DOI: 10.7270/Q2280CQZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50528779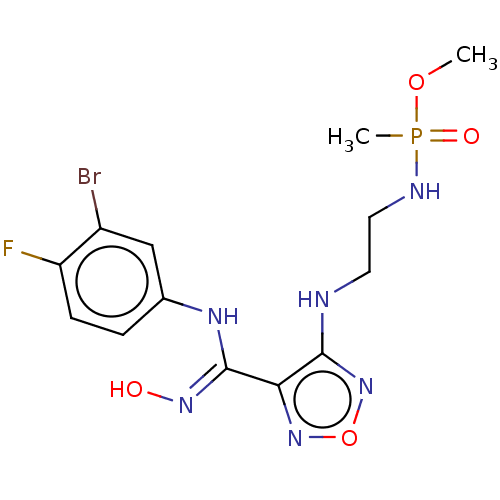 (CHEMBL4514196) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human IDO2 expressed in Escherichia coli expression system | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50606908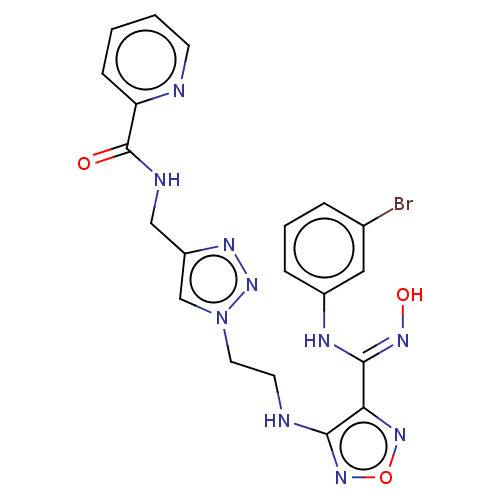 (CHEMBL5221037) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00263 BindingDB Entry DOI: 10.7270/Q2280CQZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50528780 (CHEMBL4436582) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human IDO2 expressed in Escherichia coli expression system | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587576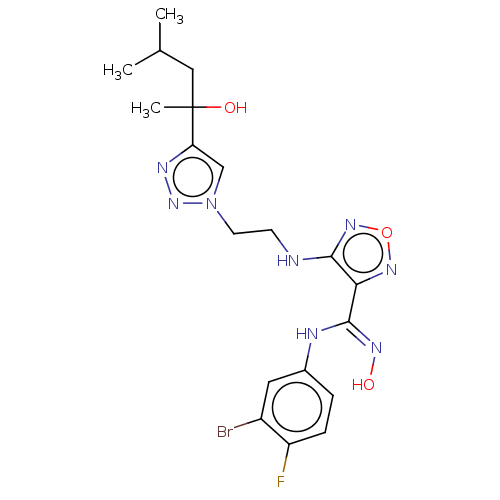 (CHEMBL5082481) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 689 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50126143 (Epacadostat | INCB-024360) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Medical University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human IDO2 expressed in Escherichia coli expression system | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111629 BindingDB Entry DOI: 10.7270/Q2BZ69GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587574 (CHEMBL5090441) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 726 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587566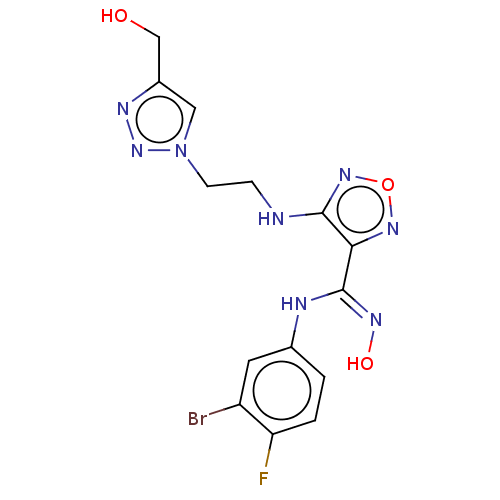 (CHEMBL5092513) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587567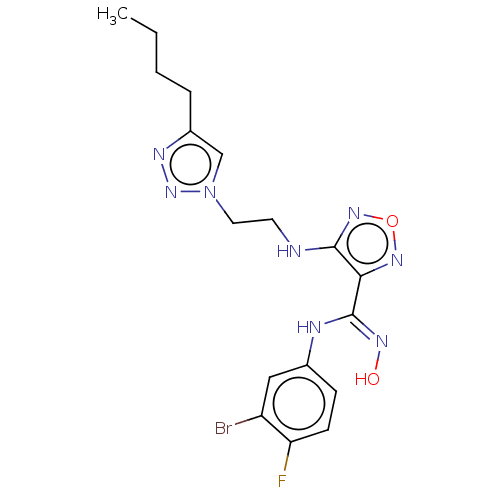 (CHEMBL5091241) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587577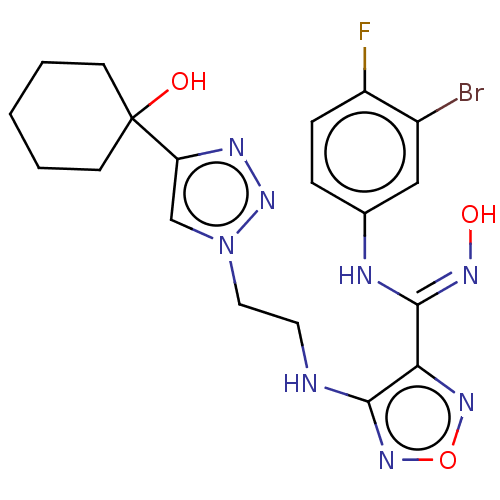 (CHEMBL5091415) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 865 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587579 (CHEMBL5091243) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587573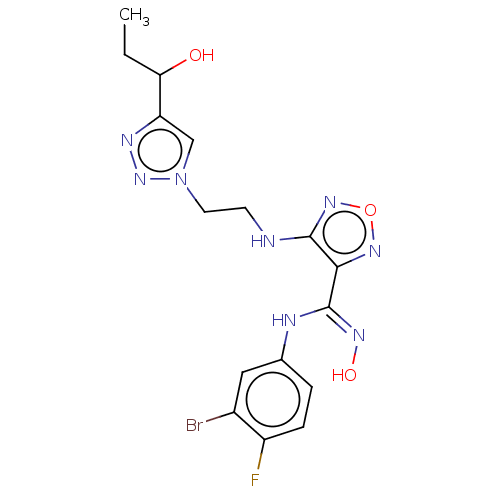 (CHEMBL5091590) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 947 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587570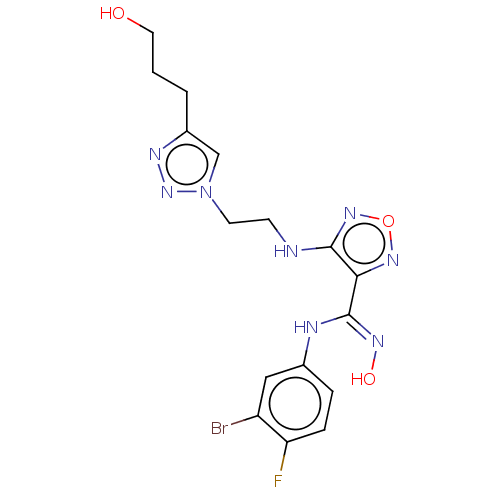 (CHEMBL5093816) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 958 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587575 (CHEMBL5084150) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 958 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM387356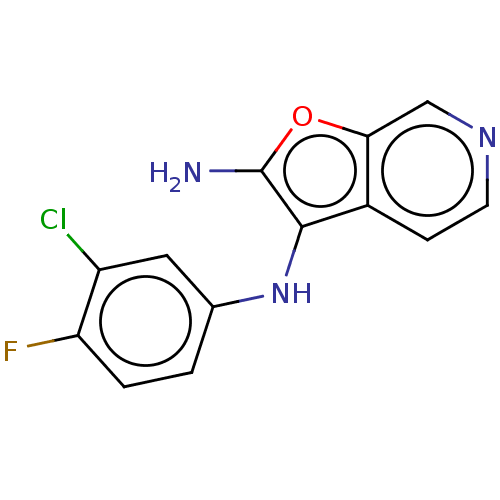 (N3-(3-Chloro- 4-fluorophenyl) furo[2,3- c]pyridine...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenase-2 (hIDO2) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formy... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM387592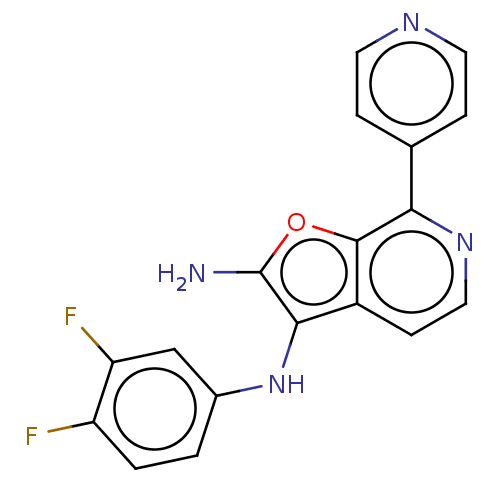 (N3-(3,4- difluoro- phenyl)-7- (pyridin-4- yl)furo[...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina | Assay Description Human indoleamine 2,3-dioxygenase-2 (hIDO2) catalyzes the oxidative cleavage of the pyrrole ring of the indole nucleus of tryptophan to yield N-formy... | J Med Chem 52: 74-86 (2009) BindingDB Entry DOI: 10.7270/Q24M96V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587571 (CHEMBL5078912) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587580 (CHEMBL5088729) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587569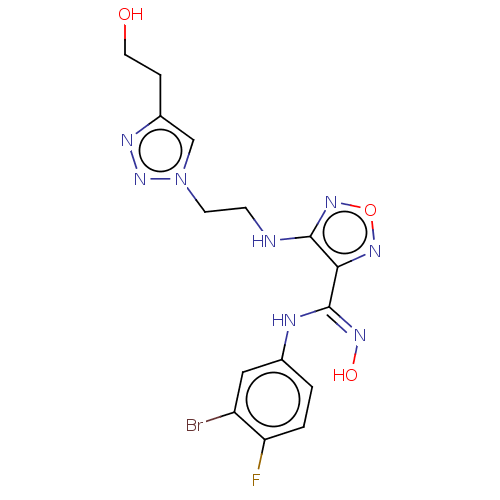 (CHEMBL5078101) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM48009 (8-fluoranylindolo[2,1-b]quinazoline-6,12-dione | 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM50336640 ((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM50402289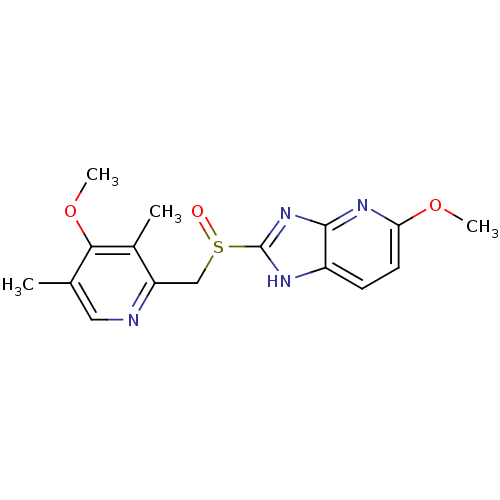 (TENATOPRAZOLE) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00263 BindingDB Entry DOI: 10.7270/Q2280CQZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442991 (CHEMBL432537 | GNF-Pf-3777 | US10669273, Compound ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50402289 (TENATOPRAZOLE) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00263 BindingDB Entry DOI: 10.7270/Q2280CQZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50587568 (CHEMBL5084767) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of IDO2 (unknown origin) assessed as reduction in kynurenine formation using L-tryptophan as substrate by methylene blue reagent based ass... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01305 BindingDB Entry DOI: 10.7270/Q2M90DJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442987 (8-Bromotryptanthrin | CHEMBL72165) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50514040 (CHEMBL4436480) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human IDO2 transfected in human U87MG cells assessed as reduction in kynurenine production using L-tryptophan as substrate incubated wi... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50514041 (CHEMBL4452150) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human IDO2 transfected in human U87MG cells assessed as reduction in kynurenine production using L-tryptophan as substrate incubated wi... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM50085045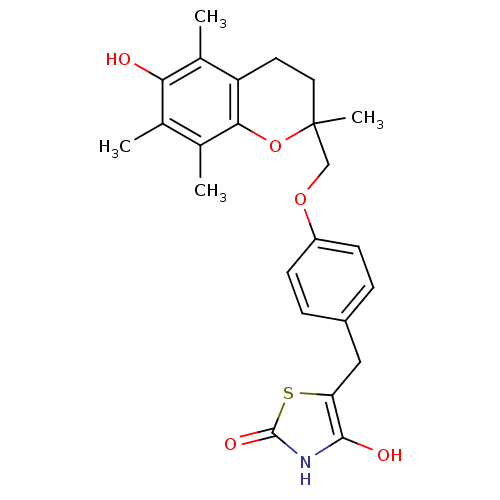 (5-((4-((6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50240612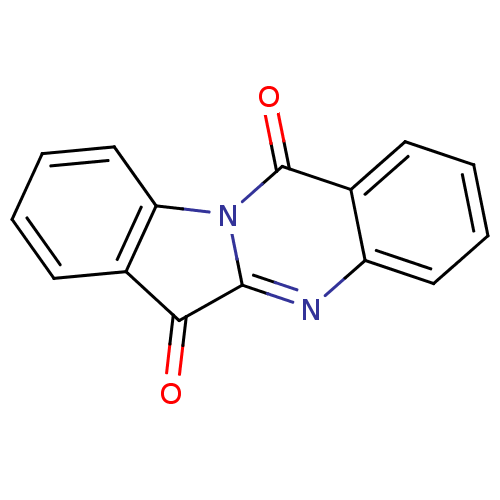 (CHEMBL306946 | GNF-PF-2691 | Indolo[2,1-b]quinazol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM31774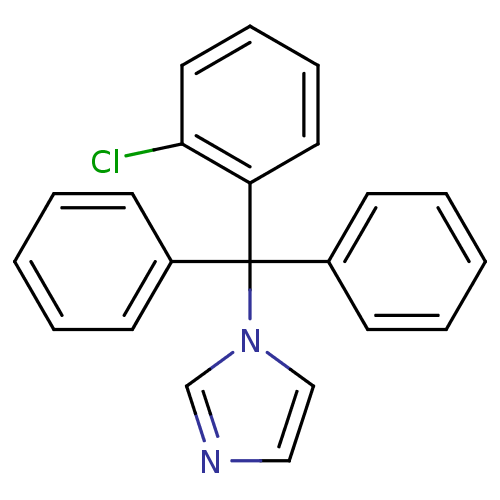 (CHEMBL104 | Canesten | Clotrimazole | Lotrimin | M...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM31772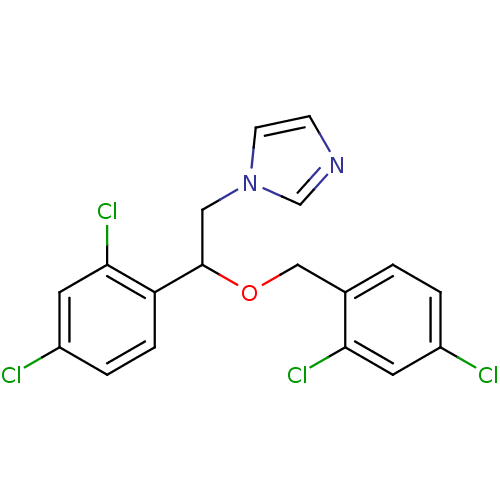 (1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM31773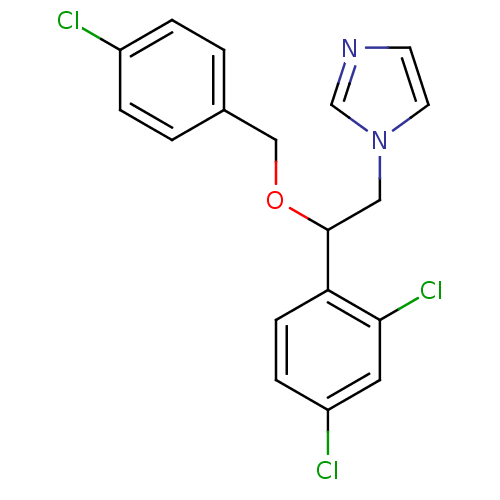 (ECONAZOLE | Econazole nitrate | Gyno-pevaryl | Pev...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM48009 (8-fluoranylindolo[2,1-b]quinazoline-6,12-dione | 8...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM50507277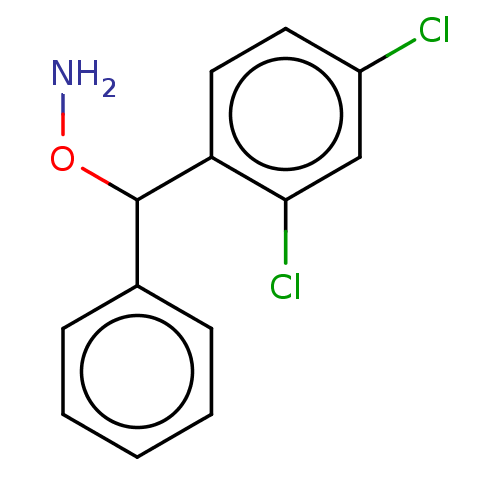 (CHEMBL4578334) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of mouse IDO2 expressed in human T-REx cells using L-tryptophan as substrate after 20 hrs | Eur J Med Chem 162: 455-464 (2019) Article DOI: 10.1016/j.ejmech.2018.11.010 BindingDB Entry DOI: 10.7270/Q2959MV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50514038 (CHEMBL4577825) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of human IDO2 transfected in human U87MG cells assessed as reduction in kynurenine production using L-tryptophan as substrate incubated wi... | J Med Chem 62: 9161-9174 (2019) Article DOI: 10.1021/acs.jmedchem.9b01079 BindingDB Entry DOI: 10.7270/Q27W6GJ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM47032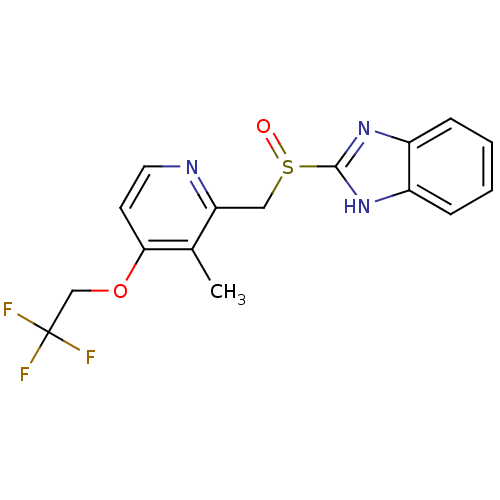 (2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Sydney Curated by ChEMBL | Assay Description Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom... | Bioorg Med Chem Lett 22: 7641-6 (2012) Article DOI: 10.1016/j.bmcl.2012.10.010 BindingDB Entry DOI: 10.7270/Q20G3M92 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442988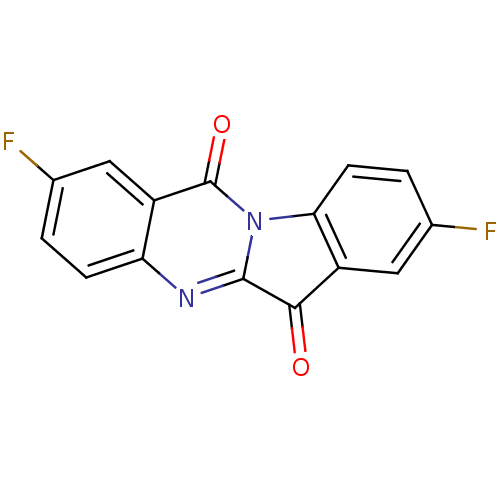 (CHEMBL3087009) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human IDO2 expressed in human U87MG cells assessed as reduction in kynurenine formation using L-tryptophan as substrate aft... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Homo sapiens (Human)) | BDBM50442987 (8-Bromotryptanthrin | CHEMBL72165) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant human C-terminal His6-tagged IDO2 (14-420 residues) expressed in Escherichia coli BL21(DE3) assessed as reduction in L-kynu... | Eur J Med Chem 123: 171-179 (2016) Article DOI: 10.1016/j.ejmech.2016.07.013 BindingDB Entry DOI: 10.7270/Q2M90BNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 2 (Mus musculus) | BDBM50507288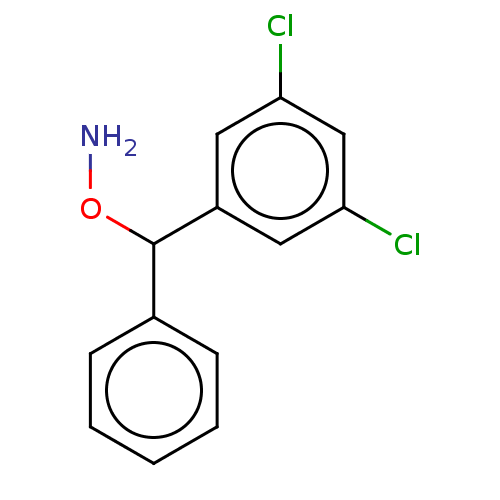 (CHEMBL4473408) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College Curated by ChEMBL | Assay Description Inhibition of mouse IDO2 expressed in human T-REx cells using L-tryptophan as substrate after 20 hrs | Eur J Med Chem 162: 455-464 (2019) Article DOI: 10.1016/j.ejmech.2018.11.010 BindingDB Entry DOI: 10.7270/Q2959MV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 133 total ) | Next | Last >> |