

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipoxygenase (Glycine max) | BDBM50505285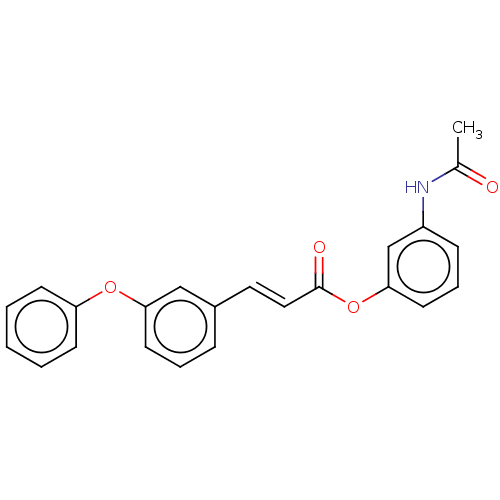 (CHEMBL4450603) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of soya bean LOX assessed as reduction in formation of 13-hydroperoxylinoleic acid using sodium linoleate as substrate | J Med Chem 62: 8881-8914 (2019) Article DOI: 10.1021/acs.jmedchem.9b00017 BindingDB Entry DOI: 10.7270/Q27084PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM32020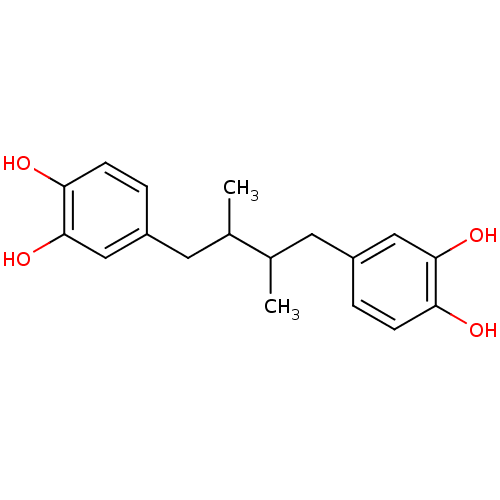 (4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
IQOG, CSIC Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase using sodium linoleate as substrate by UV-based analysis | J Med Chem 62: 2184-2201 (2019) Article DOI: 10.1021/acs.jmedchem.8b01987 BindingDB Entry DOI: 10.7270/Q2MC93HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50276365 (CHEMBL4127281) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86082 (Tetraketone, 19) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50282770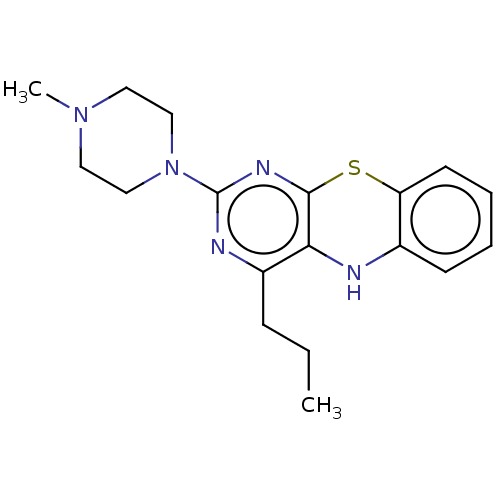 (CHEMBL2296824) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Medical University of Silesia Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase | Eur J Med Chem 138: 774-806 (2017) Article DOI: 10.1016/j.ejmech.2017.07.009 BindingDB Entry DOI: 10.7270/Q26H4KZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM32020 (4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86085 (Tetraketone, 22) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86070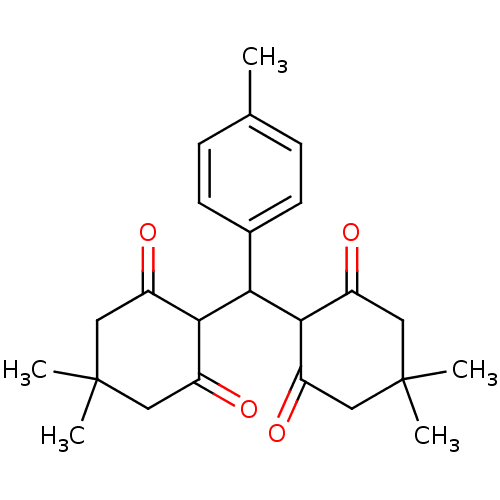 (Tetraketone, 3) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86077 (Tetraketone, 11) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50276370 (CHEMBL4126039) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM192766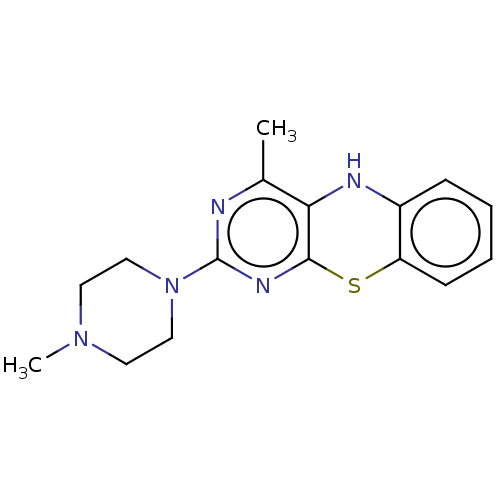 (4-MMPB) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Medical University of Silesia Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase using linoleic acid as substrate preincubated for 5 mins followed by substrate addition measuredf after 7 mins by ... | Eur J Med Chem 138: 774-806 (2017) Article DOI: 10.1016/j.ejmech.2017.07.009 BindingDB Entry DOI: 10.7270/Q26H4KZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86074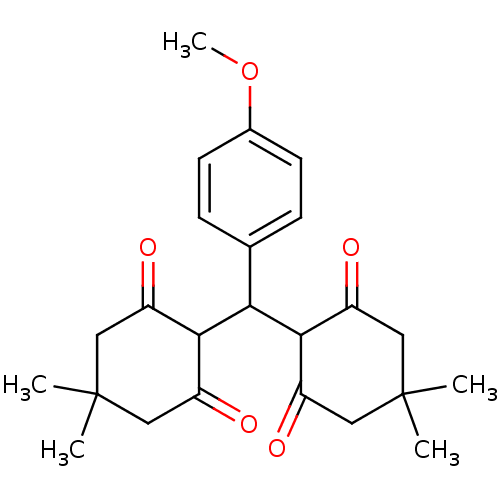 (Tetraketone, 8) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM50009001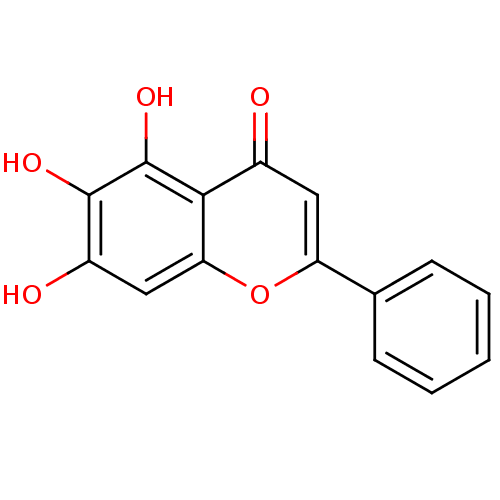 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86072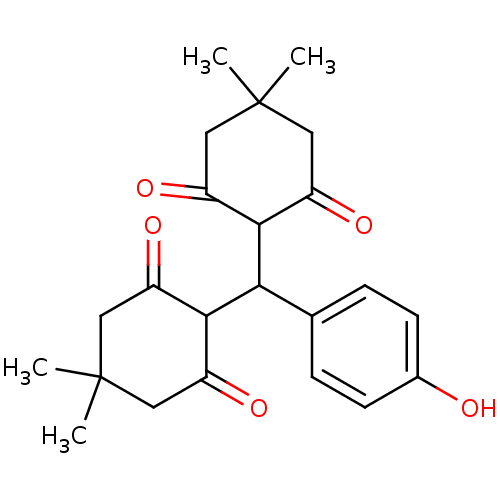 (Tetraketone, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86080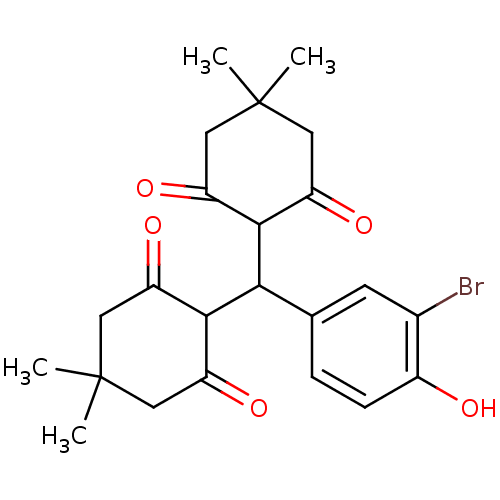 (Tetraketone, 15) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.71E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM32020 (4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbottabad University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase | Bioorg Med Chem 26: 3731-3762 (2018) Article DOI: 10.1016/j.bmc.2018.05.042 BindingDB Entry DOI: 10.7270/Q29889HN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM32020 (4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zagreb Curated by ChEMBL | Assay Description Inhibition of soybean LOX using sodium linoleate as substrate by spectrophotometry | Eur J Med Chem 86: 502-14 (2014) Article DOI: 10.1016/j.ejmech.2014.09.013 BindingDB Entry DOI: 10.7270/Q2Z89F0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86078 (Tetraketone, 12) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.84E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50021468 (CHEMBL3289934) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86103 (3'-Hydroxyepiglucoisatisin, 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86076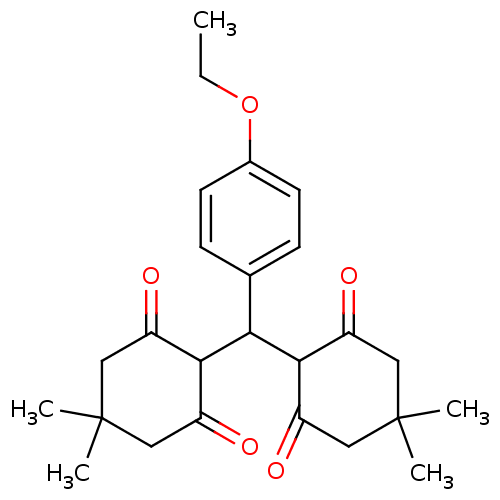 (Tetraketone, 10) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.08E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50531971 (CHEMBL4539028) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IQOG, CSIC Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase using sodium linoleate as substrate by UV-based analysis | J Med Chem 62: 2184-2201 (2019) Article DOI: 10.1021/acs.jmedchem.8b01987 BindingDB Entry DOI: 10.7270/Q2MC93HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86069 (Tetraketone, 2) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86075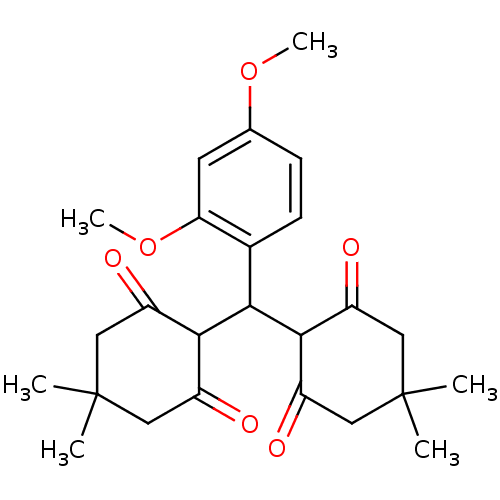 (Tetraketone, 9) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86102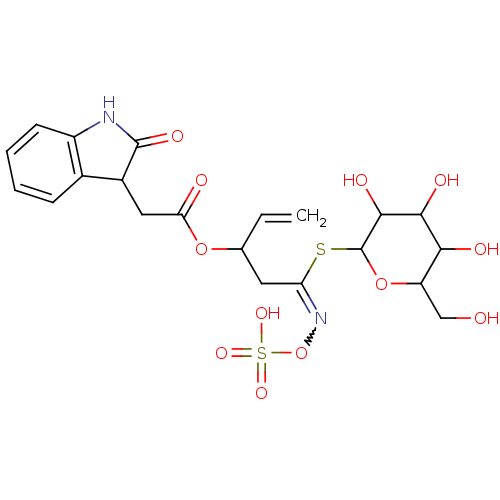 (Epiglucoisatisin, 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86083 (Tetraketone, 20) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.54E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50531968 (CHEMBL4575147) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IQOG, CSIC Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase using sodium linoleate as substrate by UV-based analysis | J Med Chem 62: 2184-2201 (2019) Article DOI: 10.1021/acs.jmedchem.8b01987 BindingDB Entry DOI: 10.7270/Q2MC93HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50037298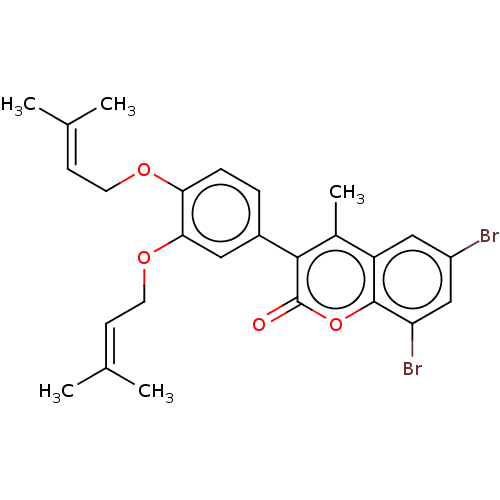 (CHEMBL3356691) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of soybean LOX assessed as reduction in conversion of sodium linoleate to 13-hydroperoxylinoleic acid by UV spectroscopy | Bioorg Med Chem 22: 6586-94 (2015) Article DOI: 10.1016/j.bmc.2014.10.008 BindingDB Entry DOI: 10.7270/Q2MK6FHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50045849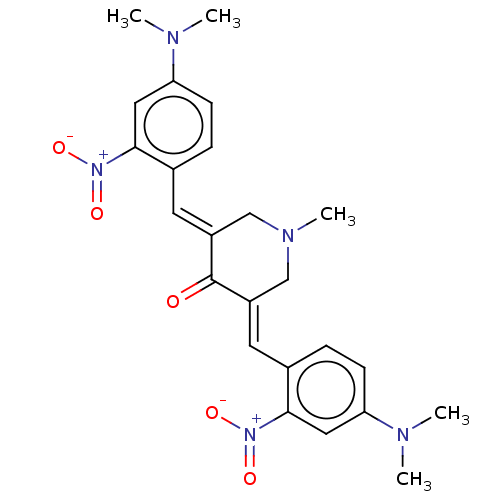 (CHEMBL3309949) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86101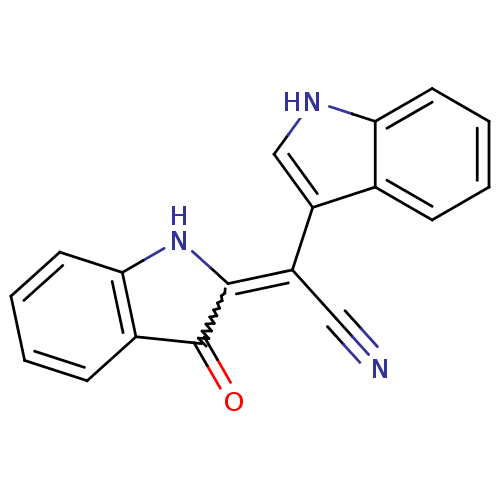 (2-[Cyano(3-indolyl) methylene]-3-indolone, 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.91E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50045848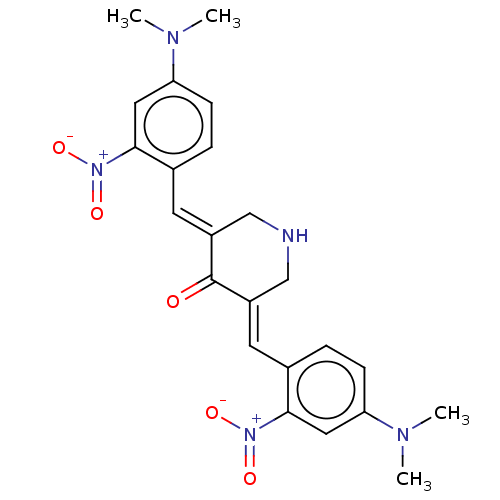 (CHEMBL3309948) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86087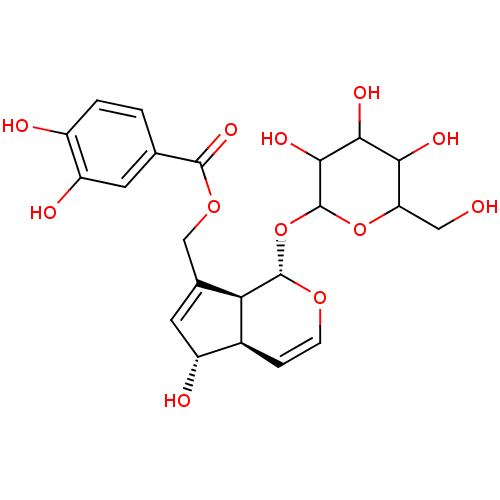 (Buddlejoside B, 2) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.97E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Chinese Academy of Medical Sciences | Assay Description Lipoxygenase inhibiting activity was measured by slightly modifying the spectrometric method. Lipoxygenase type I-B and linoleic acid were purchased... | J Enzyme Inhib Med Chem 23: 140-3 (2008) Article DOI: 10.1080/14756360701342532 BindingDB Entry DOI: 10.7270/Q2JH3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50037297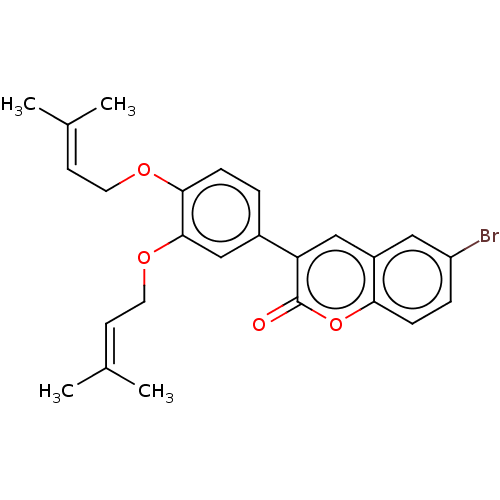 (CHEMBL3356692) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of soybean LOX assessed as reduction in conversion of sodium linoleate to 13-hydroperoxylinoleic acid by UV spectroscopy | Bioorg Med Chem 22: 6586-94 (2015) Article DOI: 10.1016/j.bmc.2014.10.008 BindingDB Entry DOI: 10.7270/Q2MK6FHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86092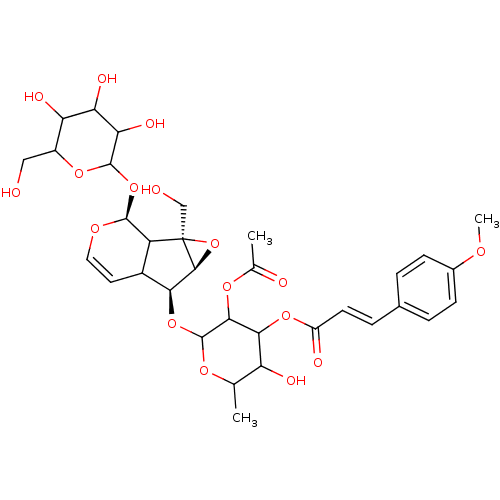 (Buddlejoside A5, 7) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.04E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Chinese Academy of Medical Sciences | Assay Description Lipoxygenase inhibiting activity was measured by slightly modifying the spectrometric method. Lipoxygenase type I-B and linoleic acid were purchased... | J Enzyme Inhib Med Chem 23: 140-3 (2008) Article DOI: 10.1080/14756360701342532 BindingDB Entry DOI: 10.7270/Q2JH3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50282833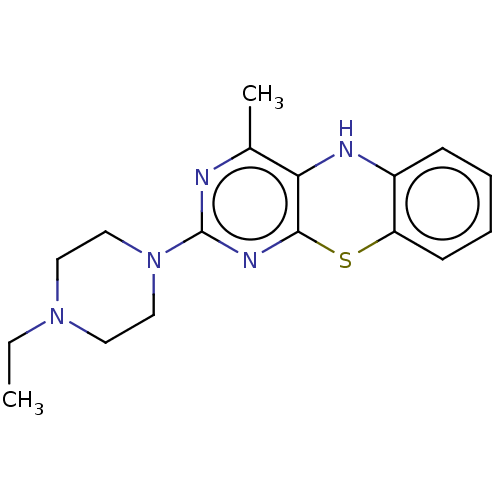 (CHEMBL226300) | UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Medical University of Silesia Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase using linoleic acid as substrate preincubated for 5 mins followed by substrate addition measuredf after 7 mins by ... | Eur J Med Chem 138: 774-806 (2017) Article DOI: 10.1016/j.ejmech.2017.07.009 BindingDB Entry DOI: 10.7270/Q26H4KZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86104 (Sulfoglucobrassicin, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86091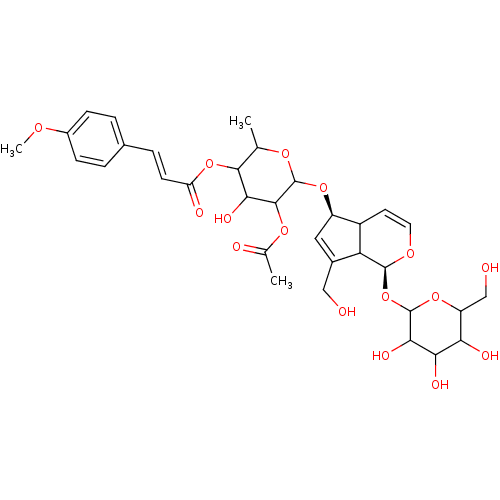 (Buddlejoside A2, 6) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.19E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Chinese Academy of Medical Sciences | Assay Description Lipoxygenase inhibiting activity was measured by slightly modifying the spectrometric method. Lipoxygenase type I-B and linoleic acid were purchased... | J Enzyme Inhib Med Chem 23: 140-3 (2008) Article DOI: 10.1080/14756360701342532 BindingDB Entry DOI: 10.7270/Q2JH3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86086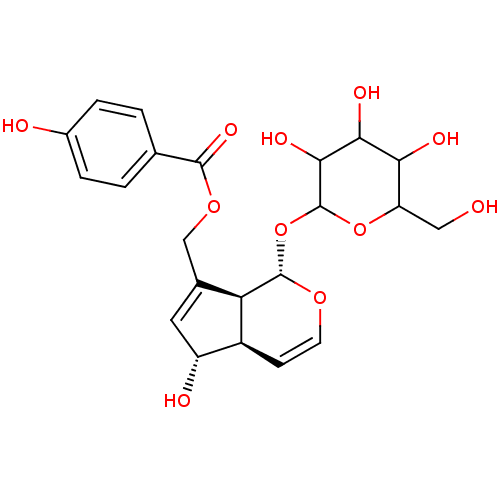 (Buddlejoside A, 1) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.43E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Chinese Academy of Medical Sciences | Assay Description Lipoxygenase inhibiting activity was measured by slightly modifying the spectrometric method. Lipoxygenase type I-B and linoleic acid were purchased... | J Enzyme Inhib Med Chem 23: 140-3 (2008) Article DOI: 10.1080/14756360701342532 BindingDB Entry DOI: 10.7270/Q2JH3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50045847 (CHEMBL3309947) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86090 (Beta-gardiol, 5) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.73E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Chinese Academy of Medical Sciences | Assay Description Lipoxygenase inhibiting activity was measured by slightly modifying the spectrometric method. Lipoxygenase type I-B and linoleic acid were purchased... | J Enzyme Inhib Med Chem 23: 140-3 (2008) Article DOI: 10.1080/14756360701342532 BindingDB Entry DOI: 10.7270/Q2JH3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86105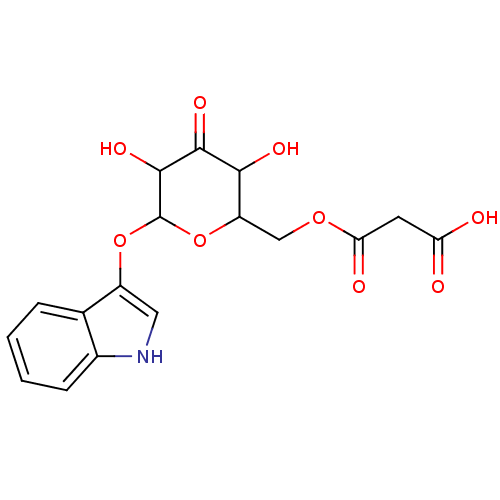 (Isatan A, 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.93E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86089 (Genipin, 4) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Chinese Academy of Medical Sciences | Assay Description Lipoxygenase inhibiting activity was measured by slightly modifying the spectrometric method. Lipoxygenase type I-B and linoleic acid were purchased... | J Enzyme Inhib Med Chem 23: 140-3 (2008) Article DOI: 10.1080/14756360701342532 BindingDB Entry DOI: 10.7270/Q2JH3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM32020 (4-[4-(3,4-dihydroxyphenyl)-2,3-dimethyl-butyl]pyro...) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of soybean LOX assessed as reduction in conversion of sodium linoleate to 13-hydroperoxylinoleic acid by UV spectroscopy | Bioorg Med Chem 22: 6586-94 (2015) Article DOI: 10.1016/j.bmc.2014.10.008 BindingDB Entry DOI: 10.7270/Q2MK6FHF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86084 (Tetraketone, 21) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.15E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50021467 (CHEMBL3289933) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universiti Kebangsaan Malaysia Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase after 5 mins by colorimetric assay | Bioorg Med Chem 22: 4151-61 (2014) Article DOI: 10.1016/j.bmc.2014.05.052 BindingDB Entry DOI: 10.7270/Q2GX4D6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86088 (Buddlejoside C, 3) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Chinese Academy of Medical Sciences | Assay Description Lipoxygenase inhibiting activity was measured by slightly modifying the spectrometric method. Lipoxygenase type I-B and linoleic acid were purchased... | J Enzyme Inhib Med Chem 23: 140-3 (2008) Article DOI: 10.1080/14756360701342532 BindingDB Entry DOI: 10.7270/Q2JH3JRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase, 12R-type (Homo sapiens (Human)) | BDBM86079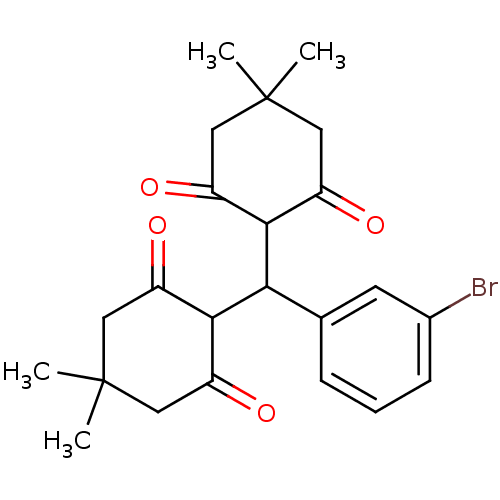 (Tetraketone, 14) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.29E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Pharmaceutical Research Centre | Assay Description In vitro liposygenase inhibition assay activity was measured by modifying the spectrophotometric method developed by Tappel. The compound was prepar... | J Enzyme Inhib Med Chem 23: 62-9 (2008) Article DOI: 10.1080/14756360701408754 BindingDB Entry DOI: 10.7270/Q2P849FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Linoleate 9S-lipoxygenase-4 (Glycine max (Soybean) (Glycine hispida)) | BDBM86106 (Isatan B, 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.38E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex | Assay Description LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. | J Enzyme Inhib Med Chem 23: 313-6 (2008) Article DOI: 10.1080/14756360701536455 BindingDB Entry DOI: 10.7270/Q2930RRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50531970 (DISUFENTON SODIUM) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
IQOG, CSIC Curated by ChEMBL | Assay Description Inhibition of soybean lipoxygenase using sodium linoleate as substrate by UV-based analysis | J Med Chem 62: 2184-2201 (2019) Article DOI: 10.1021/acs.jmedchem.8b01987 BindingDB Entry DOI: 10.7270/Q2MC93HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lipoxygenase (Glycine max) | BDBM50025855 (CHEMBL3330910) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Zagreb Curated by ChEMBL | Assay Description Inhibition of soybean LOX using sodium linoleate as substrate by spectrophotometry | Eur J Med Chem 86: 502-14 (2014) Article DOI: 10.1016/j.ejmech.2014.09.013 BindingDB Entry DOI: 10.7270/Q2Z89F0V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 87 total ) | Next | Last >> |