 Found 63 hits of ic50 data for polymerid = 5124
Found 63 hits of ic50 data for polymerid = 5124 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243463
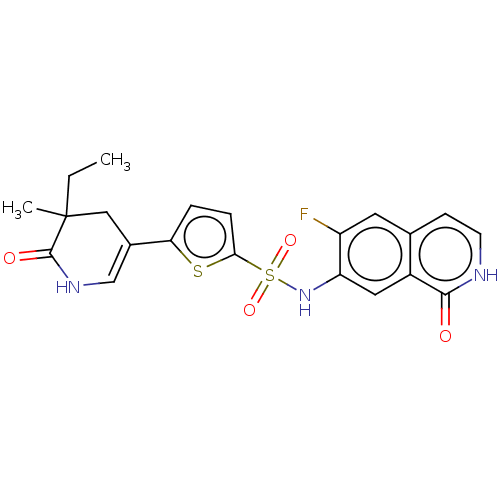
(CHEMBL4100363)Show SMILES CCC1(C)CC(=CNC1=O)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |c:5| Show InChI InChI=1S/C21H20FN3O4S2/c1-3-21(2)10-13(11-24-20(21)27)17-4-5-18(30-17)31(28,29)25-16-9-14-12(8-15(16)22)6-7-23-19(14)26/h4-9,11,25H,3,10H2,1-2H3,(H,23,26)(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243461
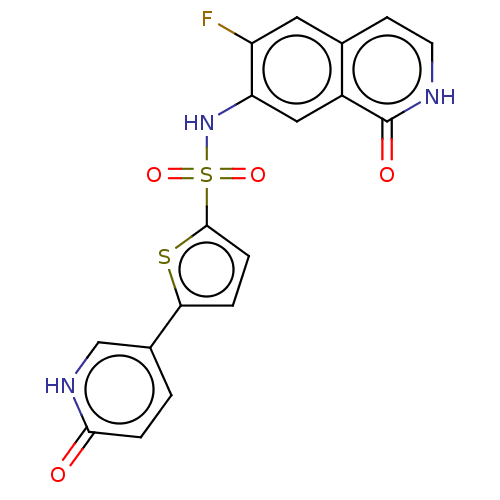
(CHEMBL4075503)Show SMILES Fc1cc2cc[nH]c(=O)c2cc1NS(=O)(=O)c1ccc(s1)-c1ccc(=O)[nH]c1 Show InChI InChI=1S/C18H12FN3O4S2/c19-13-7-10-5-6-20-18(24)12(10)8-14(13)22-28(25,26)17-4-2-15(27-17)11-1-3-16(23)21-9-11/h1-9,22H,(H,20,24)(H,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243462
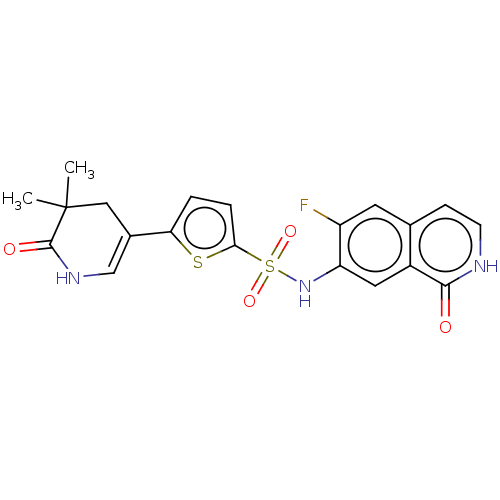
(CHEMBL4083899)Show SMILES CC1(C)CC(=CNC1=O)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |c:4| Show InChI InChI=1S/C20H18FN3O4S2/c1-20(2)9-12(10-23-19(20)26)16-3-4-17(29-16)30(27,28)24-15-8-13-11(7-14(15)21)5-6-22-18(13)25/h3-8,10,24H,9H2,1-2H3,(H,22,25)(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243486
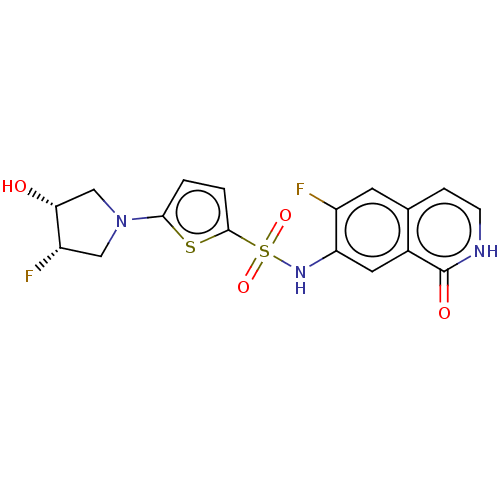
(CHEMBL4081385)Show SMILES O[C@@H]1CN(C[C@@H]1F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H15F2N3O4S2/c18-11-5-9-3-4-20-17(24)10(9)6-13(11)21-28(25,26)16-2-1-15(27-16)22-7-12(19)14(23)8-22/h1-6,12,14,21,23H,7-8H2,(H,20,24)/t12-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243487

(CHEMBL4091668)Show SMILES O[C@@H]1CN(CC1(F)F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H14F3N3O4S2/c18-11-5-9-3-4-21-16(25)10(9)6-12(11)22-29(26,27)15-2-1-14(28-15)23-7-13(24)17(19,20)8-23/h1-6,13,22,24H,7-8H2,(H,21,25)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243441
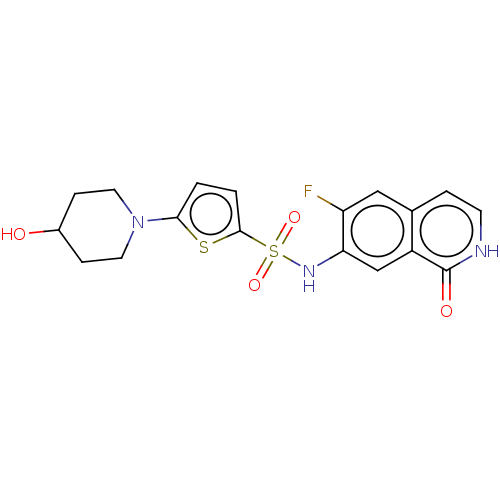
(CHEMBL4076500)Show SMILES OC1CCN(CC1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F Show InChI InChI=1S/C18H18FN3O4S2/c19-14-9-11-3-6-20-18(24)13(11)10-15(14)21-28(25,26)17-2-1-16(27-17)22-7-4-12(23)5-8-22/h1-3,6,9-10,12,21,23H,4-5,7-8H2,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243443
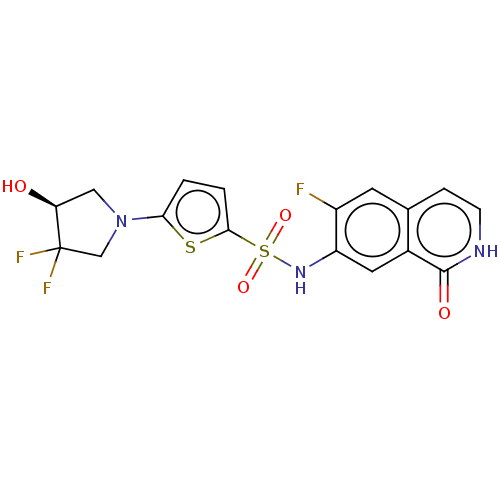
(CHEMBL4070790)Show SMILES O[C@H]1CN(CC1(F)F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H14F3N3O4S2/c18-11-5-9-3-4-21-16(25)10(9)6-12(11)22-29(26,27)15-2-1-14(28-15)23-7-13(24)17(19,20)8-23/h1-6,13,22,24H,7-8H2,(H,21,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243485
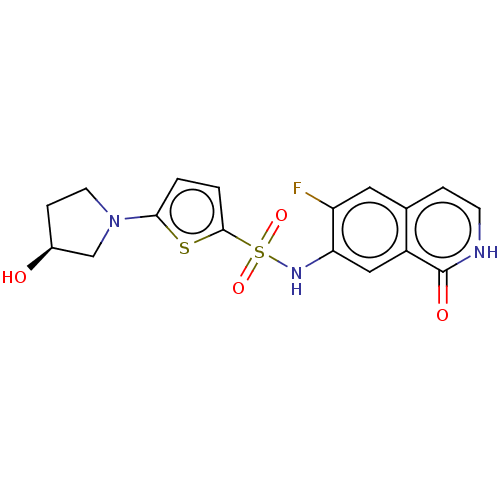
(CHEMBL4074469)Show SMILES O[C@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243415
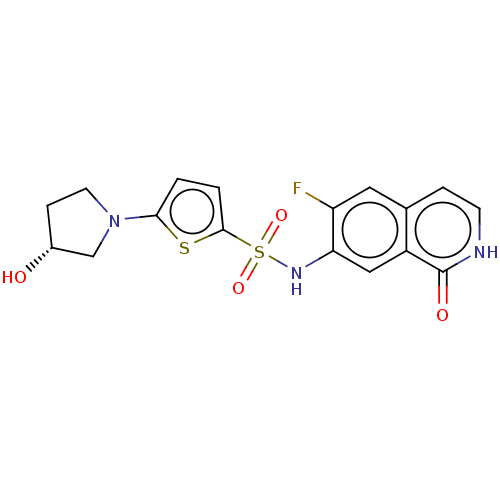
(CHEMBL4063104)Show SMILES O[C@@H]1CCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H16FN3O4S2/c18-13-7-10-3-5-19-17(23)12(10)8-14(13)20-27(24,25)16-2-1-15(26-16)21-6-4-11(22)9-21/h1-3,5,7-8,11,20,22H,4,6,9H2,(H,19,23)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243434
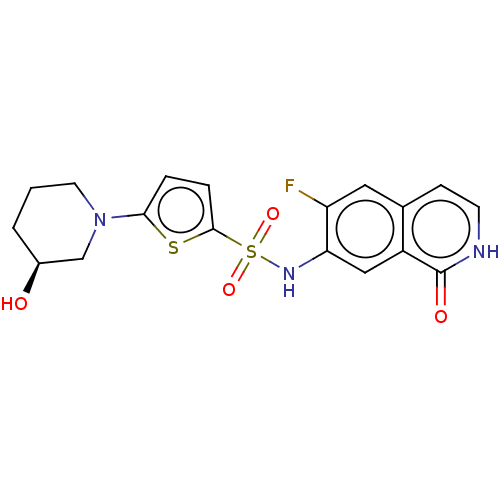
(CHEMBL4079085)Show SMILES O[C@H]1CCCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C18H18FN3O4S2/c19-14-8-11-5-6-20-18(24)13(11)9-15(14)21-28(25,26)17-4-3-16(27-17)22-7-1-2-12(23)10-22/h3-6,8-9,12,21,23H,1-2,7,10H2,(H,20,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243442
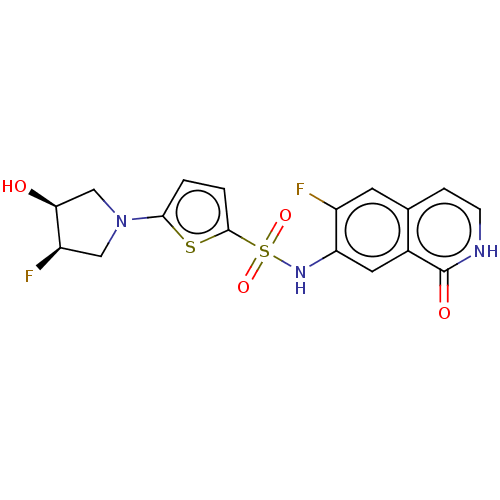
(CHEMBL4099409)Show SMILES O[C@H]1CN(C[C@H]1F)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C17H15F2N3O4S2/c18-11-5-9-3-4-20-17(24)10(9)6-13(11)21-28(25,26)16-2-1-15(27-16)22-7-12(19)14(23)8-22/h1-6,12,14,21,23H,7-8H2,(H,20,24)/t12-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243433
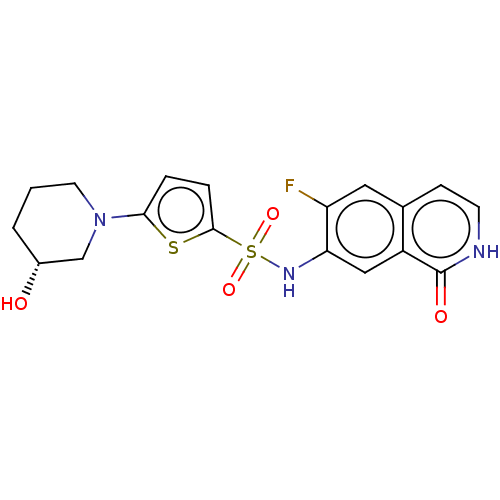
(CHEMBL4101204)Show SMILES O[C@@H]1CCCN(C1)c1ccc(s1)S(=O)(=O)Nc1cc2c(cc[nH]c2=O)cc1F |r| Show InChI InChI=1S/C18H18FN3O4S2/c19-14-8-11-5-6-20-18(24)13(11)9-15(14)21-28(25,26)17-4-3-16(27-17)22-7-1-2-12(23)10-22/h3-6,8-9,12,21,23H,1-2,7,10H2,(H,20,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50369444
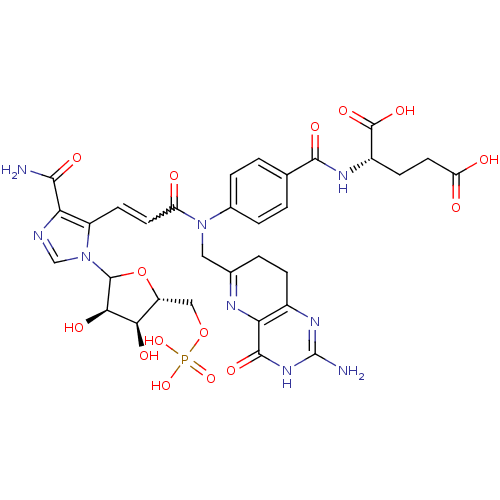
(CHEMBL608337)Show SMILES NC(=O)c1ncn(C2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c1C=CC(=O)N(CC1=Nc2c(CC1)nc(N)[nH]c2=O)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r,w:22.24,t:29| Show InChI InChI=1S/C32H36N9O15P/c33-27(47)24-19(41(13-35-24)30-26(46)25(45)20(56-30)12-55-57(52,53)54)8-9-21(42)40(11-15-3-6-17-23(36-15)29(49)39-32(34)38-17)16-4-1-14(2-5-16)28(48)37-18(31(50)51)7-10-22(43)44/h1-2,4-5,8-9,13,18,20,25-26,30,45-46H,3,6-7,10-12H2,(H2,33,47)(H,37,48)(H,43,44)(H,50,51)(H2,52,53,54)(H3,34,38,39,49)/t18-,20+,25+,26+,30?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against AICAR formyltransferase |
J Med Chem 42: 3421-4 (1999)
Article DOI: 10.1021/jm990323+
BindingDB Entry DOI: 10.7270/Q23X879T |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158378
(5-(5-sulfamoyl-naphthalen-2-ylazo)-naphthalene-1-s...)Show SMILES NS(=O)(=O)c1cccc2cc(ccc12)\N=N\c1cccc2c(cccc12)S(O)(=O)=O Show InChI InChI=1S/C20H15N3O5S2/c21-29(24,25)19-8-1-4-13-12-14(10-11-15(13)19)22-23-18-7-2-6-17-16(18)5-3-9-20(17)30(26,27)28/h1-12H,(H2,21,24,25)(H,26,27,28)/b23-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243477
(CHEMBL4092503)Show SMILES O=c1[nH]ccc2ccc(NS(=O)(=O)c3ccc(cc3)C#N)cc12 Show InChI InChI=1S/C16H11N3O3S/c17-10-11-1-5-14(6-2-11)23(21,22)19-13-4-3-12-7-8-18-16(20)15(12)9-13/h1-9,19H,(H,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 608 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50005520
((S)-2-{4-[3-(2,4-Diamino-6-oxo-1,6-dihydro-pyrimid...)Show SMILES Nc1nc(N)c(CCCNc2ccc(cc2)C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(=O)[nH]1 Show InChI InChI=1S/C19H24N6O6/c20-15-12(17(29)25-19(21)24-15)2-1-9-22-11-5-3-10(4-6-11)16(28)23-13(18(30)31)7-8-14(26)27/h3-6,13,22H,1-2,7-9H2,(H,23,28)(H,26,27)(H,30,31)(H5,20,21,24,25,29)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wellcome Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition activity against Glycinamide ribonucleotide transformylase(GAR-TFase) against hog liver |
J Med Chem 33: 561-7 (1990)
BindingDB Entry DOI: 10.7270/Q2XW4HS0 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243414
(CHEMBL4084757)Show SMILES Fc1cc2cc[nH]c(=O)c2cc1NS(=O)(=O)c1ccc(cc1)C#N Show InChI InChI=1S/C16H10FN3O3S/c17-14-7-11-5-6-19-16(21)13(11)8-15(14)20-24(22,23)12-3-1-10(9-18)2-4-12/h1-8,20H,(H,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243396
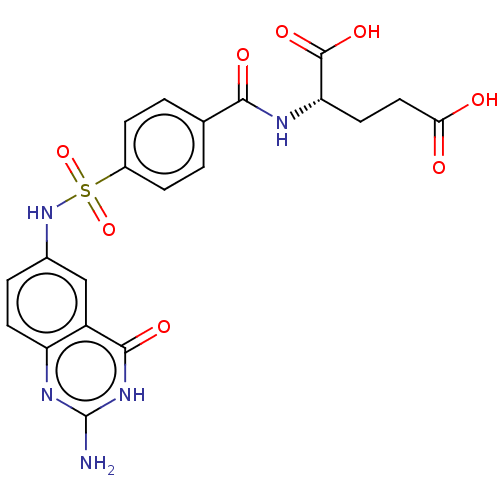
(CHEMBL1231520)Show SMILES Nc1nc2ccc(NS(=O)(=O)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H19N5O8S/c21-20-23-14-6-3-11(9-13(14)18(29)24-20)25-34(32,33)12-4-1-10(2-5-12)17(28)22-15(19(30)31)7-8-16(26)27/h1-6,9,15,25H,7-8H2,(H,22,28)(H,26,27)(H,30,31)(H3,21,23,24,29)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM22584
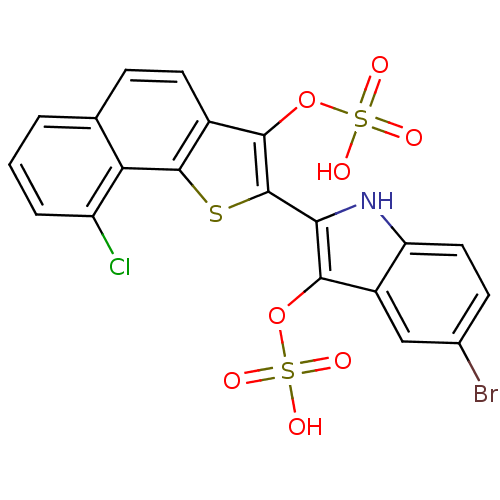
(5-bromo-2-[9-chloro-3-(sulfooxy)naphtho[1,2-b]thio...)Show SMILES OS(=O)(=O)Oc1c([nH]c2ccc(Br)cc12)-c1sc2c(ccc3cccc(Cl)c23)c1OS(O)(=O)=O Show InChI InChI=1S/C20H11BrClNO8S3/c21-10-5-7-14-12(8-10)17(30-33(24,25)26)16(23-14)20-18(31-34(27,28)29)11-6-4-9-2-1-3-13(22)15(9)19(11)32-20/h1-8,23H,(H,24,25,26)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute
| Assay Description
The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The... |
J Biol Chem 279: 50555-65 (2004)
Article DOI: 10.1074/jbc.M406801200
BindingDB Entry DOI: 10.7270/Q2MC8X97 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM22580
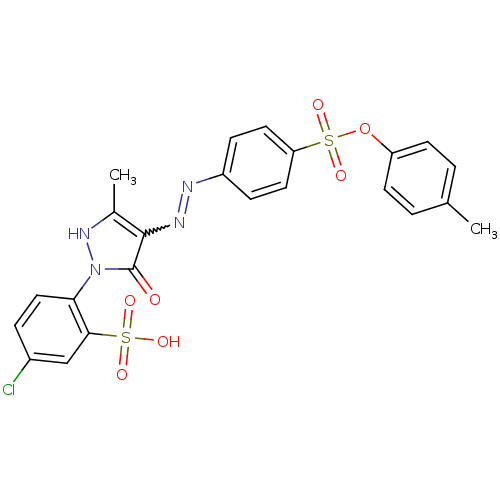
(47729-M | 5-chloro-2-{5-hydroxy-3-methyl-4-[(E)-2-...)Show SMILES Cc1[nH]n(-c2ccc(Cl)cc2S(O)(=O)=O)c(=O)c1N=Nc1ccc(cc1)S(=O)(=O)Oc1ccc(C)cc1 |w:18.19| Show InChI InChI=1S/C23H19ClN4O7S2/c1-14-3-8-18(9-4-14)35-37(33,34)19-10-6-17(7-11-19)25-26-22-15(2)27-28(23(22)29)20-12-5-16(24)13-21(20)36(30,31)32/h3-13,27H,1-2H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute
| Assay Description
The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The... |
J Biol Chem 279: 50555-65 (2004)
Article DOI: 10.1074/jbc.M406801200
BindingDB Entry DOI: 10.7270/Q2MC8X97 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM22580

(47729-M | 5-chloro-2-{5-hydroxy-3-methyl-4-[(E)-2-...)Show SMILES Cc1[nH]n(-c2ccc(Cl)cc2S(O)(=O)=O)c(=O)c1N=Nc1ccc(cc1)S(=O)(=O)Oc1ccc(C)cc1 |w:18.19| Show InChI InChI=1S/C23H19ClN4O7S2/c1-14-3-8-18(9-4-14)35-37(33,34)19-10-6-17(7-11-19)25-26-22-15(2)27-28(23(22)29)20-12-5-16(24)13-21(20)36(30,31)32/h3-13,27H,1-2H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158388
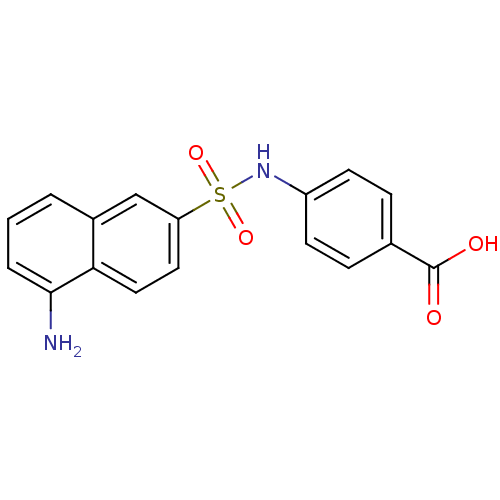
(4-(1-aminonaphthalene-6-sulfonamido)benzoic acid |...)Show SMILES Nc1cccc2cc(ccc12)S(=O)(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C17H14N2O4S/c18-16-3-1-2-12-10-14(8-9-15(12)16)24(22,23)19-13-6-4-11(5-7-13)17(20)21/h1-10,19H,18H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50080319
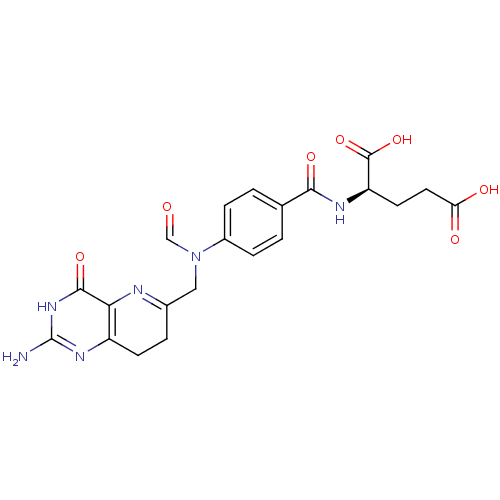
(10-Formyl-8-deazafolic acid | CHEMBL114215)Show SMILES Nc1nc2CCC(CN(C=O)c3ccc(cc3)C(=O)N[C@H](CCC(O)=O)C(O)=O)=Nc2c(=O)[nH]1 |c:29| Show InChI InChI=1S/C21H22N6O7/c22-21-25-14-6-3-12(23-17(14)19(32)26-21)9-27(10-28)13-4-1-11(2-5-13)18(31)24-15(20(33)34)7-8-16(29)30/h1-2,4-5,10,15H,3,6-9H2,(H,24,31)(H,29,30)(H,33,34)(H3,22,25,26,32)/t15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against AICAR formyltransferase |
J Med Chem 42: 3421-4 (1999)
Article DOI: 10.1021/jm990323+
BindingDB Entry DOI: 10.7270/Q23X879T |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50022388
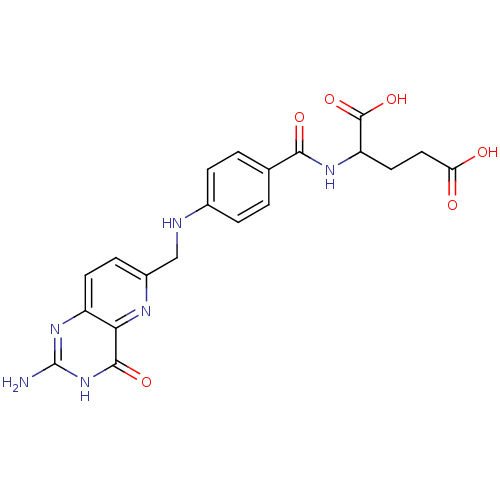
(2-{4-[(2-Amino-4-hydroxy-pyrido[3,2-d]pyrimidin-6-...)Show SMILES Nc1nc2ccc(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)nc2c(=O)[nH]1 Show InChI InChI=1S/C20H20N6O6/c21-20-25-13-6-5-12(23-16(13)18(30)26-20)9-22-11-3-1-10(2-4-11)17(29)24-14(19(31)32)7-8-15(27)28/h1-6,14,22H,7-9H2,(H,24,29)(H,27,28)(H,31,32)(H3,21,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Inhibitory activity against AICAR formyltransferase of Lactobacillus casei |
J Med Chem 31: 150-3 (1988)
BindingDB Entry DOI: 10.7270/Q2GX4C44 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158380
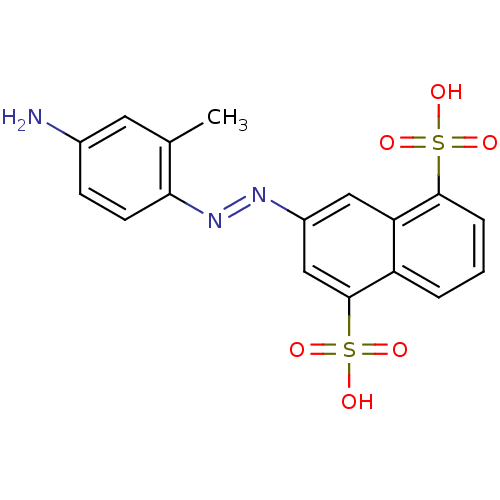
(3-(4-amino-2-methyl-phenylazo)-naphthalene-1,5-dis...)Show SMILES Cc1cc(N)ccc1\N=N\c1cc(c2cccc(c2c1)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C17H15N3O6S2/c1-10-7-11(18)5-6-15(10)20-19-12-8-14-13(17(9-12)28(24,25)26)3-2-4-16(14)27(21,22)23/h2-9H,18H2,1H3,(H,21,22,23)(H,24,25,26)/b20-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158381
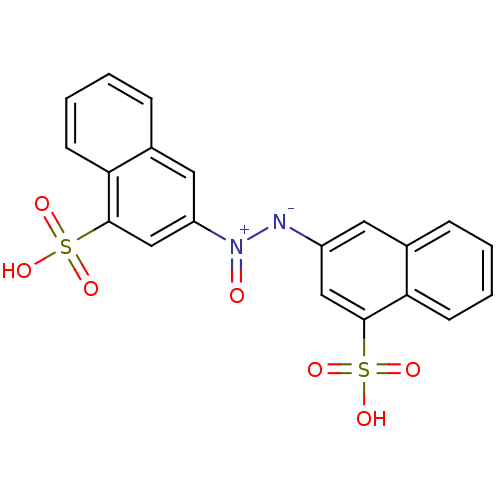
((Z)-1,2-bis(4-sulfonaphthalen-2-yl)diazene oxide |...)Show SMILES OS(=O)(=O)c1cc([N-][N+](=O)c2cc(c3ccccc3c2)S(O)(=O)=O)cc2ccccc12 Show InChI InChI=1S/C20H14N2O7S2/c23-22(16-10-14-6-2-4-8-18(14)20(12-16)31(27,28)29)21-15-9-13-5-1-3-7-17(13)19(11-15)30(24,25)26/h1-12H,(H,24,25,26)(H,27,28,29)/b22-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158387
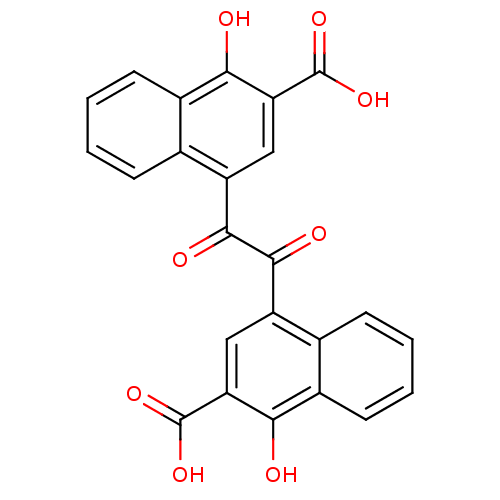
(4-[(3-carboxy-4-hydroxy-1-naphthyl)(oxo)acetyl]-1-...)Show SMILES OC(=O)c1cc(C(=O)C(=O)c2cc(C(O)=O)c(O)c3ccccc23)c2ccccc2c1O Show InChI InChI=1S/C24H14O8/c25-19-13-7-3-1-5-11(13)15(9-17(19)23(29)30)21(27)22(28)16-10-18(24(31)32)20(26)14-8-4-2-6-12(14)16/h1-10,25-26H,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
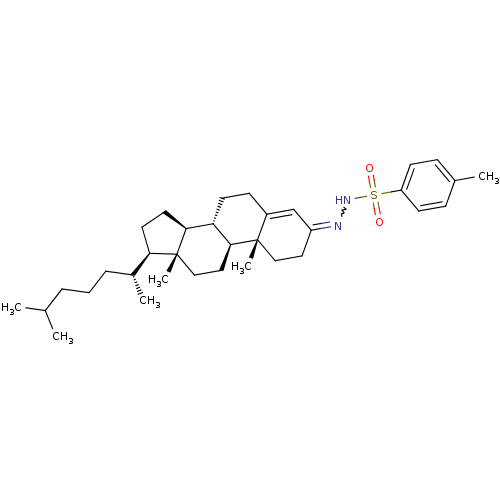
(Homo sapiens (Human)) | BDBM50158382
(CHEMBL376528 | N'-((8S,9S,10R,13R,14S,17R)-10,13-d...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CCC4=CC(CC[C@]4(C)[C@H]3CC[C@]12C)=NNS(=O)(=O)c1ccc(C)cc1 |r,w:27.31,t:15| Show InChI InChI=1S/C34H52N2O2S/c1-23(2)8-7-9-25(4)30-16-17-31-29-15-12-26-22-27(18-20-33(26,5)32(29)19-21-34(30,31)6)35-36-39(37,38)28-13-10-24(3)11-14-28/h10-11,13-14,22-23,25,29-32,36H,7-9,12,15-21H2,1-6H3/t25-,29+,30-,31+,32+,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
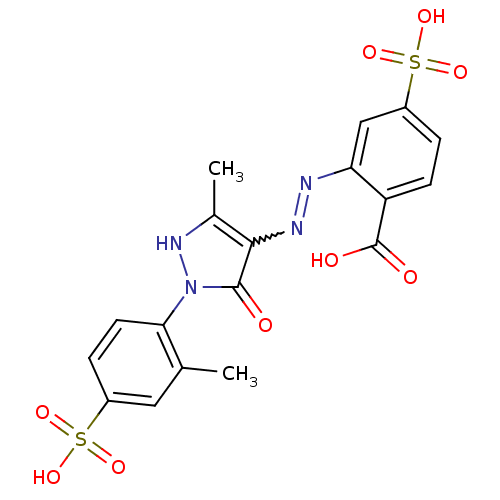
(Homo sapiens (Human)) | BDBM22578
(2-[(E)-2-[5-hydroxy-3-methyl-1-(2-methyl-4-sulfoph...)Show SMILES Cc1[nH]n(-c2ccc(cc2C)S(O)(=O)=O)c(=O)c1N=Nc1cc(ccc1C(O)=O)S(O)(=O)=O |w:18.19| Show InChI InChI=1S/C18H16N4O9S2/c1-9-7-11(32(26,27)28)4-6-15(9)22-17(23)16(10(2)21-22)20-19-14-8-12(33(29,30)31)3-5-13(14)18(24)25/h3-8,21H,1-2H3,(H,24,25)(H,26,27,28)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 7.10E+3 | -29.1 | 1.16E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute
| Assay Description
The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The... |
J Biol Chem 279: 50555-65 (2004)
Article DOI: 10.1074/jbc.M406801200
BindingDB Entry DOI: 10.7270/Q2MC8X97 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
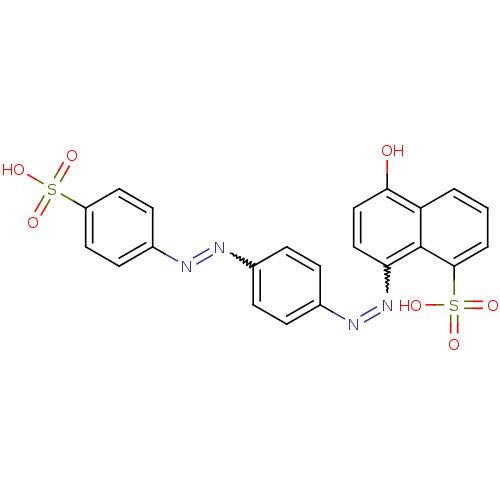
(Homo sapiens (Human)) | BDBM50158386
(5-hydroxy-8-[4-(4-sulfo-phenylazo)-phenylazo]-naph...)Show SMILES Oc1ccc(N=Nc2ccc(cc2)N=Nc2ccc(cc2)S(O)(=O)=O)c2c(cccc12)S(O)(=O)=O |w:13.13,5.4| Show InChI InChI=1S/C22H16N4O7S2/c27-20-13-12-19(22-18(20)2-1-3-21(22)35(31,32)33)26-25-15-6-4-14(5-7-15)23-24-16-8-10-17(11-9-16)34(28,29)30/h1-13,27H,(H,28,29,30)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM22582
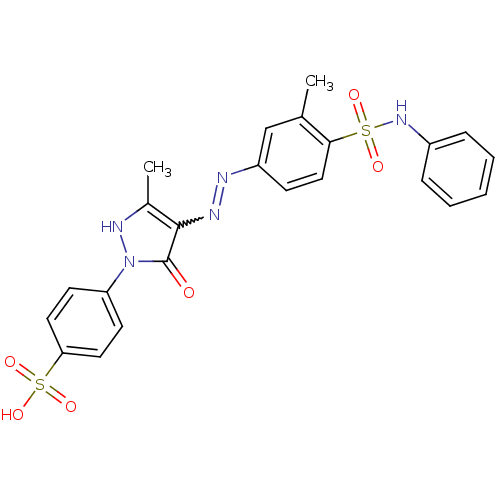
(324572-F | 4-{3-methyl-4-[(E)-2-[3-methyl-4-(pheny...)Show SMILES Cc1[nH]n(-c2ccc(cc2)S(O)(=O)=O)c(=O)c1N=Nc1ccc(c(C)c1)S(=O)(=O)Nc1ccccc1 |w:17.18| Show InChI InChI=1S/C23H21N5O6S2/c1-15-14-18(8-13-21(15)35(30,31)27-17-6-4-3-5-7-17)24-25-22-16(2)26-28(23(22)29)19-9-11-20(12-10-19)36(32,33)34/h3-14,26-27H,1-2H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM22582

(324572-F | 4-{3-methyl-4-[(E)-2-[3-methyl-4-(pheny...)Show SMILES Cc1[nH]n(-c2ccc(cc2)S(O)(=O)=O)c(=O)c1N=Nc1ccc(c(C)c1)S(=O)(=O)Nc1ccccc1 |w:17.18| Show InChI InChI=1S/C23H21N5O6S2/c1-15-14-18(8-13-21(15)35(30,31)27-17-6-4-3-5-7-17)24-25-22-16(2)26-28(23(22)29)19-9-11-20(12-10-19)36(32,33)34/h3-14,26-27H,1-2H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute
| Assay Description
The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The... |
J Biol Chem 279: 50555-65 (2004)
Article DOI: 10.1074/jbc.M406801200
BindingDB Entry DOI: 10.7270/Q2MC8X97 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158383
(2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)COS(=O)(=O)c1ccc(Br)cc1 |r,t:8| Show InChI InChI=1S/C27H33BrO5S/c1-26-13-11-19(29)15-17(26)3-8-21-22-9-10-24(27(22,2)14-12-23(21)26)25(30)16-33-34(31,32)20-6-4-18(28)5-7-20/h4-7,15,21-24H,3,8-14,16H2,1-2H3/t21-,22-,23-,24+,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
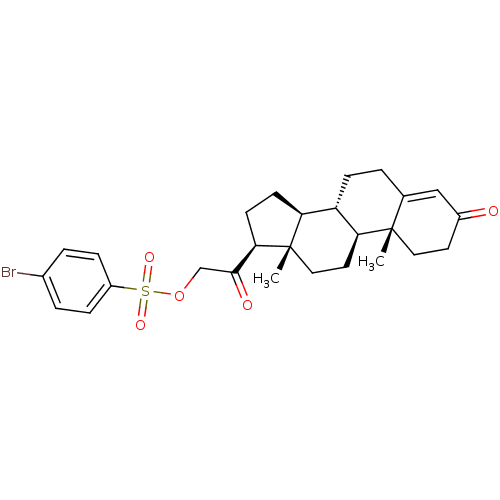
(Homo sapiens (Human)) | BDBM50158385
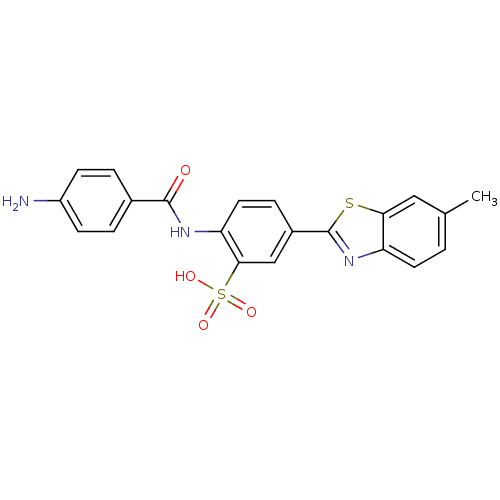
(2-(4-aminobenzamido)-5-(6-methylbenzo[d]thiazol-2-...)Show SMILES Cc1ccc2nc(sc2c1)-c1ccc(NC(=O)c2ccc(N)cc2)c(c1)S(O)(=O)=O Show InChI InChI=1S/C21H17N3O4S2/c1-12-2-8-16-18(10-12)29-21(24-16)14-5-9-17(19(11-14)30(26,27)28)23-20(25)13-3-6-15(22)7-4-13/h2-11H,22H2,1H3,(H,23,25)(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50003058
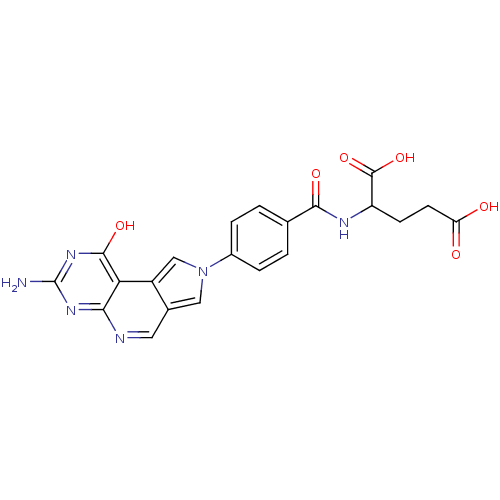
(2-[4-(7-Amino-9-oxo-8,9-dihydro-2,5,6,8-tetraaza-c...)Show SMILES Nc1nc(O)c2c3cn(cc3cnc2n1)-c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H18N6O6/c22-21-25-17-16(19(31)26-21)13-9-27(8-11(13)7-23-17)12-3-1-10(2-4-12)18(30)24-14(20(32)33)5-6-15(28)29/h1-4,7-9,14H,5-6H2,(H,24,30)(H,28,29)(H,32,33)(H3,22,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Aminoimidazole carboxamide ribonucleotide formyl transferase (AICAR) with (6R) tetrahydrofolate as subst... |
J Med Chem 35: 3678-85 (1992)
BindingDB Entry DOI: 10.7270/Q2RJ4HFH |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243413
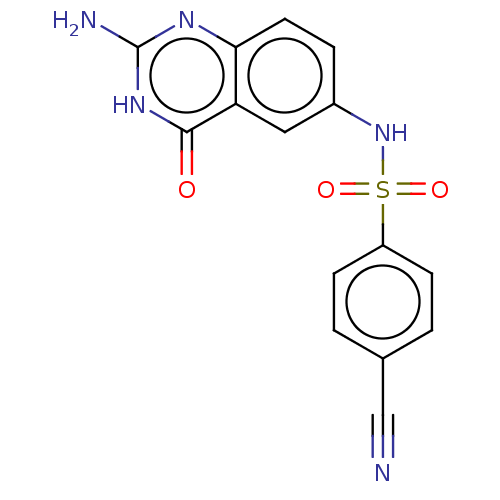
(CHEMBL4077027)Show SMILES Nc1nc2ccc(NS(=O)(=O)c3ccc(cc3)C#N)cc2c(=O)[nH]1 Show InChI InChI=1S/C15H11N5O3S/c16-8-9-1-4-11(5-2-9)24(22,23)20-10-3-6-13-12(7-10)14(21)19-15(17)18-13/h1-7,20H,(H3,17,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50022387
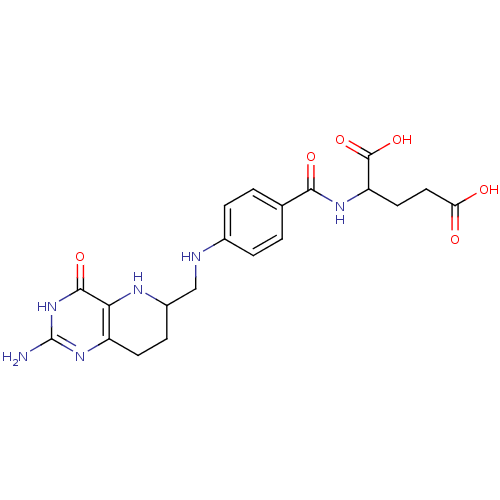
(2-{4-[(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyrido...)Show SMILES Nc1nc2CCC(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)Nc2c(=O)[nH]1 Show InChI InChI=1S/C20H24N6O6/c21-20-25-13-6-5-12(23-16(13)18(30)26-20)9-22-11-3-1-10(2-4-11)17(29)24-14(19(31)32)7-8-15(27)28/h1-4,12,14,22-23H,5-9H2,(H,24,29)(H,27,28)(H,31,32)(H3,21,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Inhibitory activity against AICAR formyltransferase of Lactobacillus casei |
J Med Chem 31: 150-3 (1988)
BindingDB Entry DOI: 10.7270/Q2GX4C44 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50022739
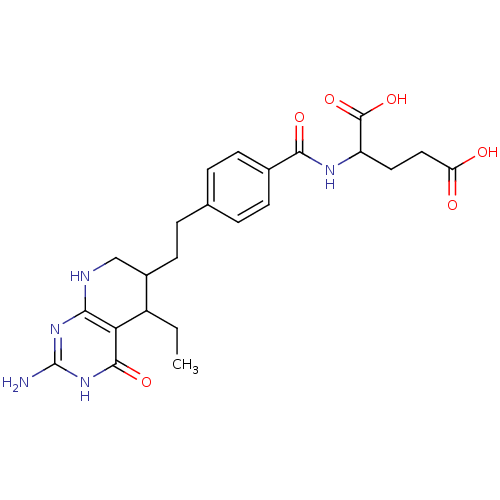
((6RS) 2-{4-[2-(2-Amino-5-ethyl-4-oxo-3,4,5,6,7,8-h...)Show SMILES CCC1C(CCc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)CNc2nc(N)[nH]c(=O)c12 Show InChI InChI=1S/C23H29N5O6/c1-2-15-14(11-25-19-18(15)21(32)28-23(24)27-19)8-5-12-3-6-13(7-4-12)20(31)26-16(22(33)34)9-10-17(29)30/h3-4,6-7,14-16H,2,5,8-11H2,1H3,(H,26,31)(H,29,30)(H,33,34)(H4,24,25,27,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of AICAR formyltransferase |
J Med Chem 31: 2164-9 (1988)
BindingDB Entry DOI: 10.7270/Q25H7F8Z |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM18050

(2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Inhibitory activity against AICAR formyltransferase of Lactobacillus casei |
J Med Chem 31: 150-3 (1988)
BindingDB Entry DOI: 10.7270/Q2GX4C44 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50022741
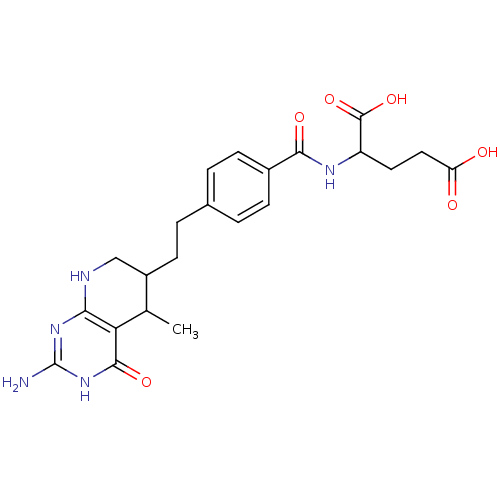
((5RS, 6RS) 2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4,5,6...)Show SMILES CC1C(CCc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)CNc2nc(N)[nH]c(=O)c12 Show InChI InChI=1S/C22H27N5O6/c1-11-14(10-24-18-17(11)20(31)27-22(23)26-18)7-4-12-2-5-13(6-3-12)19(30)25-15(21(32)33)8-9-16(28)29/h2-3,5-6,11,14-15H,4,7-10H2,1H3,(H,25,30)(H,28,29)(H,32,33)(H4,23,24,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of AICAR formyltransferase |
J Med Chem 31: 2164-9 (1988)
BindingDB Entry DOI: 10.7270/Q25H7F8Z |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
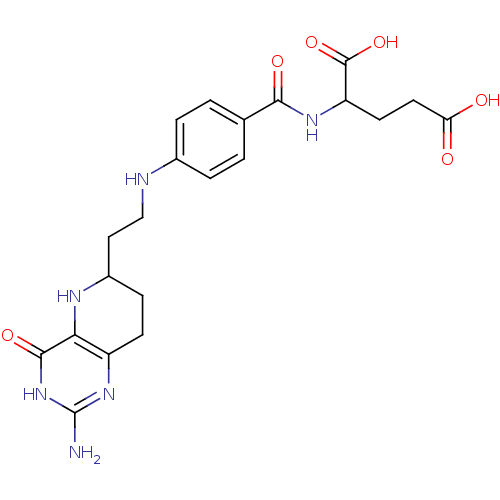
(Homo sapiens (Human)) | BDBM50022386
(2-{4-[2-(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyri...)Show SMILES Nc1nc2CCC(CCNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)Nc2c(=O)[nH]1 Show InChI InChI=1S/C21H26N6O6/c22-21-26-14-6-5-13(24-17(14)19(31)27-21)9-10-23-12-3-1-11(2-4-12)18(30)25-15(20(32)33)7-8-16(28)29/h1-4,13,15,23-24H,5-10H2,(H,25,30)(H,28,29)(H,32,33)(H3,22,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Inhibitory activity against AICAR formyltransferase of Lactobacillus casei |
J Med Chem 31: 150-3 (1988)
BindingDB Entry DOI: 10.7270/Q2GX4C44 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
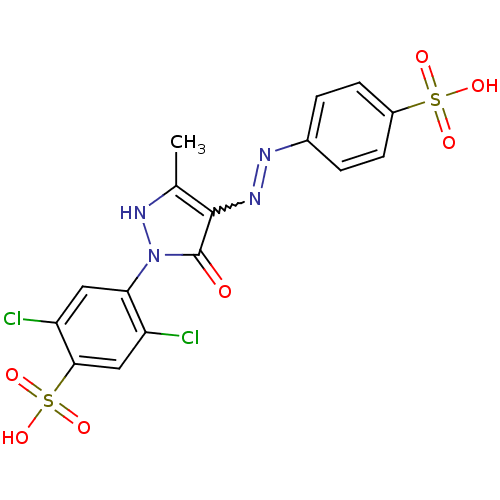
(Homo sapiens (Human)) | BDBM22583
(2,5-dichloro-4-{3-methyl-5-oxo-4-[(E)-2-(4-sulfoph...)Show SMILES Cc1[nH]n(-c2cc(Cl)c(cc2Cl)S(O)(=O)=O)c(=O)c1N=Nc1ccc(cc1)S(O)(=O)=O |w:19.20| Show InChI InChI=1S/C16H12Cl2N4O7S2/c1-8-15(20-19-9-2-4-10(5-3-9)30(24,25)26)16(23)22(21-8)13-6-12(18)14(7-11(13)17)31(27,28)29/h2-7,21H,1H3,(H,24,25,26)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM22583

(2,5-dichloro-4-{3-methyl-5-oxo-4-[(E)-2-(4-sulfoph...)Show SMILES Cc1[nH]n(-c2cc(Cl)c(cc2Cl)S(O)(=O)=O)c(=O)c1N=Nc1ccc(cc1)S(O)(=O)=O |w:19.20| Show InChI InChI=1S/C16H12Cl2N4O7S2/c1-8-15(20-19-9-2-4-10(5-3-9)30(24,25)26)16(23)22(21-8)13-6-12(18)14(7-11(13)17)31(27,28)29/h2-7,21H,1H3,(H,24,25,26)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
The Scripps Research Institute
| Assay Description
The human ATIC enzyme was used for the inhibition assay. The assay buffer was flushed with nitrogen to minimize oxidization of cofactor 10-f-THF. The... |
J Biol Chem 279: 50555-65 (2004)
Article DOI: 10.1074/jbc.M406801200
BindingDB Entry DOI: 10.7270/Q2MC8X97 |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
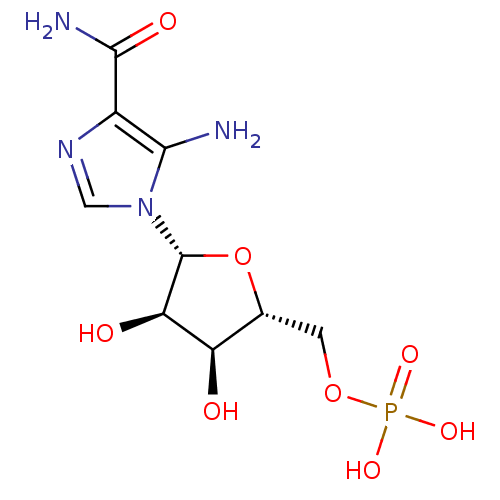
(Homo sapiens (Human)) | BDBM22579
(AICAR | Aminoimidazole-4-carboxamide ribonucleotid...)Show SMILES NC(=O)c1ncn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c1N Show InChI InChI=1S/C9H15N4O8P/c10-7-4(8(11)16)12-2-13(7)9-6(15)5(14)3(21-9)1-20-22(17,18)19/h2-3,5-6,9,14-15H,1,10H2,(H2,11,16)(H2,17,18,19)/t3-,5-,6-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University
Curated by ChEMBL
| Assay Description
Inhibitory activity against AICAR formyltransferase |
J Med Chem 42: 3421-4 (1999)
Article DOI: 10.1021/jm990323+
BindingDB Entry DOI: 10.7270/Q23X879T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50041176

(2-{4-[(2,4-Diamino-furo[2,3-d]pyrimidin-5-ylmethyl...)Show SMILES CN(Cc1coc2nc(N)nc(N)c12)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N6O6/c1-26(8-11-9-32-18-15(11)16(21)24-20(22)25-18)12-4-2-10(3-5-12)17(29)23-13(19(30)31)6-7-14(27)28/h2-5,9,13H,6-8H2,1H3,(H,23,29)(H,27,28)(H,30,31)(H4,21,22,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against L. casei AICAR formyltransferase |
J Med Chem 37: 1169-76 (1994)
BindingDB Entry DOI: 10.7270/Q2K64H4G |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
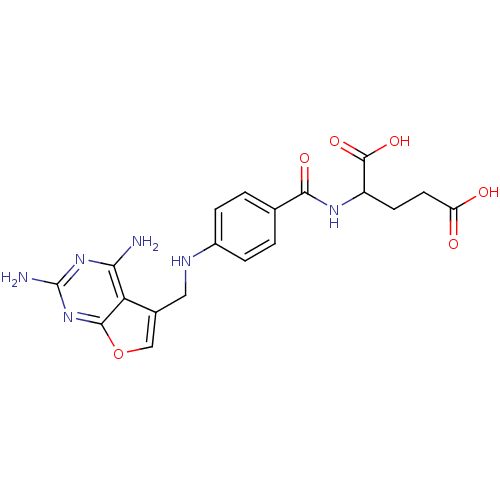
(Homo sapiens (Human)) | BDBM50041179
(2-{4-[(2,4-Diamino-furo[2,3-d]pyrimidin-5-ylmethyl...)Show SMILES Nc1nc(N)c2c(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)coc2n1 Show InChI InChI=1S/C19H20N6O6/c20-15-14-10(8-31-17(14)25-19(21)24-15)7-22-11-3-1-9(2-4-11)16(28)23-12(18(29)30)5-6-13(26)27/h1-4,8,12,22H,5-7H2,(H,23,28)(H,26,27)(H,29,30)(H4,20,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against L. casei AICAR formyltransferase |
J Med Chem 37: 1169-76 (1994)
BindingDB Entry DOI: 10.7270/Q2K64H4G |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50243508
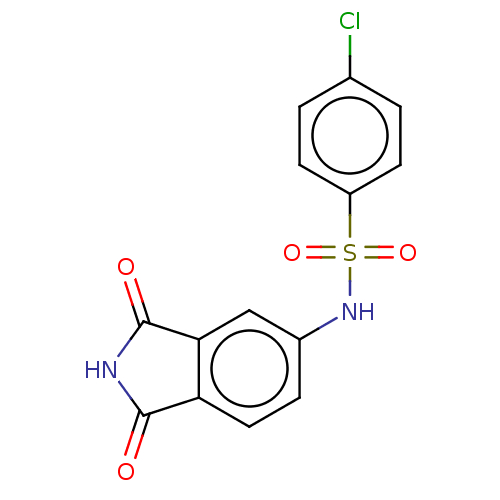
(CHEMBL4101760)Show SMILES Clc1ccc(cc1)S(=O)(=O)Nc1ccc2C(=O)NC(=O)c2c1 Show InChI InChI=1S/C14H9ClN2O4S/c15-8-1-4-10(5-2-8)22(20,21)17-9-3-6-11-12(7-9)14(19)16-13(11)18/h1-7,17H,(H,16,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human full length N-terminal His-tagged AICARFT expressed in Escherichia coli BL21 (DE3) using ZMP/10-formyltetrahydrofolate as substra... |
J Med Chem 60: 9599-9616 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01046
BindingDB Entry DOI: 10.7270/Q2222X6J |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158390
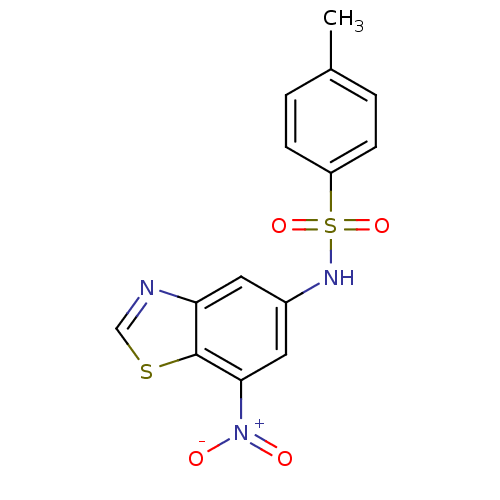
(4-methyl-N-(7-nitrobenzo[d]thiazol-5-yl)benzenesul...)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1cc([N+]([O-])=O)c2scnc2c1 Show InChI InChI=1S/C14H11N3O4S2/c1-9-2-4-11(5-3-9)23(20,21)16-10-6-12-14(22-8-15-12)13(7-10)17(18)19/h2-8,16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50003059
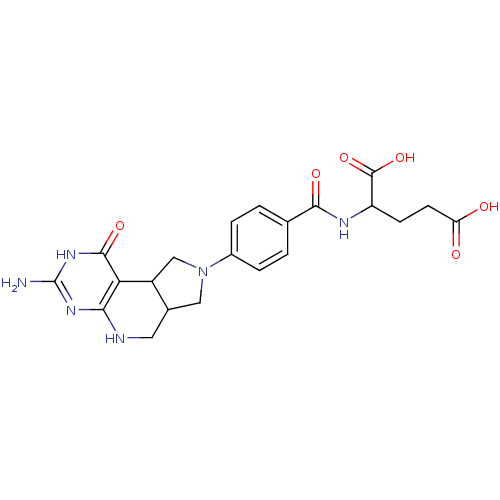
(2-[4-(7-Amino-9-oxo-1,3,3a,4,5,8,9,9b-octahydro-2,...)Show SMILES Nc1nc2NCC3CN(CC3c2c(=O)[nH]1)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C21H24N6O6/c22-21-25-17-16(19(31)26-21)13-9-27(8-11(13)7-23-17)12-3-1-10(2-4-12)18(30)24-14(20(32)33)5-6-15(28)29/h1-4,11,13-14H,5-9H2,(H,24,30)(H,28,29)(H,32,33)(H4,22,23,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Aminoimidazole carboxamide ribonucleotide formyl transferase (AICAR) with (6R) tetrahydrofolate as subst... |
J Med Chem 35: 3678-85 (1992)
BindingDB Entry DOI: 10.7270/Q2RJ4HFH |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50022385
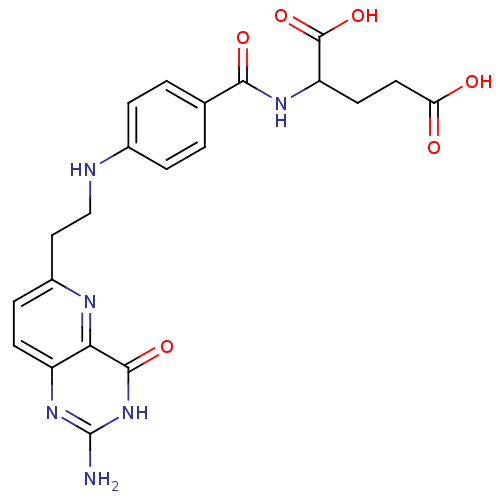
(2-{4-[2-(2-Amino-4-hydroxy-pyrido[3,2-d]pyrimidin-...)Show SMILES Nc1nc2ccc(CCNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)nc2c(=O)[nH]1 Show InChI InChI=1S/C21H22N6O6/c22-21-26-14-6-5-13(24-17(14)19(31)27-21)9-10-23-12-3-1-11(2-4-12)18(30)25-15(20(32)33)7-8-16(28)29/h1-6,15,23H,7-10H2,(H,25,30)(H,28,29)(H,32,33)(H3,22,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Inhibitory activity against AICAR formyltransferase of Lactobacillus casei |
J Med Chem 31: 150-3 (1988)
BindingDB Entry DOI: 10.7270/Q2GX4C44 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data