 Found 471 hits of ic50 data for polymerid = 5112
Found 471 hits of ic50 data for polymerid = 5112 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488086
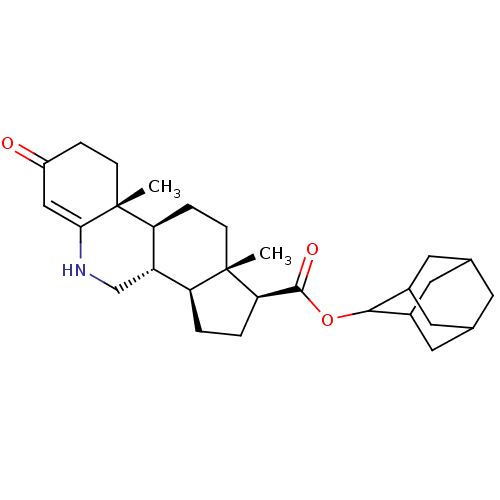
(CHEMBL2282783)Show SMILES C[C@]12CC[C@H]3[C@@H](CNC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)OC1C2CC3CC(C2)CC1C3 |r,wU:4.3,16.19,1.0,19.23,14.16,wD:5.5,t:8,TLB:32:31:29:25.26.27,22:23:29:25.26.27,THB:32:26:23.31.30:29,27:26:23:30.28.29,27:28:23:25.32.26,22:23:25.32.26:30.28.29,(20.54,-3.16,;20.55,-4.7,;19.22,-3.92,;17.87,-4.68,;17.87,-6.22,;19.2,-7.01,;19.2,-8.54,;17.87,-9.3,;16.54,-8.54,;15.21,-9.3,;13.88,-8.54,;12.55,-9.31,;13.88,-7,;15.21,-6.22,;16.54,-7,;16.53,-5.46,;20.54,-6.25,;22,-6.74,;22.93,-5.5,;22.03,-4.23,;22.52,-2.77,;21.5,-1.62,;24.03,-2.47,;24.5,-1,;26.25,-1.06,;26.76,-2.38,;27.94,-3.2,;29.1,-2.2,;28.61,-.77,;27.36,-.08,;27,-.81,;25.81,-1.86,;26.31,-3.28,)| Show InChI InChI=1S/C29H41NO3/c1-28-8-6-23-21(15-30-25-14-20(31)5-7-29(23,25)2)22(28)3-4-24(28)27(32)33-26-18-10-16-9-17(12-18)13-19(26)11-16/h14,16-19,21-24,26,30H,3-13,15H2,1-2H3/t16?,17?,18?,19?,21-,22-,23-,24+,26?,28-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043607
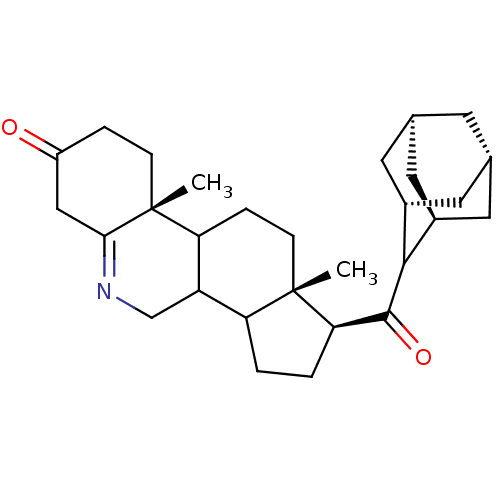
(1-(Adamantane-2-carbonyl)-9a,11a-dimethyl-1,2,3,3a...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)C1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2 |wU:19.23,27.31,1.0,14.16,wD:25.35,29.32,23.27,t:7,THB:28:27:24:22.29.30,26:27:22:25.24.30,26:25:22:27.28.31,(3.55,-4.37,;3.55,-5.91,;2.22,-5.13,;.87,-5.89,;.85,-7.43,;2.18,-8.22,;2.18,-9.75,;.85,-10.51,;-.48,-9.74,;-1.81,-10.51,;-3.13,-9.74,;-4.47,-10.51,;-3.13,-8.2,;-1.81,-7.43,;-.48,-8.2,;-.48,-6.66,;3.52,-7.47,;4.98,-7.96,;5.91,-6.73,;5.02,-5.47,;5.51,-4,;4.49,-2.85,;7.03,-3.69,;7.68,-2.32,;9.26,-2.41,;10.44,-3.52,;10.04,-2.15,;8.55,-2.06,;7.98,-3.37,;8.24,-4.78,;9.78,-4.88,;7.35,-.8,)| Show InChI InChI=1S/C29H41NO2/c1-28-8-6-23-21(15-30-25-14-20(31)5-7-29(23,25)2)22(28)3-4-24(28)27(32)26-18-10-16-9-17(12-18)13-19(26)11-16/h16-19,21-24,26H,3-15H2,1-2H3/t16-,17+,18-,19+,21?,22?,23?,24-,26?,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-alpha reductase-2 at a concentration of 5 microL after preincubation for 10 minutes |
J Med Chem 36: 4313-5 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F76 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043608
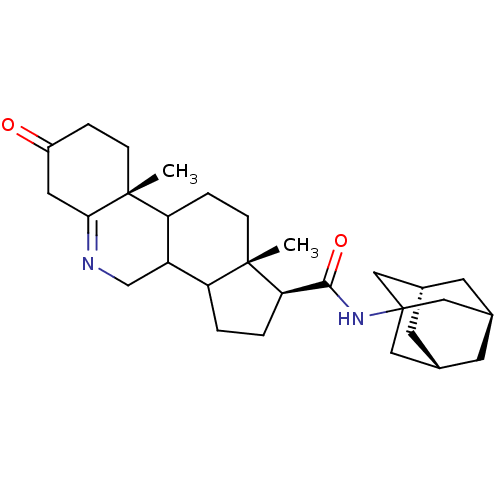
(9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,7,8,9,9a,9b,10...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC12C[C@H]3C[C@H](C[C@H](C3)C1)C2 |t:7,TLB:30:29:32:25.24.26,30:25:32:29.31.28| Show InChI InChI=1S/C29H42N2O2/c1-27-8-6-23-21(16-30-25-12-20(32)5-7-28(23,25)2)22(27)3-4-24(27)26(33)31-29-13-17-9-18(14-29)11-19(10-17)15-29/h17-19,21-24H,3-16H2,1-2H3,(H,31,33)/t17-,18+,19-,21?,22?,23?,24-,27+,28-,29?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-alpha reductase-2 at a concentration of 5 microL after preincubation for 10 minutes |
J Med Chem 36: 4313-5 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F76 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488064
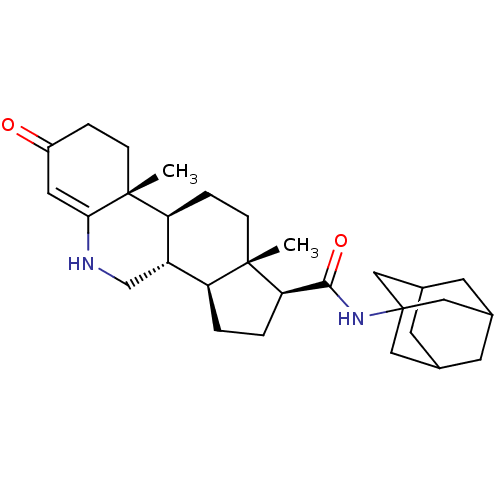
(CHEMBL2282782)Show SMILES C[C@]12CC[C@H]3[C@@H](CNC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)NC12CC3CC(CC(C3)C1)C2 |r,t:8,TLB:26:27:24.25.30:31,THB:26:25:31:32.27.28,28:27:24:30.29.31,28:29:24:32.26.27| Show InChI InChI=1S/C29H42N2O2/c1-27-8-6-23-21(16-30-25-12-20(32)5-7-28(23,25)2)22(27)3-4-24(27)26(33)31-29-13-17-9-18(14-29)11-19(10-17)15-29/h12,17-19,21-24,30H,3-11,13-16H2,1-2H3,(H,31,33)/t17?,18?,19?,21-,22-,23-,24+,27-,28+,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039257
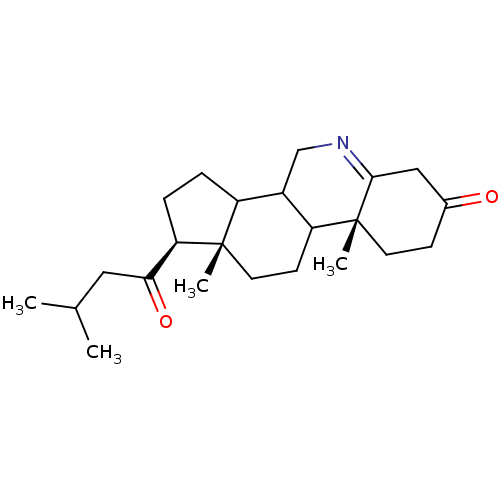
((1S,9aR,11aS)-9a,11a-Dimethyl-1-(3-methyl-butyryl)...)Show SMILES CC(C)CC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:12| Show InChI InChI=1S/C23H35NO2/c1-14(2)11-20(26)19-6-5-17-16-13-24-21-12-15(25)7-9-23(21,4)18(16)8-10-22(17,19)3/h14,16-19H,5-13H2,1-4H3/t16?,17?,18?,19-,22+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-alpha reductase-2 at a concentration of 5 microL after preincubation for 10 minutes |
J Med Chem 36: 4313-5 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F76 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488078

(CHEMBL2282766)Show SMILES CC(C)CC(=O)[C@H]1CC[C@H]2[C@@H]3CNC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r,t:13| Show InChI InChI=1S/C23H35NO2/c1-14(2)11-20(26)19-6-5-17-16-13-24-21-12-15(25)7-9-23(21,4)18(16)8-10-22(17,19)3/h12,14,16-19,24H,5-11,13H2,1-4H3/t16-,17-,18-,19+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488112

(CHEMBL2282775)Show SMILES C[C@]12CC[C@H]3[C@@H](CNC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)NC(c1ccccc1)(c1ccccc1)c1ccccc1 |r,t:8| Show InChI InChI=1S/C38H42N2O2/c1-36-23-21-32-30(25-39-34-24-29(41)20-22-37(32,34)2)31(36)18-19-33(36)35(42)40-38(26-12-6-3-7-13-26,27-14-8-4-9-15-27)28-16-10-5-11-17-28/h3-17,24,30-33,39H,18-23,25H2,1-2H3,(H,40,42)/t30-,31-,32-,33+,36-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488110
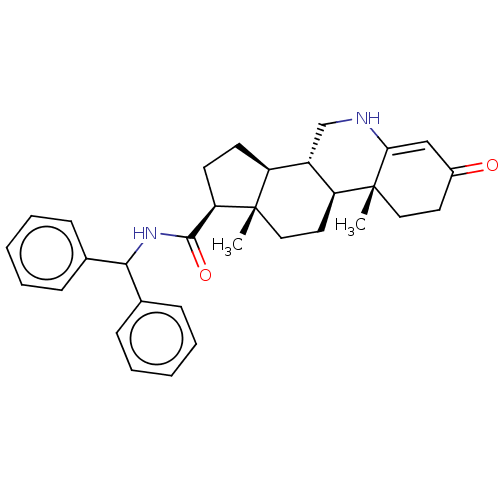
(CHEMBL2282776)Show SMILES C[C@]12CC[C@H]3[C@@H](CNC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)NC(c1ccccc1)c1ccccc1 |r,t:8| Show InChI InChI=1S/C32H38N2O2/c1-31-18-16-26-24(20-33-28-19-23(35)15-17-32(26,28)2)25(31)13-14-27(31)30(36)34-29(21-9-5-3-6-10-21)22-11-7-4-8-12-22/h3-12,19,24-27,29,33H,13-18,20H2,1-2H3,(H,34,36)/t24-,25-,26-,27+,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039268
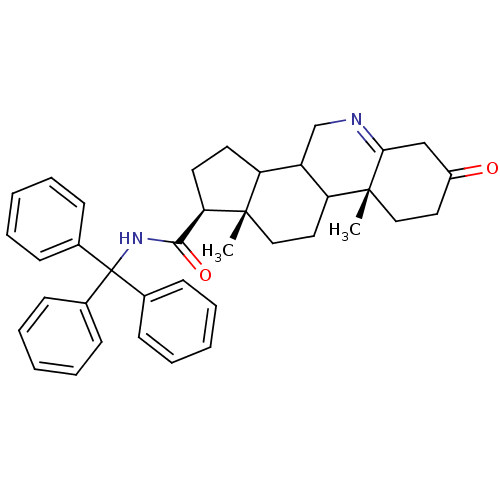
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC(c1ccccc1)(c1ccccc1)c1ccccc1 |t:7| Show InChI InChI=1S/C38H42N2O2/c1-36-23-21-32-30(25-39-34-24-29(41)20-22-37(32,34)2)31(36)18-19-33(36)35(42)40-38(26-12-6-3-7-13-26,27-14-8-4-9-15-27)28-16-10-5-11-17-28/h3-17,30-33H,18-25H2,1-2H3,(H,40,42)/t30?,31?,32?,33-,36+,37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-alpha reductase-2 at a concentration of 5 microL after preincubation for 10 minutes |
J Med Chem 36: 4313-5 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F76 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039298

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC(c1ccccc1)c1ccccc1 |t:7| Show InChI InChI=1S/C32H38N2O2/c1-31-18-16-26-24(20-33-28-19-23(35)15-17-32(26,28)2)25(31)13-14-27(31)30(36)34-29(21-9-5-3-6-10-21)22-11-7-4-8-12-22/h3-12,24-27,29H,13-20H2,1-2H3,(H,34,36)/t24?,25?,26?,27-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-alpha reductase-2 at a concentration of 5 microL after preincubation for 10 minutes |
J Med Chem 36: 4313-5 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F76 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032782

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc(c1)C(F)(F)F |c:12| Show InChI InChI=1S/C26H31F3N2O2/c1-24-12-10-19-17(6-9-21-25(19,2)13-11-22(32)31-21)18(24)7-8-20(24)23(33)30-16-5-3-4-15(14-16)26(27,28)29/h3-5,11,13-14,17-21H,6-10,12H2,1-2H3,(H,30,33)(H,31,32)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039315

((1S,9aR,11aS)-5,9a,11a-Trimethyl-1-(3-methyl-butyr...)Show SMILES CC(C)CC(=O)[C@H]1CCC2C3CN(C)C4=CC(=O)CC[C@]4(C)C3CC[C@]12C |t:14| Show InChI InChI=1S/C24H37NO2/c1-15(2)12-21(27)20-7-6-18-17-14-25(5)22-13-16(26)8-10-24(22,4)19(17)9-11-23(18,20)3/h13,15,17-20H,6-12,14H2,1-5H3/t17?,18?,19?,20-,23+,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 2 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50340481
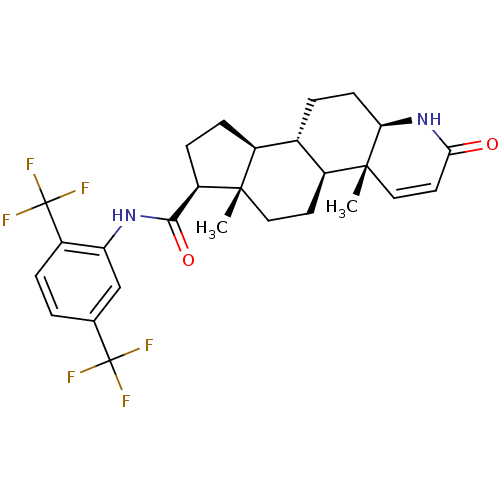
(Avodart | CHEMBL1200969 | DUTASTERIDE | GG-745 | G...)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@H]4NC(=O)C=C[C@]34C)[C@@H]1CC[C@@H]2C(=O)Nc1cc(ccc1C(F)(F)F)C(F)(F)F |r,c:12| Show InChI InChI=1S/C27H30F6N2O2/c1-24-11-9-17-15(4-8-21-25(17,2)12-10-22(36)35-21)16(24)6-7-19(24)23(37)34-20-13-14(26(28,29)30)3-5-18(20)27(31,32)33/h3,5,10,12-13,15-17,19,21H,4,6-9,11H2,1-2H3,(H,34,37)(H,35,36)/t15-,16-,17-,19+,21+,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human type 2 5alpha reductase |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50031883
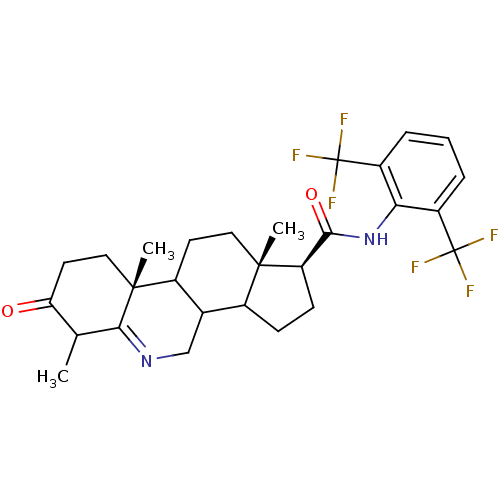
((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CC1C(=O)CC[C@]2(C)C3CC[C@@]4(C)C(CC[C@@H]4C(=O)Nc4c(cccc4C(F)(F)F)C(F)(F)F)C3CN=C12 |t:39| Show InChI InChI=1S/C28H32F6N2O2/c1-14-21(37)10-12-26(3)17-9-11-25(2)16(15(17)13-35-23(14)26)7-8-20(25)24(38)36-22-18(27(29,30)31)5-4-6-19(22)28(32,33)34/h4-6,14-17,20H,7-13H2,1-3H3,(H,36,38)/t14?,15?,16?,17?,20-,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity measured on human steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488097
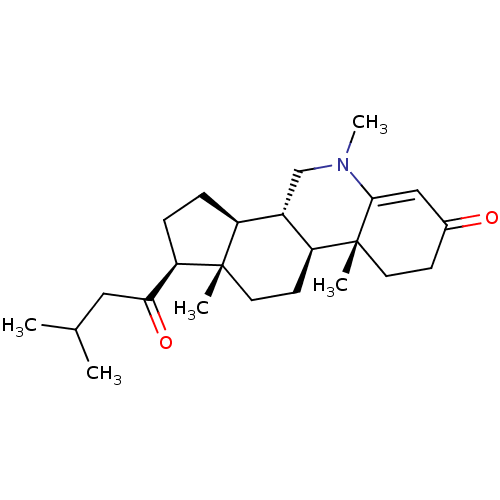
(CHEMBL2282649)Show SMILES CC(C)CC(=O)[C@H]1CC[C@H]2[C@@H]3CN(C)C4=CC(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r,t:14| Show InChI InChI=1S/C24H37NO2/c1-15(2)12-21(27)20-7-6-18-17-14-25(5)22-13-16(26)8-10-24(22,4)19(17)9-11-23(18,20)3/h13,15,17-20H,6-12,14H2,1-5H3/t17-,18-,19-,20+,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032789

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc(Cl)c1 |c:12| Show InChI InChI=1S/C25H31ClN2O2/c1-24-12-10-19-17(6-9-21-25(19,2)13-11-22(29)28-21)18(24)7-8-20(24)23(30)27-16-5-3-4-15(26)14-16/h3-5,11,13-14,17-21H,6-10,12H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032773

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES Cc1ccc(NC(=O)C2CCC3C4CCC5NC(=O)C=C[C@]5(C)C4CC[C@]23C)cc1 |c:19| Show InChI InChI=1S/C26H34N2O2/c1-16-4-6-17(7-5-16)27-24(30)21-10-9-19-18-8-11-22-26(3,15-13-23(29)28-22)20(18)12-14-25(19,21)2/h4-7,13,15,18-22H,8-12,14H2,1-3H3,(H,27,30)(H,28,29)/t18?,19?,20?,21?,22?,25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039259
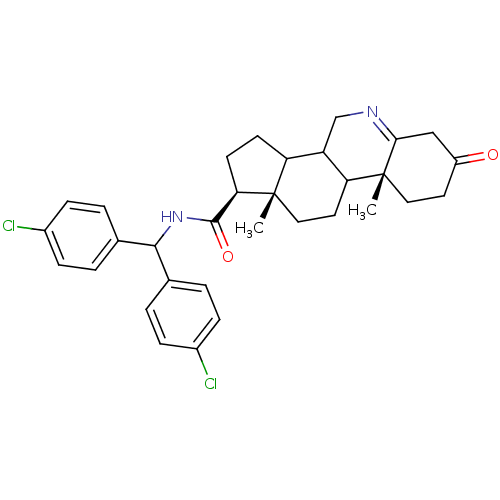
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C32H36Cl2N2O2/c1-31-16-14-26-24(18-35-28-17-23(37)13-15-32(26,28)2)25(31)11-12-27(31)30(38)36-29(19-3-7-21(33)8-4-19)20-5-9-22(34)10-6-20/h3-10,24-27,29H,11-18H2,1-2H3,(H,36,38)/t24?,25?,26?,27-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-alpha reductase-2 at a concentration of 5 microL after preincubation for 10 minutes |
J Med Chem 36: 4313-5 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F76 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488082

(CHEMBL2282773)Show SMILES C[C@]12CC[C@H]3[C@@H](CNC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)NC(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |r,t:8| Show InChI InChI=1S/C32H36Cl2N2O2/c1-31-16-14-26-24(18-35-28-17-23(37)13-15-32(26,28)2)25(31)11-12-27(31)30(38)36-29(19-3-7-21(33)8-4-19)20-5-9-22(34)10-6-20/h3-10,17,24-27,29,35H,11-16,18H2,1-2H3,(H,36,38)/t24-,25-,26-,27+,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039259

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C32H36Cl2N2O2/c1-31-16-14-26-24(18-35-28-17-23(37)13-15-32(26,28)2)25(31)11-12-27(31)30(38)36-29(19-3-7-21(33)8-4-19)20-5-9-22(34)10-6-20/h3-10,24-27,29H,11-18H2,1-2H3,(H,36,38)/t24?,25?,26?,27-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 2 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039259

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC(c1ccc(Cl)cc1)c1ccc(Cl)cc1 |t:7| Show InChI InChI=1S/C32H36Cl2N2O2/c1-31-16-14-26-24(18-35-28-17-23(37)13-15-32(26,28)2)25(31)11-12-27(31)30(38)36-29(19-3-7-21(33)8-4-19)20-5-9-22(34)10-6-20/h3-10,24-27,29H,11-18H2,1-2H3,(H,36,38)/t24?,25?,26?,27-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-alpha reductase 2 isozyme |
J Med Chem 40: 1293-315 (1997)
Article DOI: 10.1021/jm960697s
BindingDB Entry DOI: 10.7270/Q2W096MN |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488068

(CHEMBL2282777)Show SMILES C[C@]12CC[C@H]3[C@@H](CNC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)N(Cc1ccccc1)Cc1ccccc1 |r,t:8| Show InChI InChI=1S/C33H40N2O2/c1-32-18-16-28-26(20-34-30-19-25(36)15-17-33(28,30)2)27(32)13-14-29(32)31(37)35(21-23-9-5-3-6-10-23)22-24-11-7-4-8-12-24/h3-12,19,26-29,34H,13-18,20-22H2,1-2H3/t26-,27-,28-,29+,32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043605
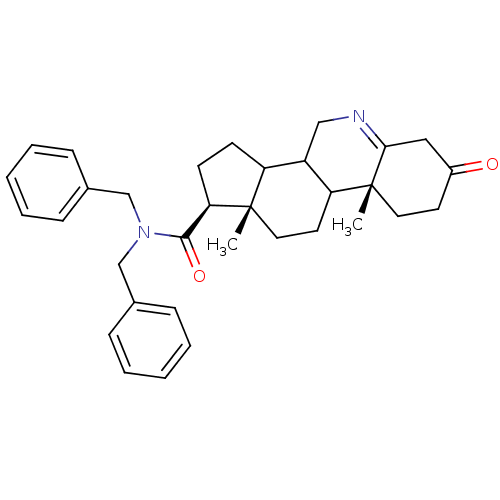
(9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,7,8,9,9a,9b,10...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)N(Cc1ccccc1)Cc1ccccc1 |t:7| Show InChI InChI=1S/C33H40N2O2/c1-32-18-16-28-26(20-34-30-19-25(36)15-17-33(28,30)2)27(32)13-14-29(32)31(37)35(21-23-9-5-3-6-10-23)22-24-11-7-4-8-12-24/h3-12,26-29H,13-22H2,1-2H3/t26?,27?,28?,29-,32+,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-alpha reductase-2 at a concentration of 5 microL after preincubation for 10 minutes |
J Med Chem 36: 4313-5 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F76 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039294
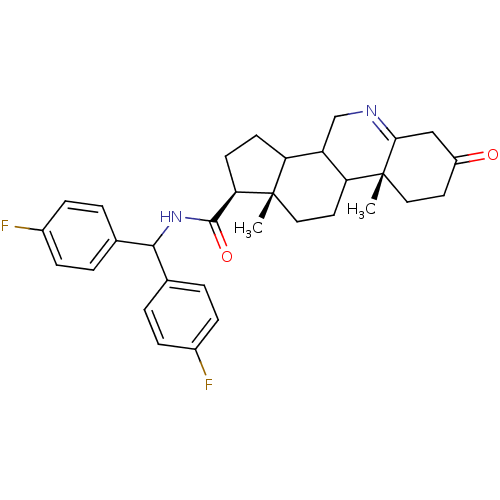
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC(c1ccc(F)cc1)c1ccc(F)cc1 |t:7| Show InChI InChI=1S/C32H36F2N2O2/c1-31-16-14-26-24(18-35-28-17-23(37)13-15-32(26,28)2)25(31)11-12-27(31)30(38)36-29(19-3-7-21(33)8-4-19)20-5-9-22(34)10-6-20/h3-10,24-27,29H,11-18H2,1-2H3,(H,36,38)/t24?,25?,26?,27-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 2 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488063

(CHEMBL2282774)Show SMILES C[C@]12CC[C@H]3[C@@H](CNC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)NC(c1ccc(F)cc1)c1ccc(F)cc1 |r,t:8| Show InChI InChI=1S/C32H36F2N2O2/c1-31-16-14-26-24(18-35-28-17-23(37)13-15-32(26,28)2)25(31)11-12-27(31)30(38)36-29(19-3-7-21(33)8-4-19)20-5-9-22(34)10-6-20/h3-10,17,24-27,29,35H,11-16,18H2,1-2H3,(H,36,38)/t24-,25-,26-,27+,31-,32+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039294

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NC(c1ccc(F)cc1)c1ccc(F)cc1 |t:7| Show InChI InChI=1S/C32H36F2N2O2/c1-31-16-14-26-24(18-35-28-17-23(37)13-15-32(26,28)2)25(31)11-12-27(31)30(38)36-29(19-3-7-21(33)8-4-19)20-5-9-22(34)10-6-20/h3-10,24-27,29H,11-18H2,1-2H3,(H,36,38)/t24?,25?,26?,27-,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-alpha reductase-2 at a concentration of 5 microL after preincubation for 10 minutes |
J Med Chem 36: 4313-5 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F76 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604
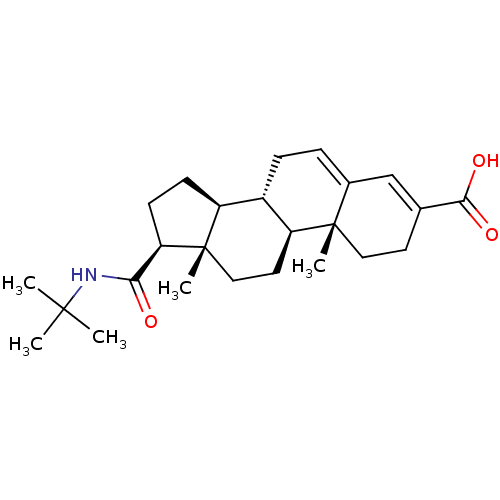
((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 2 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50025356
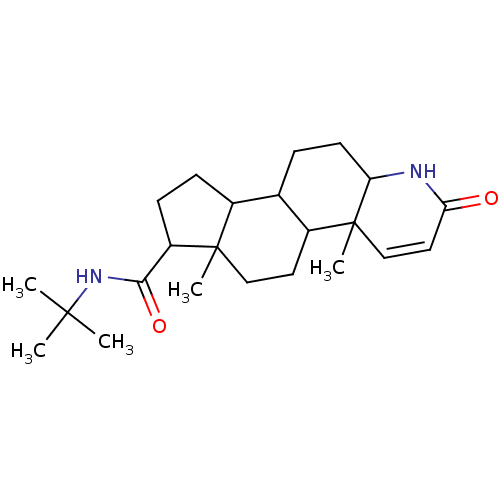
(4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10...)Show SMILES CC(C)(C)NC(=O)C1CCC2C3CCC4NC(=O)C=CC4(C)C3CCC12C |c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human 5-alpha reductase-2 at a concentration of 5 microL after preincubation for 10 minutes |
J Med Chem 36: 4313-5 (1994)
BindingDB Entry DOI: 10.7270/Q2PK0F76 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50031876
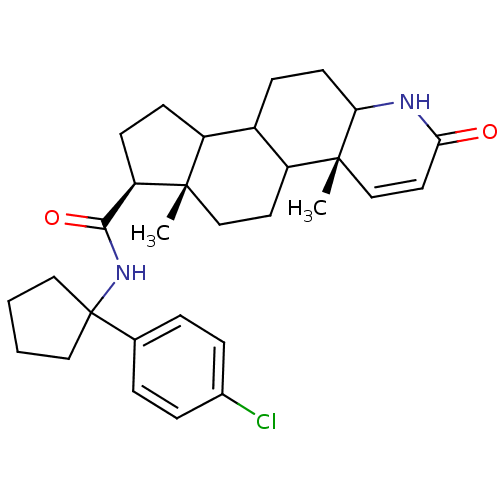
((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CC[C@@H]2C(=O)NC1(CCCC1)c1ccc(Cl)cc1 |c:12| Show InChI InChI=1S/C30H39ClN2O2/c1-28-17-13-23-21(9-12-25-29(23,2)18-14-26(34)32-25)22(28)10-11-24(28)27(35)33-30(15-3-4-16-30)19-5-7-20(31)8-6-19/h5-8,14,18,21-25H,3-4,9-13,15-17H2,1-2H3,(H,32,34)(H,33,35)/t21?,22?,23?,24-,25?,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity measured on human steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human type 2 5alpha reductase |
J Med Chem 61: 5822-5880 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01788
BindingDB Entry DOI: 10.7270/Q232008T |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 2 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50043604

((8S,9S,10R,13S,14S,17S)-17-(tert-butylcarbamoyl)-1...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC=C4C=C(CC[C@]4(C)[C@H]3CC[C@]12C)C(O)=O |c:15,t:13| Show InChI InChI=1S/C25H37NO3/c1-23(2,3)26-21(27)20-9-8-18-17-7-6-16-14-15(22(28)29)10-12-24(16,4)19(17)11-13-25(18,20)5/h6,14,17-20H,7-13H2,1-5H3,(H,26,27)(H,28,29)/t17-,18-,19-,20+,24-,25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity measured on human steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity for human 5-alpha reductase 2 isozyme |
J Med Chem 40: 1293-315 (1997)
Article DOI: 10.1021/jm960697s
BindingDB Entry DOI: 10.7270/Q2W096MN |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50334788

((17beta-(N-tert-butylcarbamoyl)-4-aza-5alpha-andro...)Show SMILES CC(C)(C)NC(=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4NC(=O)C=C[C@]4(C)[C@H]3CC[C@]12C |r,c:18| Show InChI InChI=1S/C23H36N2O2/c1-21(2,3)25-20(27)17-8-7-15-14-6-9-18-23(5,13-11-19(26)24-18)16(14)10-12-22(15,17)4/h11,13-18H,6-10,12H2,1-5H3,(H,24,26)(H,25,27)/t14-,15-,16-,17+,18+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity measured on human steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488076

(CHEMBL2282763)Show SMILES C[C@]12CC[C@H]3[C@@H](CNC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F |r,t:8| Show InChI InChI=1S/C27H30F6N2O2/c1-24-8-6-20-18(13-34-22-12-17(36)5-7-25(20,22)2)19(24)3-4-21(24)23(37)35-16-10-14(26(28,29)30)9-15(11-16)27(31,32)33/h9-12,18-21,34H,3-8,13H2,1-2H3,(H,35,37)/t18-,19-,20-,21+,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488096

(CHEMBL2282644)Show SMILES CN1C[C@H]2[C@@H]3CC[C@H](C(=O)NC(c4ccccc4)c4ccccc4)[C@@]3(C)CC[C@@H]2[C@@]2(C)CCC(=O)C=C12 |r,t:39| Show InChI InChI=1S/C33H40N2O2/c1-32-19-17-27-25(21-35(3)29-20-24(36)16-18-33(27,29)2)26(32)14-15-28(32)31(37)34-30(22-10-6-4-7-11-22)23-12-8-5-9-13-23/h4-13,20,25-28,30H,14-19,21H2,1-3H3,(H,34,37)/t25-,26-,27-,28+,32-,33+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50488103
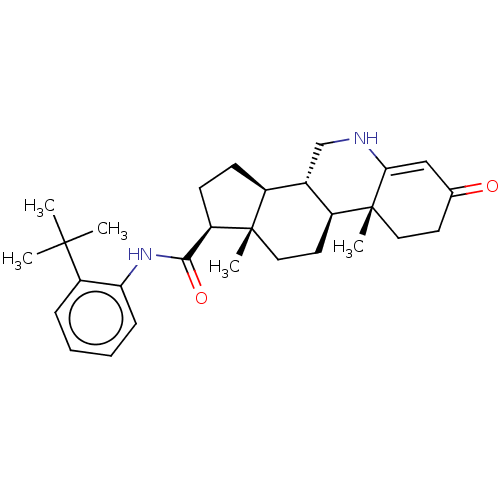
(CHEMBL2282765)Show SMILES CC(C)(C)c1ccccc1NC(=O)[C@H]1CC[C@H]2[C@@H]3CNC4=CC(=O)CC[C@]4(C)[C@H]3CC[C@]12C |r,t:21| Show InChI InChI=1S/C29H40N2O2/c1-27(2,3)22-8-6-7-9-24(22)31-26(33)23-11-10-20-19-17-30-25-16-18(32)12-14-29(25,5)21(19)13-15-28(20,23)4/h6-9,16,19-21,23,30H,10-15,17H2,1-5H3,(H,31,33)/t19-,20-,21-,23+,28-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Homo sapiens (human) Steroid 5-alpha-reductase type 2 |
Citation and Details
Article DOI: 10.1007/s00044-012-0006-1
BindingDB Entry DOI: 10.7270/Q29889WK |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057475
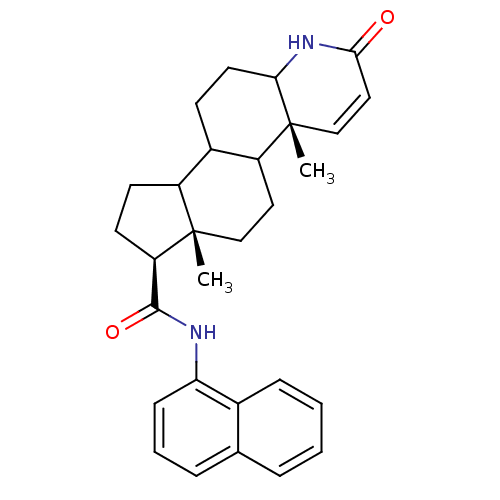
((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CC[C@@H]2C(=O)Nc1cccc2ccccc12 |c:12| Show InChI InChI=1S/C29H34N2O2/c1-28-16-14-22-20(10-13-25-29(22,2)17-15-26(32)31-25)21(28)11-12-23(28)27(33)30-24-9-5-7-18-6-3-4-8-19(18)24/h3-9,15,17,20-23,25H,10-14,16H2,1-2H3,(H,30,33)(H,31,32)/t20?,21?,22?,23-,25?,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5-alpha reductase 2 isozyme. |
J Med Chem 40: 1293-315 (1997)
Article DOI: 10.1021/jm960697s
BindingDB Entry DOI: 10.7270/Q2W096MN |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50057476
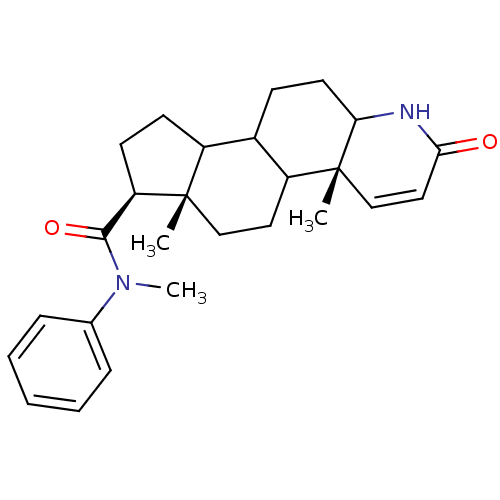
((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...)Show SMILES CN(C(=O)[C@H]1CCC2C3CCC4NC(=O)C=C[C@]4(C)C3CC[C@]12C)c1ccccc1 |c:15| Show InChI InChI=1S/C26H34N2O2/c1-25-15-13-20-18(9-12-22-26(20,2)16-14-23(29)27-22)19(25)10-11-21(25)24(30)28(3)17-7-5-4-6-8-17/h4-8,14,16,18-22H,9-13,15H2,1-3H3,(H,27,29)/t18?,19?,20?,21-,22?,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human 5-alpha reductase 2 isozyme. |
J Med Chem 40: 1293-315 (1997)
Article DOI: 10.1021/jm960697s
BindingDB Entry DOI: 10.7270/Q2W096MN |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50031891
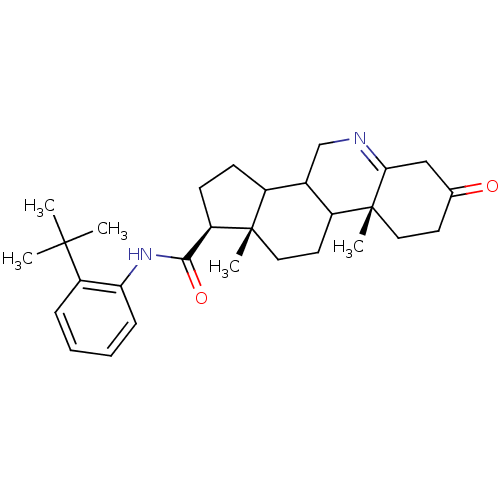
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES CC(C)(C)c1ccccc1NC(=O)[C@H]1CCC2C3CN=C4CC(=O)CC[C@]4(C)C3CC[C@]12C |t:20| Show InChI InChI=1S/C29H40N2O2/c1-27(2,3)22-8-6-7-9-24(22)31-26(33)23-11-10-20-19-17-30-25-16-18(32)12-14-29(25,5)21(19)13-15-28(20,23)4/h6-9,19-21,23H,10-17H2,1-5H3,(H,31,33)/t19?,20?,21?,23-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity measured on human steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50031901

((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)Nc1cc(cc(c1)C(F)(F)F)C(F)(F)F |t:7| Show InChI InChI=1S/C27H30F6N2O2/c1-24-8-6-20-18(13-34-22-12-17(36)5-7-25(20,22)2)19(24)3-4-21(24)23(37)35-16-10-14(26(28,29)30)9-15(11-16)27(31,32)33/h9-11,18-21H,3-8,12-13H2,1-2H3,(H,35,37)/t18?,19?,20?,21-,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity measured on human steroid 5-alpha-reductase type 2 |
J Med Chem 38: 2621-7 (1995)
BindingDB Entry DOI: 10.7270/Q2C829XC |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032763
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc(Br)c1 |c:12| Show InChI InChI=1S/C25H31BrN2O2/c1-24-12-10-19-17(6-9-21-25(19,2)13-11-22(29)28-21)18(24)7-8-20(24)23(30)27-16-5-3-4-15(26)14-16/h3-5,11,13-14,17-21H,6-10,12H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032774
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc(F)c1 |c:12| Show InChI InChI=1S/C25H31FN2O2/c1-24-12-10-19-17(6-9-21-25(19,2)13-11-22(29)28-21)18(24)7-8-20(24)23(30)27-16-5-3-4-15(26)14-16/h3-5,11,13-14,17-21H,6-10,12H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032785

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc2ccccc12 |c:12| Show InChI InChI=1S/C29H34N2O2/c1-28-16-14-22-20(10-13-25-29(22,2)17-15-26(32)31-25)21(28)11-12-23(28)27(33)30-24-9-5-7-18-6-3-4-8-19(18)24/h3-9,15,17,20-23,25H,10-14,16H2,1-2H3,(H,30,33)(H,31,32)/t20?,21?,22?,23?,25?,28-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032786
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1ccc2ccccc2c1 |c:12| Show InChI InChI=1S/C29H34N2O2/c1-28-15-13-23-21(9-12-25-29(23,2)16-14-26(32)31-25)22(28)10-11-24(28)27(33)30-20-8-7-18-5-3-4-6-19(18)17-20/h3-8,14,16-17,21-25H,9-13,15H2,1-2H3,(H,30,33)(H,31,32)/t21?,22?,23?,24?,25?,28-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032778
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1ccccc1 |c:12| Show InChI InChI=1S/C25H32N2O2/c1-24-14-12-19-17(8-11-21-25(19,2)15-13-22(28)27-21)18(24)9-10-20(24)23(29)26-16-6-4-3-5-7-16/h3-7,13,15,17-21H,8-12,14H2,1-2H3,(H,26,29)(H,27,28)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032787
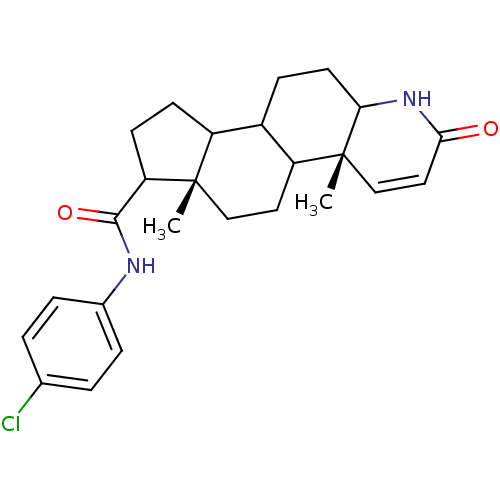
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1ccc(Cl)cc1 |c:12| Show InChI InChI=1S/C25H31ClN2O2/c1-24-13-11-19-17(7-10-21-25(19,2)14-12-22(29)28-21)18(24)8-9-20(24)23(30)27-16-5-3-15(26)4-6-16/h3-6,12,14,17-21H,7-11,13H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032781

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1ccc(F)cc1 |c:12| Show InChI InChI=1S/C25H31FN2O2/c1-24-13-11-19-17(7-10-21-25(19,2)14-12-22(29)28-21)18(24)8-9-20(24)23(30)27-16-5-3-15(26)4-6-16/h3-6,12,14,17-21H,7-11,13H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039288
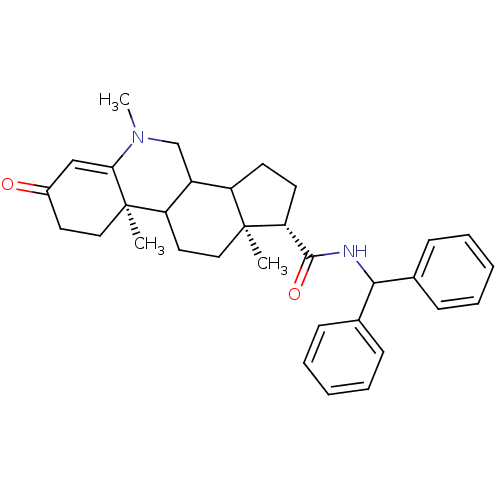
((1S,9aR,11aS)-5,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...)Show SMILES CN1CC2C3CC[C@H](C(=O)NC(c4ccccc4)c4ccccc4)[C@@]3(C)CCC2[C@@]2(C)CCC(=O)C=C12 |t:39| Show InChI InChI=1S/C33H40N2O2/c1-32-19-17-27-25(21-35(3)29-20-24(36)16-18-33(27,29)2)26(32)14-15-28(32)31(37)34-30(22-10-6-4-7-11-22)23-12-8-5-9-13-23/h4-13,20,25-28,30H,14-19,21H2,1-3H3,(H,34,37)/t25?,26?,27?,28-,32+,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 2 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50039295
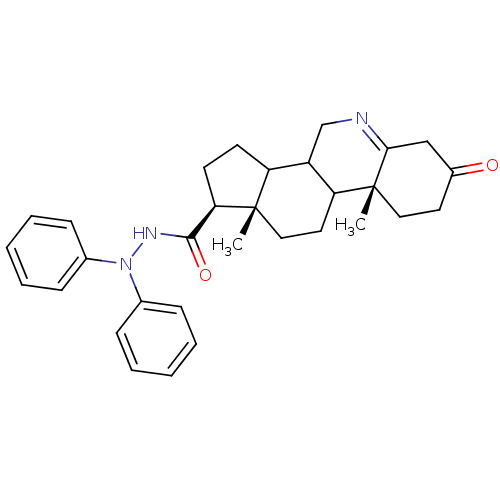
((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...)Show SMILES C[C@]12CCC3C(CN=C4CC(=O)CC[C@]34C)C1CC[C@@H]2C(=O)NN(c1ccccc1)c1ccccc1 |t:7| Show InChI InChI=1S/C31H37N3O2/c1-30-18-16-26-24(20-32-28-19-23(35)15-17-31(26,28)2)25(30)13-14-27(30)29(36)33-34(21-9-5-3-6-10-21)22-11-7-4-8-12-22/h3-12,24-27H,13-20H2,1-2H3,(H,33,36)/t24?,25?,26?,27-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human type 2 5-alpha reductase |
J Med Chem 37: 2352-60 (1994)
BindingDB Entry DOI: 10.7270/Q228088W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data