

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554035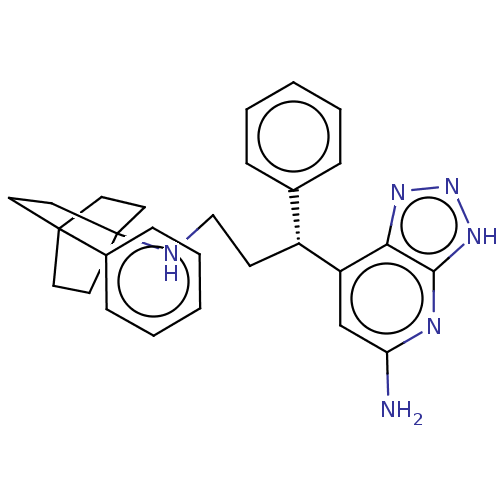 (CHEMBL4790231) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MPO (unknown origin) chlorination activity incubated for 10 mins followed by NaCl addition by aminophenyl fluorescein assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554034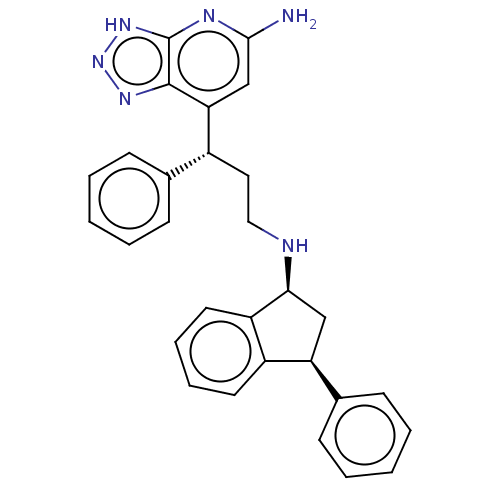 (CHEMBL4747269) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554035 (CHEMBL4790231) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554052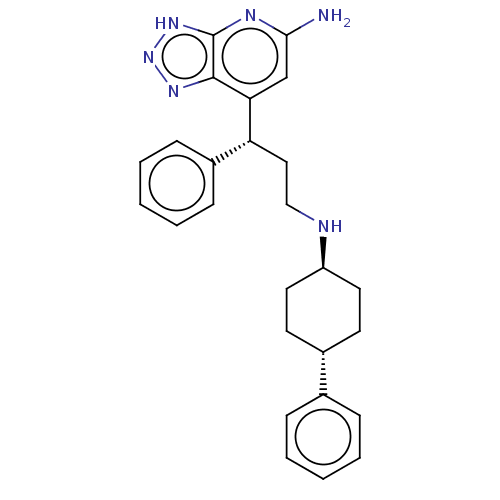 (CHEMBL4763667) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554050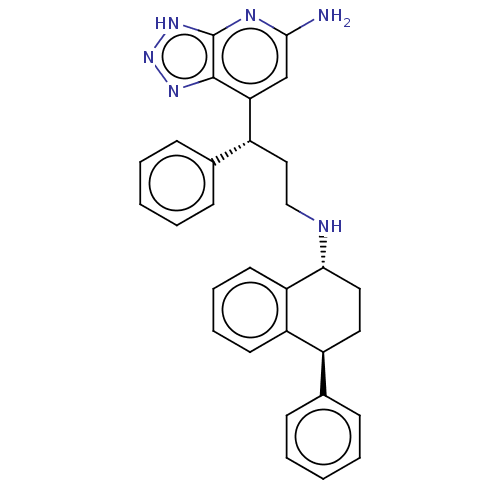 (CHEMBL4752248) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50432573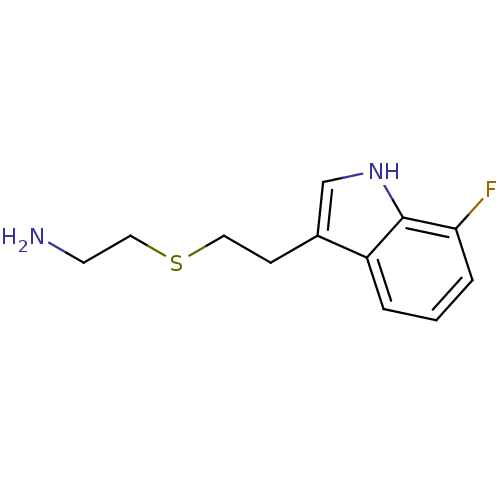 (CHEMBL2347170) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of human recombinant MPO-mediated taurine chlorination after 5 mins by microplate assay | J Med Chem 56: 3943-58 (2013) Article DOI: 10.1021/jm4001538 BindingDB Entry DOI: 10.7270/Q21C1Z7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50595667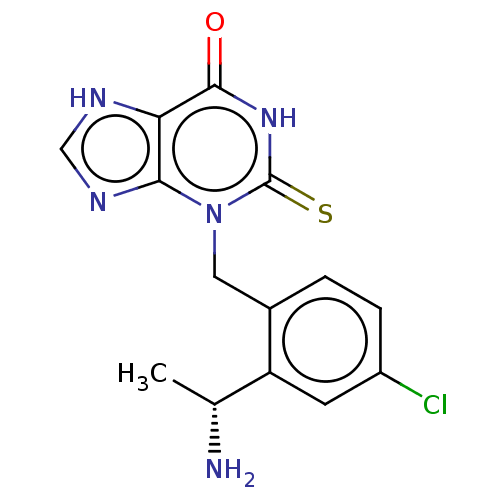 (CHEMBL5181350) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50432575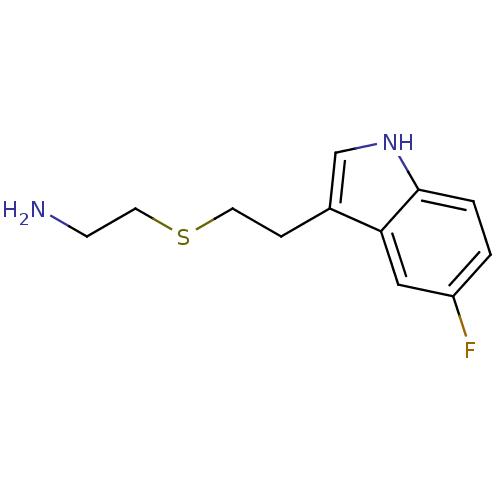 (CHEMBL2347168) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of human recombinant MPO-mediated taurine chlorination after 5 mins by microplate assay | J Med Chem 56: 3943-58 (2013) Article DOI: 10.1021/jm4001538 BindingDB Entry DOI: 10.7270/Q21C1Z7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50507392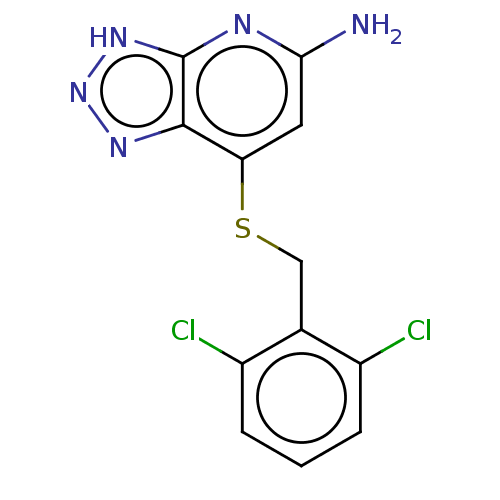 (CHEMBL4548537 | US10981879, Example 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human PMN leukocytes MPO peroxidation activity using H2O2 as substrate preincubated for 10 mins followed by H2O2 addition and measured ... | ACS Med Chem Lett 9: 1175-1180 (2018) Article DOI: 10.1021/acsmedchemlett.8b00308 BindingDB Entry DOI: 10.7270/Q2W0997Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM434866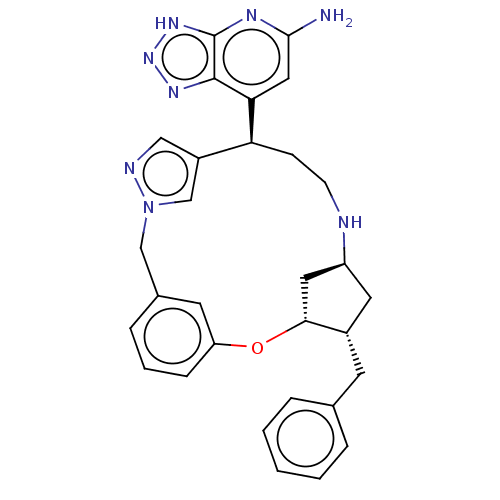 (7-[(3R,4S,6S,10R)-4-Benzyl-2-oxa-7,13,14-triazatet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MPO (unknown origin) chlorination activity incubated for 10 mins followed by NaCl addition by aminophenyl fluorescein assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50595661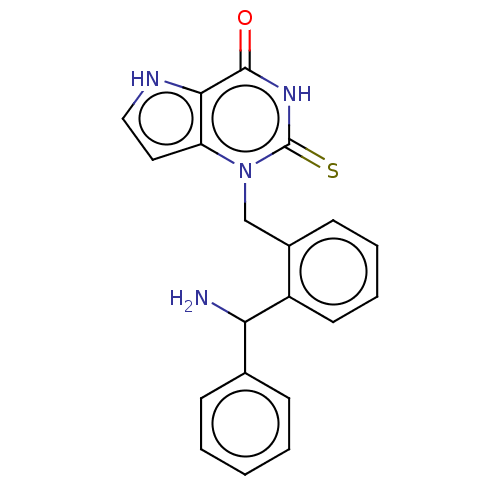 (CHEMBL5197968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50595661 (CHEMBL5197968) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02141 BindingDB Entry DOI: 10.7270/Q2WD44KM | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554057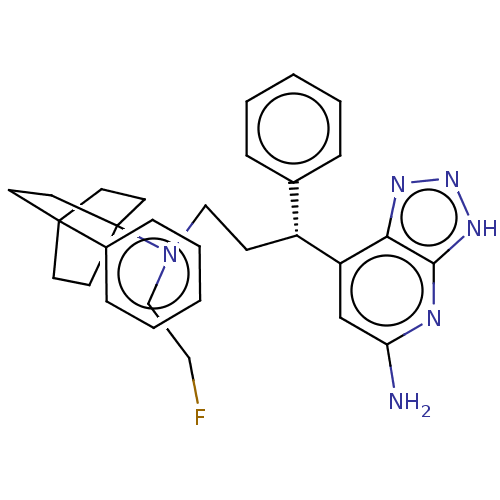 (CHEMBL4740830) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50432568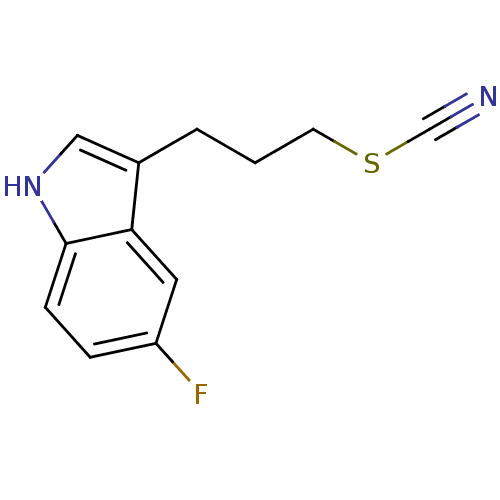 (CHEMBL2347177) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of human recombinant MPO-mediated taurine chlorination after 5 mins by microplate assay | J Med Chem 56: 3943-58 (2013) Article DOI: 10.1021/jm4001538 BindingDB Entry DOI: 10.7270/Q21C1Z7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50332590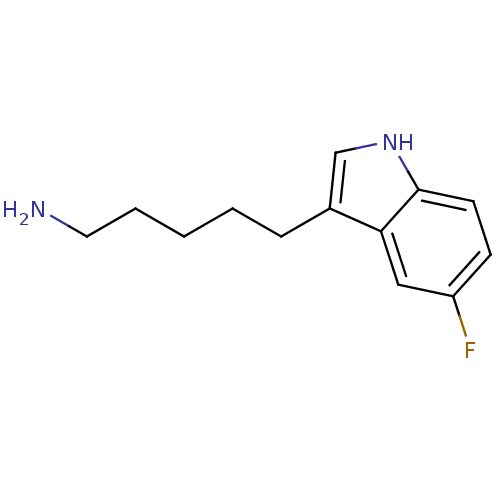 (5-(5-fluoro-1H-indol-3-yl)pentan-1-amine | CHEMBL1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of recombinant MPO (unknown origin) assessed as reduction in taurine chloramine production preincubated with enzyme and taurine followed b... | J Med Chem 60: 6563-6586 (2017) Article DOI: 10.1021/acs.jmedchem.7b00285 BindingDB Entry DOI: 10.7270/Q27D2XD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50238843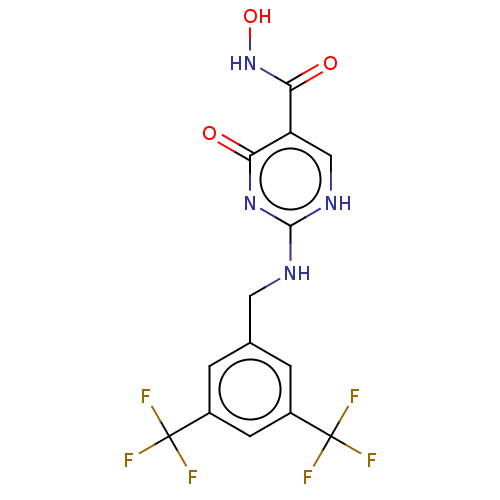 (CHEMBL4068067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of recombinant MPO (unknown origin) assessed as reduction in taurine chloramine production preincubated with enzyme and taurine followed b... | J Med Chem 60: 6563-6586 (2017) Article DOI: 10.1021/acs.jmedchem.7b00285 BindingDB Entry DOI: 10.7270/Q27D2XD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50332590 (5-(5-fluoro-1H-indol-3-yl)pentan-1-amine | CHEMBL1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£? Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of recombinant MPO mediated LDL oxidation using MPO/Cl-/H2O2 system | J Med Chem 53: 8747-59 (2010) Article DOI: 10.1021/jm1009988 BindingDB Entry DOI: 10.7270/Q24T6JNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM217354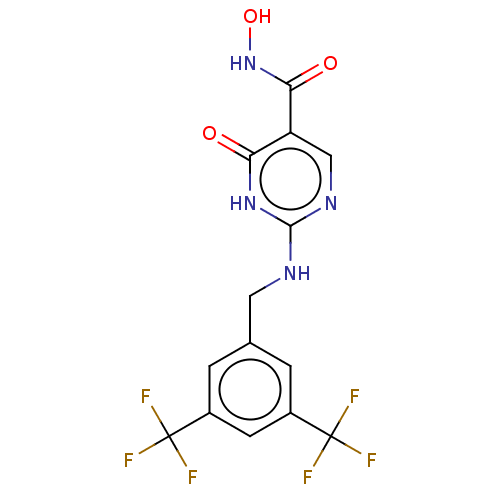 (2-(3,5-bistrifluoromethylbenzylamino)-6-oxo-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | 6.5 | n/a |
University of Otago Christchurch | Assay Description Assays were performed at 22 °C with 2 nM MPO and 10 μM hydrogen peroxide (H2O2) in 20 mM NaH2PO4 buffer, pH 6.5 containing 140 mM NaCl, 10 mM ta... | J Biol Chem 288: 36636-47 (2013) Article DOI: 10.1074/jbc.M113.507756 BindingDB Entry DOI: 10.7270/Q20K27DH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554056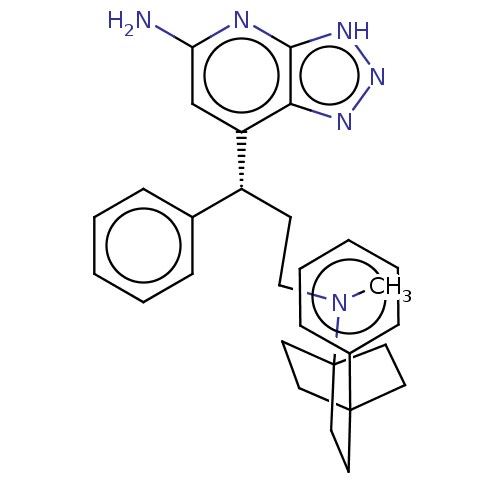 (CHEMBL4782307) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50432573 (CHEMBL2347170) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of human recombinant MPO-mediated LDL oxidation after 5 mins by ELISA | J Med Chem 56: 3943-58 (2013) Article DOI: 10.1021/jm4001538 BindingDB Entry DOI: 10.7270/Q21C1Z7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50432580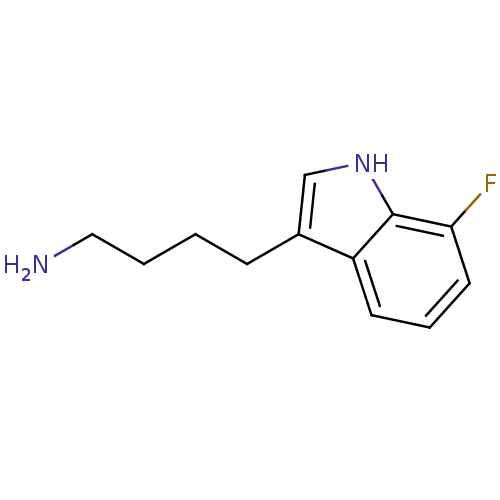 (CHEMBL2347032) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of human recombinant MPO-mediated taurine chlorination after 5 mins by microplate assay | J Med Chem 56: 3943-58 (2013) Article DOI: 10.1021/jm4001538 BindingDB Entry DOI: 10.7270/Q21C1Z7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50507396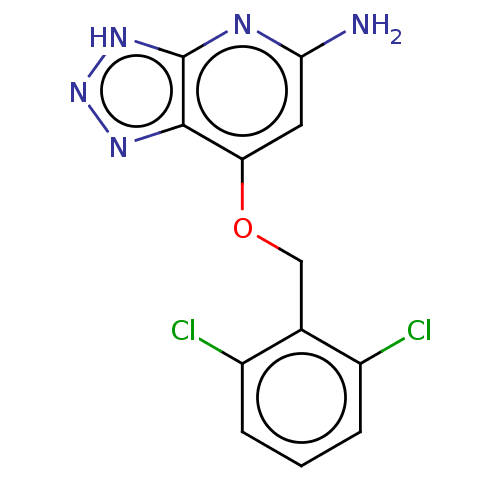 (CHEMBL4453728) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human PMN leukocytes MPO peroxidation activity using H2O2 as substrate preincubated for 10 mins followed by H2O2 addition and measured ... | ACS Med Chem Lett 9: 1175-1180 (2018) Article DOI: 10.1021/acsmedchemlett.8b00308 BindingDB Entry DOI: 10.7270/Q2W0997Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50507390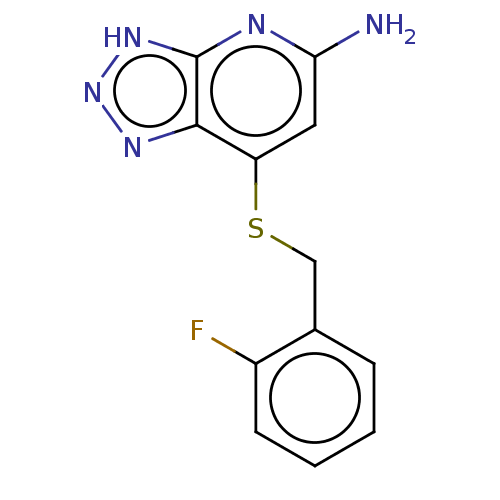 (CHEMBL4482878 | US10981879, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human PMN leukocytes MPO chlorination activity using H2O2 as substrate preincubated for 10 mins followed by H2O2 addition and measured ... | ACS Med Chem Lett 9: 1175-1180 (2018) Article DOI: 10.1021/acsmedchemlett.8b00308 BindingDB Entry DOI: 10.7270/Q2W0997Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM434867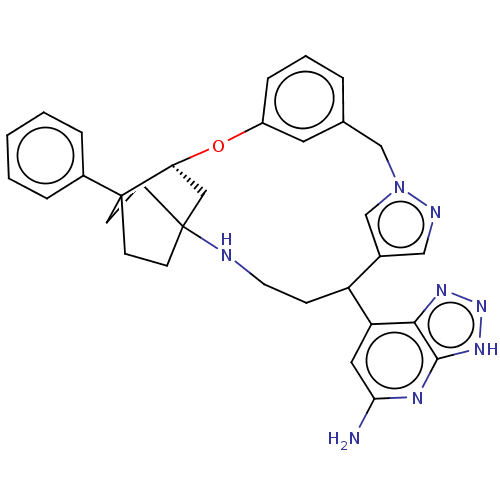 (7-[(17S)-18-Phenyl-16-oxa-2,8,9-triazapentacyclo[1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MPO (unknown origin) chlorination activity incubated for 10 mins followed by NaCl addition by aminophenyl fluorescein assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50507390 (CHEMBL4482878 | US10981879, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description MPO peroxidation activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Amplex Red (Invitrogen catalog #A12222) which ... | US Patent US10981879 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM492844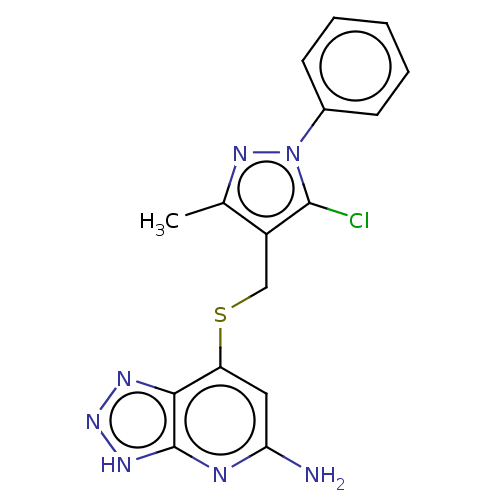 (7-(((5-chloro-3-methyl-1- phenyl-1H-pyrazol-4- yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description MPO chlorination activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Aminophenyl fluorescein (APF, Invitrogen catal... | US Patent US10981879 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312171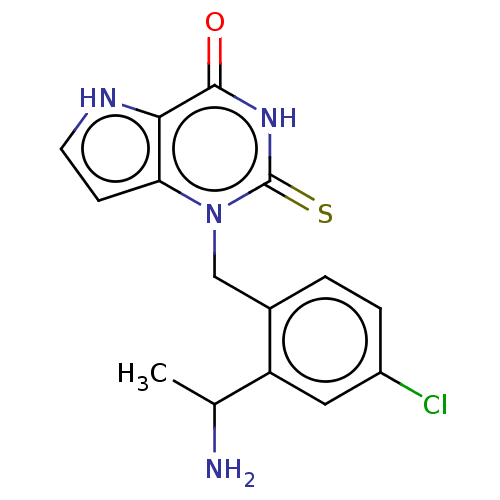 (1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312171 (1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312171 (1-[2-(1-Aminoethyl)-4-chlorobenzyl]-2-thioxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312172 (Alternative Preparation | US10016430, Example 3 | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM434851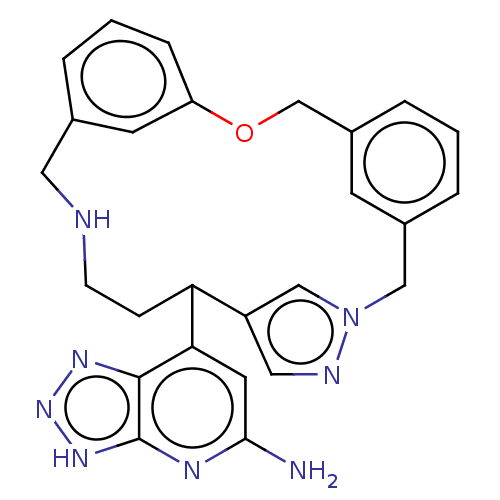 (7-{17-Oxa-3,4,10-triazatetracyclo[17.3.1.13,6.112,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MPO (unknown origin) chlorination activity incubated for 10 mins followed by NaCl addition by aminophenyl fluorescein assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128010 BindingDB Entry DOI: 10.7270/Q2PZ5DKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM413387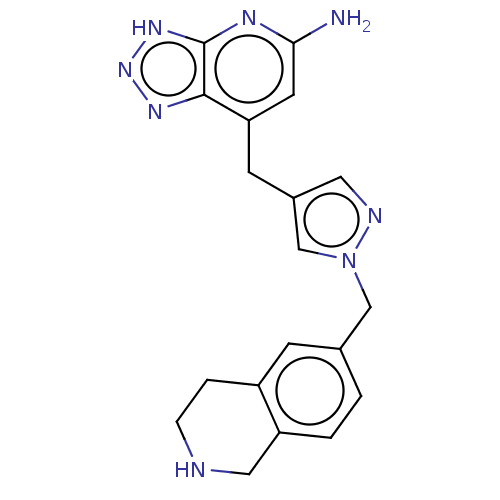 (US10407422, Example 130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi... | US Patent US10407422 (2019) BindingDB Entry DOI: 10.7270/Q28G8P24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50432575 (CHEMBL2347168) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of human recombinant MPO-mediated LDL oxidation after 5 mins by ELISA | J Med Chem 56: 3943-58 (2013) Article DOI: 10.1021/jm4001538 BindingDB Entry DOI: 10.7270/Q21C1Z7T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50332590 (5-(5-fluoro-1H-indol-3-yl)pentan-1-amine | CHEMBL1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universite£? Libre de Bruxelles Curated by ChEMBL | Assay Description Inhibition of recombinant MPO mediated taurine chlorination by microplate reader method | J Med Chem 53: 8747-59 (2010) Article DOI: 10.1021/jm1009988 BindingDB Entry DOI: 10.7270/Q24T6JNJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50332589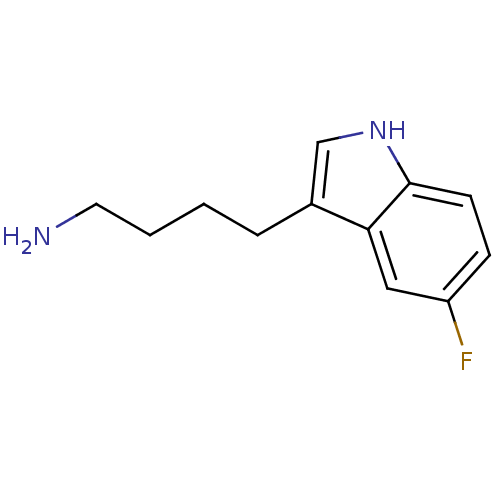 (4-(5-fluoro-1H-indol-3-yl)butan-1-amine | CHEMBL16...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Libre de Bruxelles Curated by ChEMBL | Assay Description In vitro antagonist activity against rat prostatic androgen receptor (AR) | J Med Chem 60: 6563-6586 (2017) Article DOI: 10.1021/acs.jmedchem.7b00285 BindingDB Entry DOI: 10.7270/Q27D2XD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50507391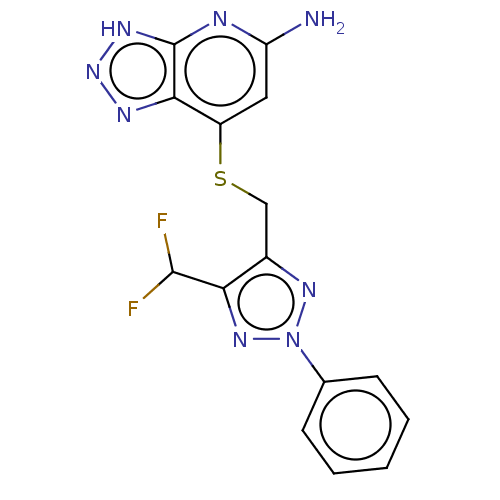 (CHEMBL4594116 | US10981879, Example 153) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human PMN leukocytes MPO peroxidation activity using H2O2 as substrate preincubated for 10 mins followed by H2O2 addition and measured ... | ACS Med Chem Lett 9: 1175-1180 (2018) Article DOI: 10.1021/acsmedchemlett.8b00308 BindingDB Entry DOI: 10.7270/Q2W0997Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312157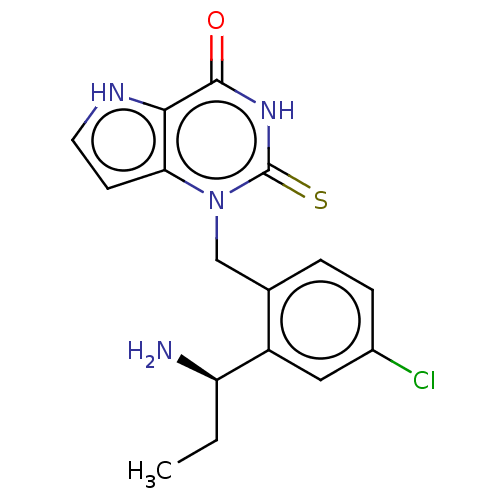 (1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the... | US Patent US10016430 (2018) BindingDB Entry DOI: 10.7270/Q2WW7M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312157 (1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres... | US Patent US9616063 (2017) BindingDB Entry DOI: 10.7270/Q2Q24296 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM312157 (1-{2-[(1R)-1-Aminopropyl]-4-chlorobenzyl}-2-thioxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa... | US Patent US11000525 (2021) BindingDB Entry DOI: 10.7270/Q2QJ7MD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50507390 (CHEMBL4482878 | US10981879, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human PMN leukocytes MPO peroxidation activity using H2O2 as substrate preincubated for 10 mins followed by H2O2 addition and measured ... | ACS Med Chem Lett 9: 1175-1180 (2018) Article DOI: 10.1021/acsmedchemlett.8b00308 BindingDB Entry DOI: 10.7270/Q2W0997Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM492845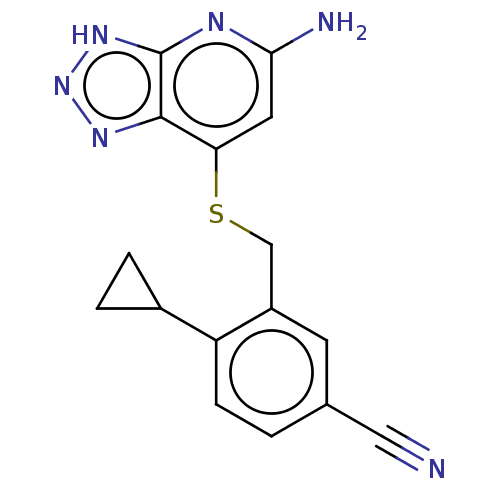 (3-(((5-amino-3H-[1,2,3] triazolo[4,5-b]pyridin-7- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description MPO chlorination activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Aminophenyl fluorescein (APF, Invitrogen catal... | US Patent US10981879 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50554054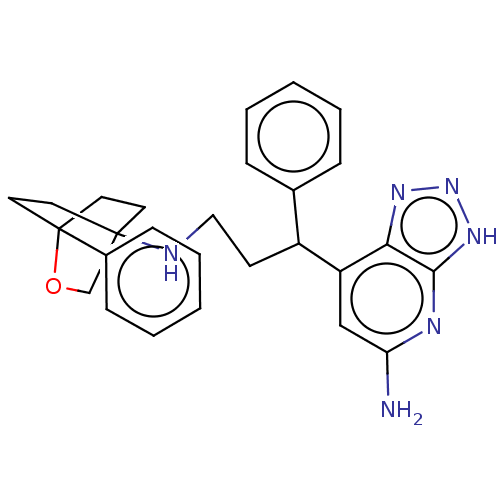 (CHEMBL4751166) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human MPO incubated for 10 mins in presence of 120 mM NaCl by aminophenyl fluorescein based assay | Citation and Details Article DOI: 10.1016/j.bmc.2020.115723 BindingDB Entry DOI: 10.7270/Q21Z4829 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM492832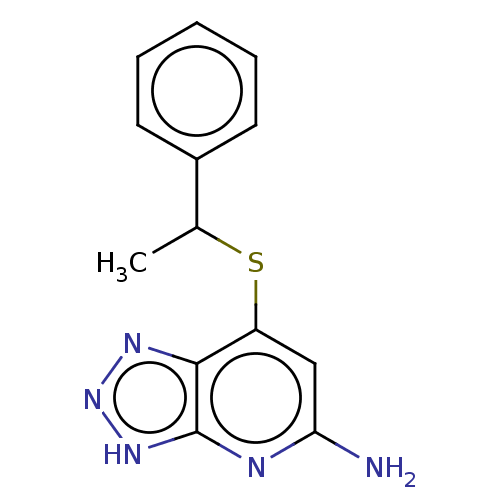 (7-((1-phenylethyl)thio)- 3H-[1,2,3]triazolo[4,5- b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description MPO chlorination activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Aminophenyl fluorescein (APF, Invitrogen catal... | US Patent US10981879 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM492832 (7-((1-phenylethyl)thio)- 3H-[1,2,3]triazolo[4,5- b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description MPO peroxidation activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Amplex Red (Invitrogen catalog #A12222) which ... | US Patent US10981879 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM50507390 (CHEMBL4482878 | US10981879, Example 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description MPO chlorination activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Aminophenyl fluorescein (APF, Invitrogen catal... | US Patent US10981879 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Myeloperoxidase (Homo sapiens (Human)) | BDBM492834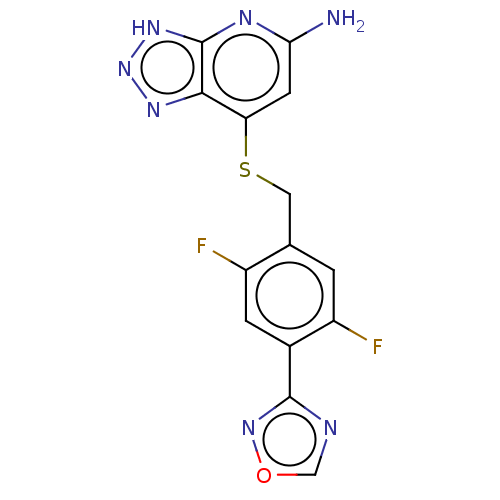 (7-((2,5-difluoro-4-(1,2,4- oxadiazol-3-yl)benzyl)t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description MPO peroxidation activity was measured in 100 mM KPi (pH 7.4) by utilizing the non-fluorescent reagent Amplex Red (Invitrogen catalog #A12222) which ... | US Patent US10981879 (2021) BindingDB Entry DOI: 10.7270/Q2SN0D3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1008 total ) | Next | Last >> |