

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459214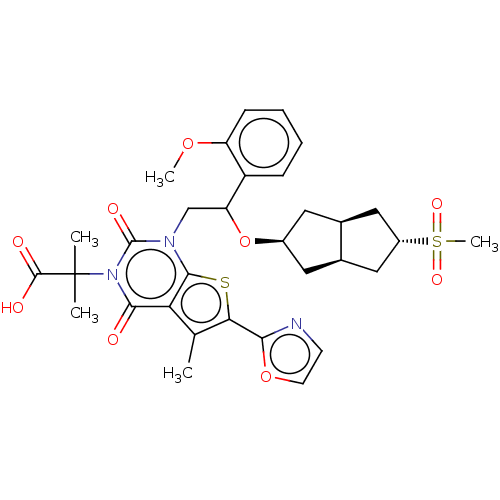 (2-[1-[2-[[(3aR,6aS)-5-methylsulfonyl-1,2,3,3a,4,5,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50069756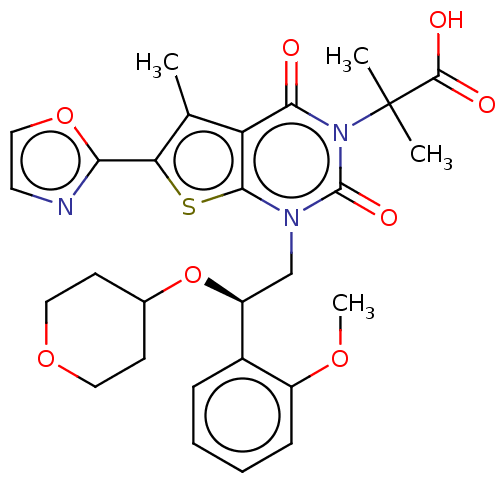 (CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247130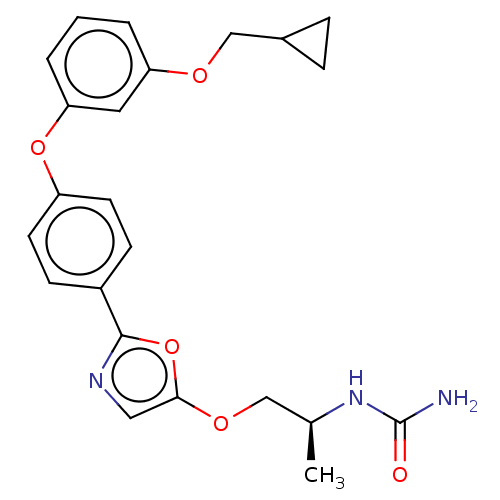 (CHEMBL4073202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527539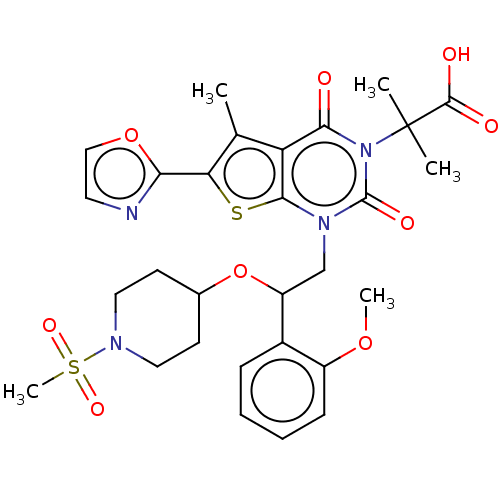 (US11186587, Example 22) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459211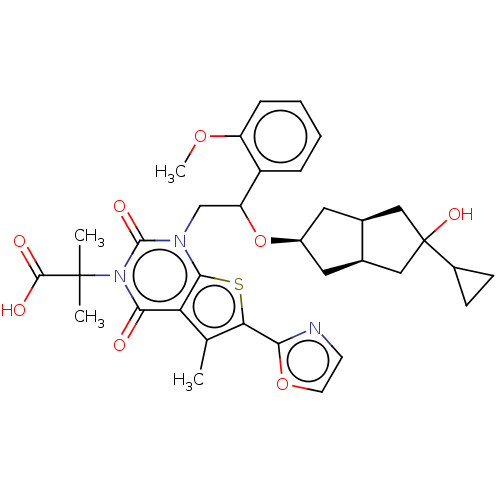 (2-[1-[2-[[(3aR,6aS)-5-cyclopropyl-5-hydroxy-2,3,3a...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527001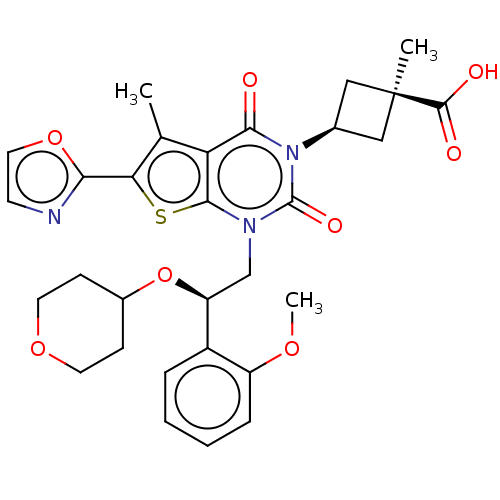 (US11186587, Example 4) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM526973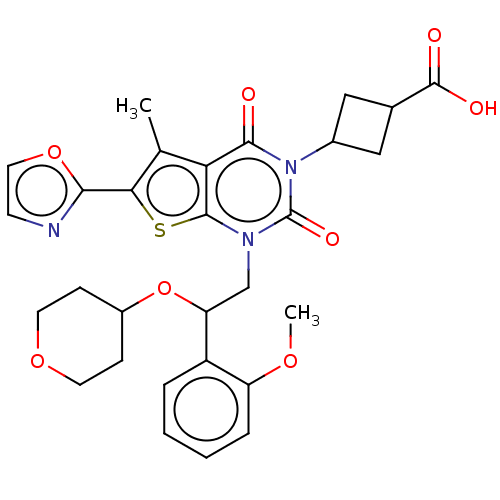 (US11186587, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527538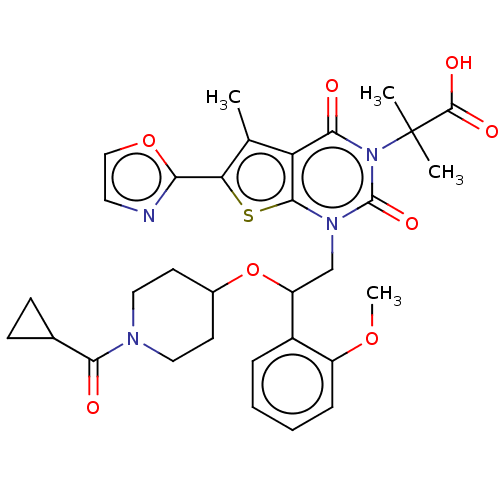 (US11186587, Example 21) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081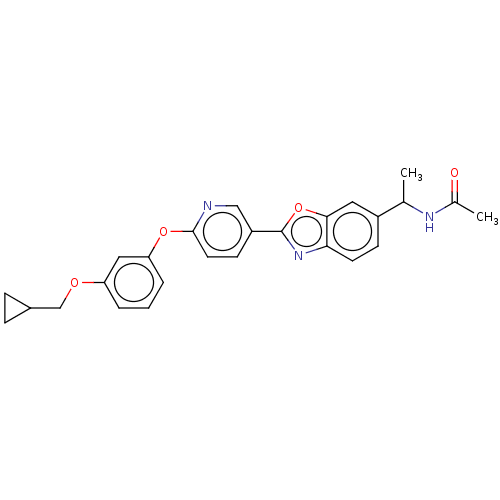 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459201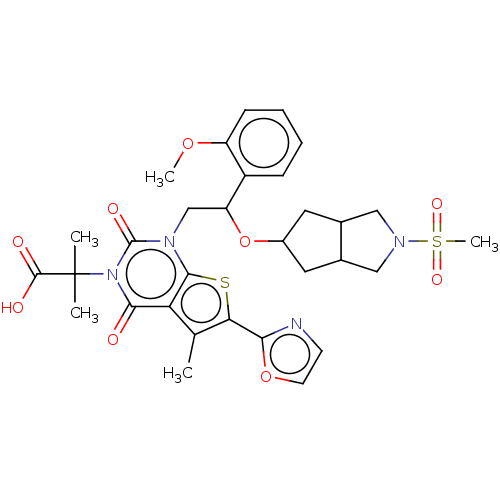 (2-[1-[2-(2-methoxyphenyl)-2-[(2-methylsulfonyl-3,3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527543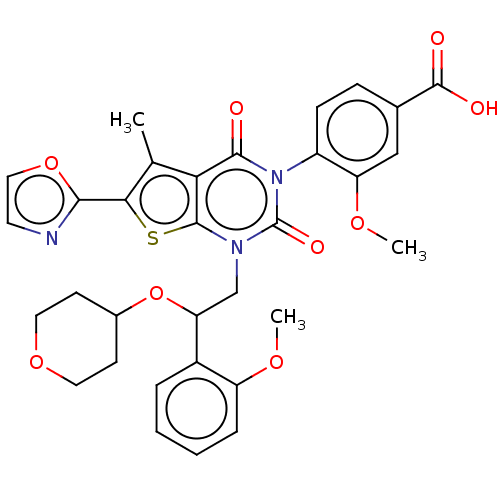 (US11186587, Example 26) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459208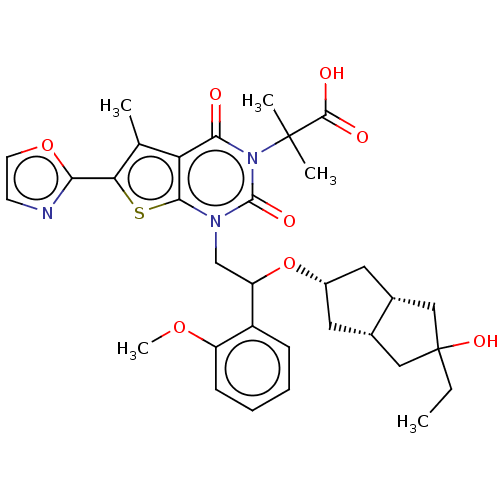 (2-[1-[2-[[(3aR,6aS)-5-ethyl-5-hydroxy-2,3,3a,4,6,6...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459206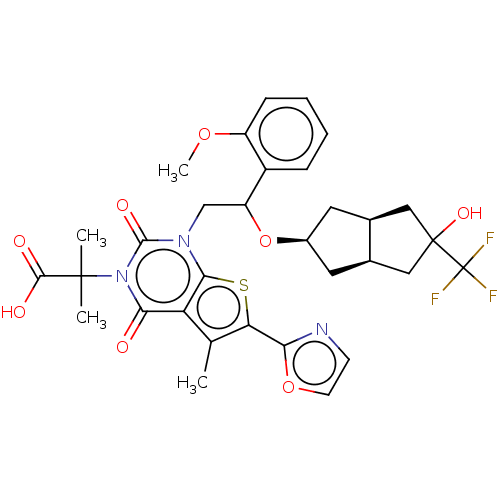 (2-[1-[2-[[(3aR,6aS)-5-hydroxy-5-(trifluoromethyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527537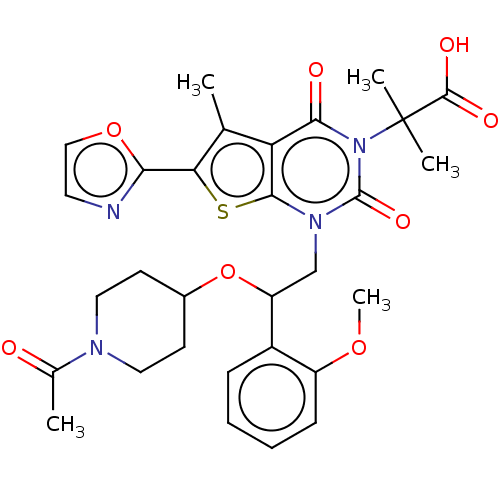 (US11186587, Example 20) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459200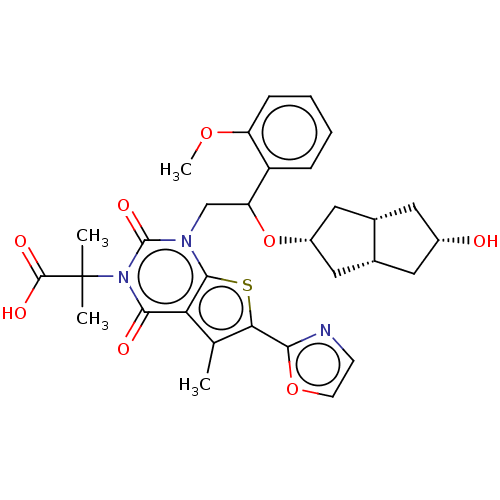 (2-[1-[2-[[(3aS,6aR)-5-hydroxy-1,2,3,3a,4,5,6,6a-oc...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247114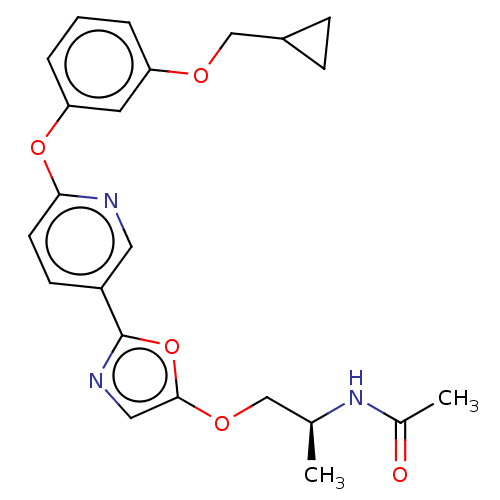 (CHEMBL4086127) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247112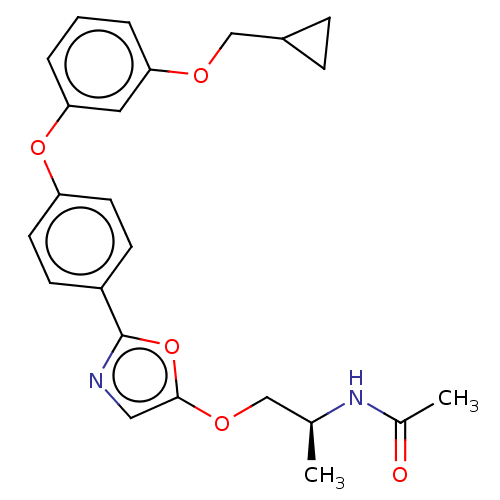 (CHEMBL4085633) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM496965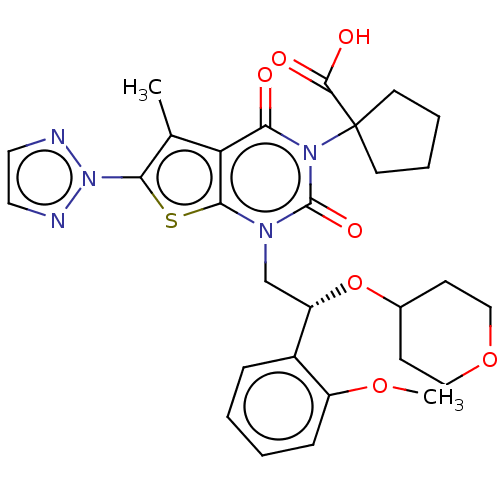 (US10995099, Example 55) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Ruijie Pharmatech Co., Ltd. US Patent | Assay Description An exemplary procedure for the in vitro ACC inhibition assay, which can be used to determine the inhibitory action of compounds of the invention towa... | US Patent US10995099 (2021) BindingDB Entry DOI: 10.7270/Q2028VPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM496979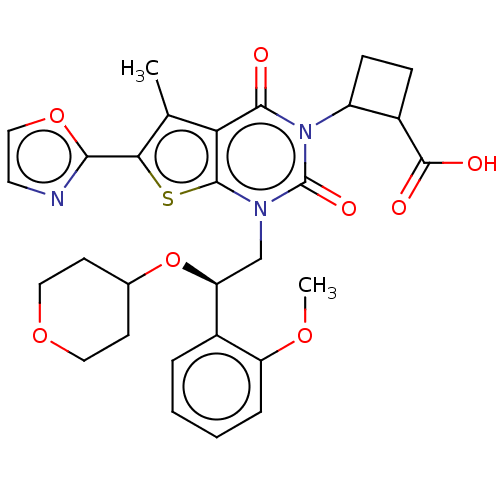 (US10995099, Example 69) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Ruijie Pharmatech Co., Ltd. US Patent | Assay Description An exemplary procedure for the in vitro ACC inhibition assay, which can be used to determine the inhibitory action of compounds of the invention towa... | US Patent US10995099 (2021) BindingDB Entry DOI: 10.7270/Q2028VPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM489789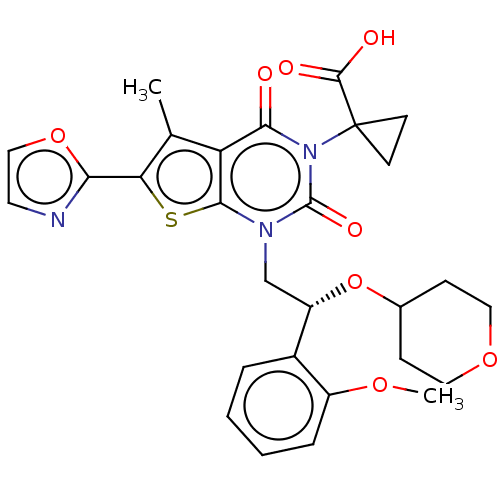 (US10995099, Example 33) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Ruijie Pharmatech Co., Ltd. US Patent | Assay Description An exemplary procedure for the in vitro ACC inhibition assay, which can be used to determine the inhibitory action of compounds of the invention towa... | US Patent US10995099 (2021) BindingDB Entry DOI: 10.7270/Q2028VPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM489782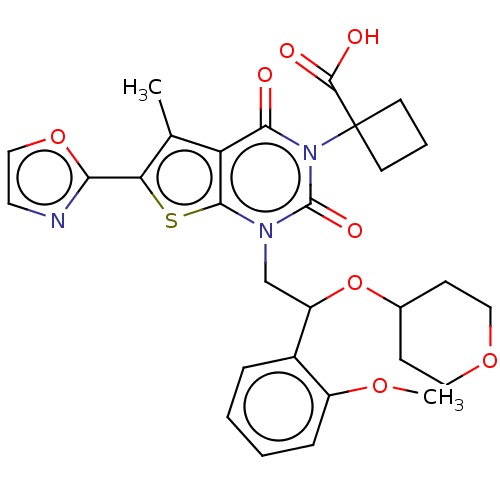 (US10995099, Example 26 | US10995099, Example 37) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Ruijie Pharmatech Co., Ltd. US Patent | Assay Description An exemplary procedure for the in vitro ACC inhibition assay, which can be used to determine the inhibitory action of compounds of the invention towa... | US Patent US10995099 (2021) BindingDB Entry DOI: 10.7270/Q2028VPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50069756 (CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Ruijie Pharmatech Co., Ltd. US Patent | Assay Description An exemplary procedure for the in vitro ACC inhibition assay, which can be used to determine the inhibitory action of compounds of the invention towa... | US Patent US10995099 (2021) BindingDB Entry DOI: 10.7270/Q2028VPN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM496904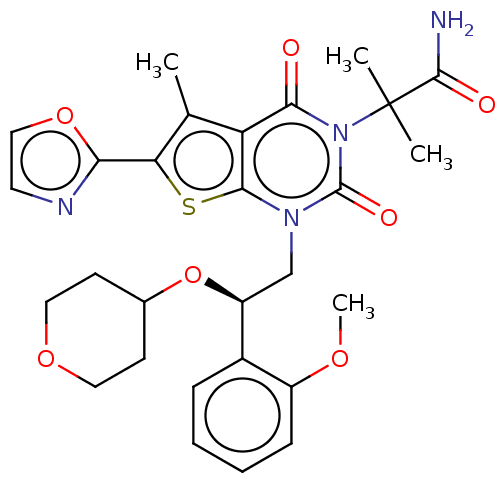 (US10995099, Example 42) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanjing Ruijie Pharmatech Co., Ltd. US Patent | Assay Description An exemplary procedure for the in vitro ACC inhibition assay, which can be used to determine the inhibitory action of compounds of the invention towa... | US Patent US10995099 (2021) BindingDB Entry DOI: 10.7270/Q2028VPN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM526976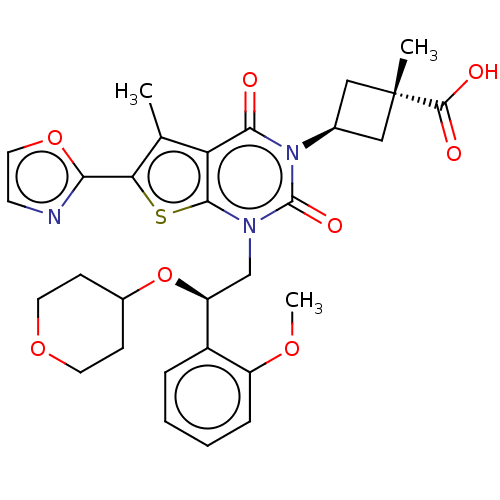 (US11186587, Example 3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527016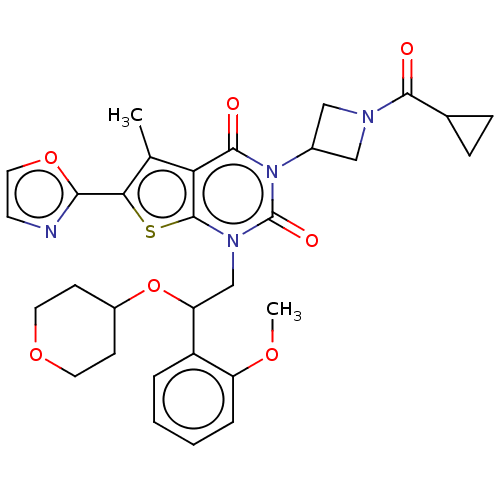 (US11186587, Example 8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459215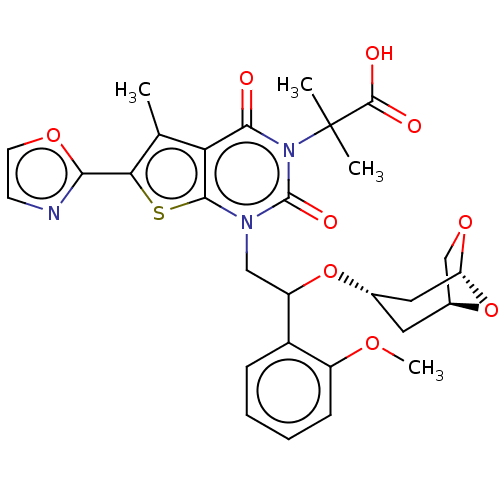 (2-[1-[2-[[(1S,3S,5R)-6,8-dioxabicyclo[3.2.1]oct-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527069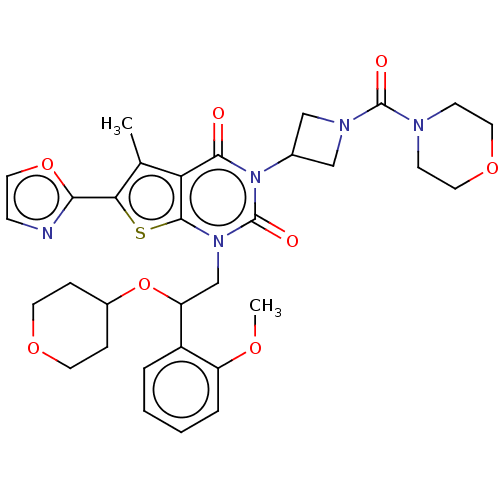 (US11186587, Example 12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50562563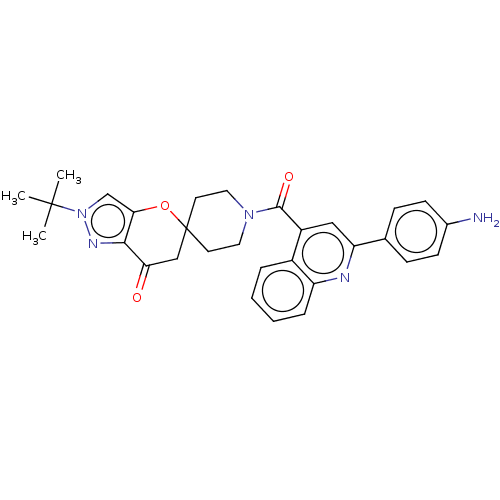 (CHEMBL4777068) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal His-tagged ACC1 (1 to 2383 residues) expressed in baculovirus infected Sf9 insect cells preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113036 BindingDB Entry DOI: 10.7270/Q2GX4G85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527058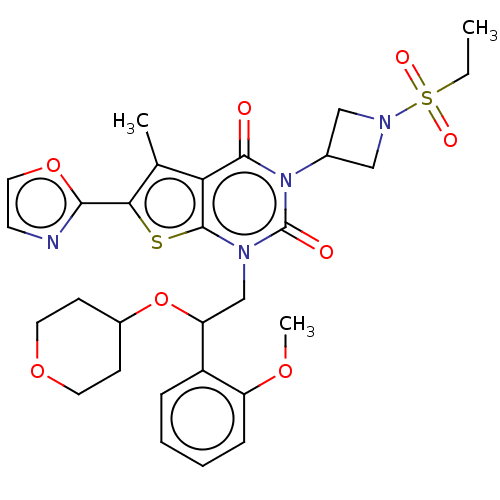 (US11186587, Example 10) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459203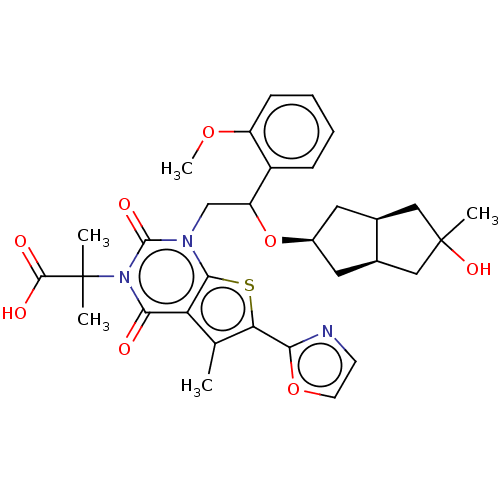 (2-[1-[2-[[(3aR,6aS)-5-hydroxy-5-methyl-2,3,3a,4,6,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459209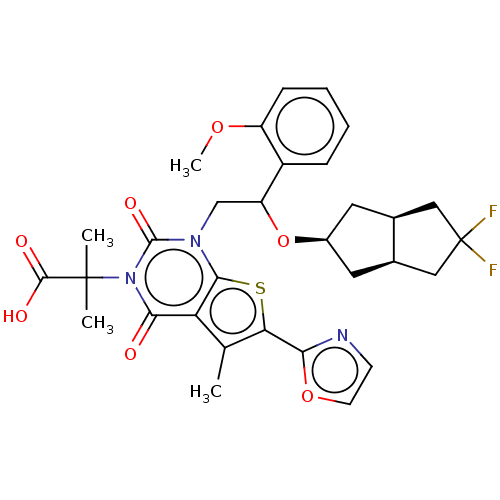 (2-[1-[2-[[(3aR,6aS)-5,5-difluoro-2,3,3a,4,6,6a-hex...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459212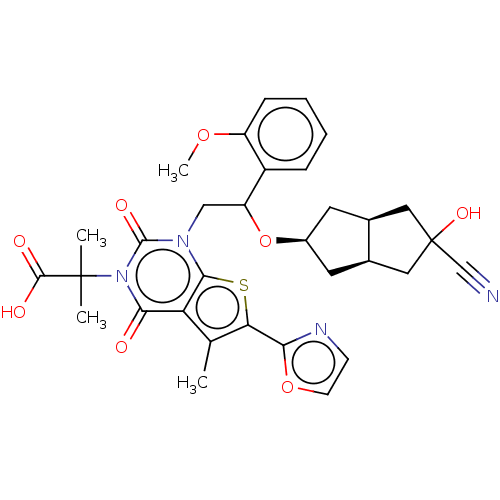 (2-[1-[2-[[(3aR,6aS)-5-cyano-5-hydroxy-2,3,3a,4,6,6...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527545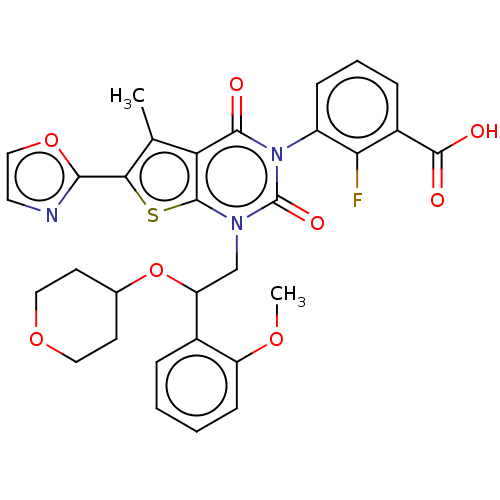 (US11186587, Example 28) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50529222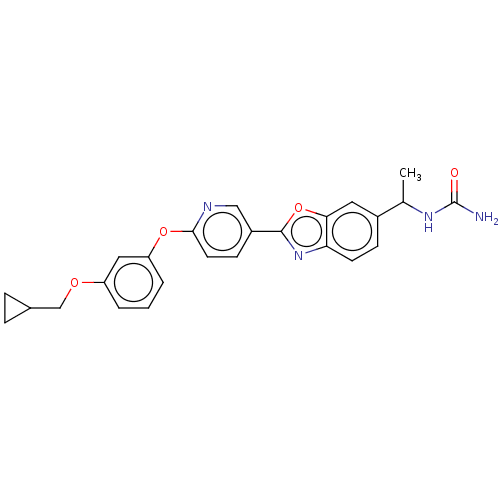 (CHEMBL4528475) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459210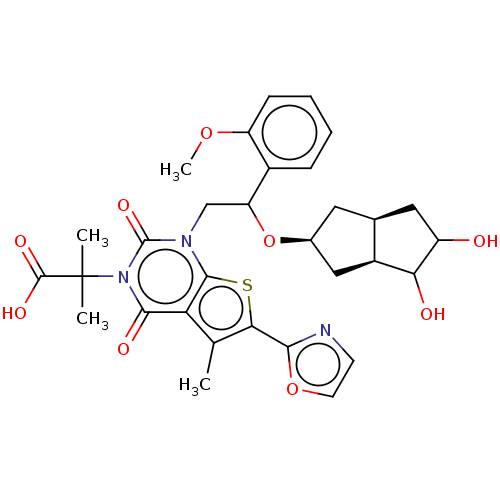 (2-[1-[2-[[(2S,3aS,6aR)-4,5-dihydroxy-1,2,3,3a,4,5,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459213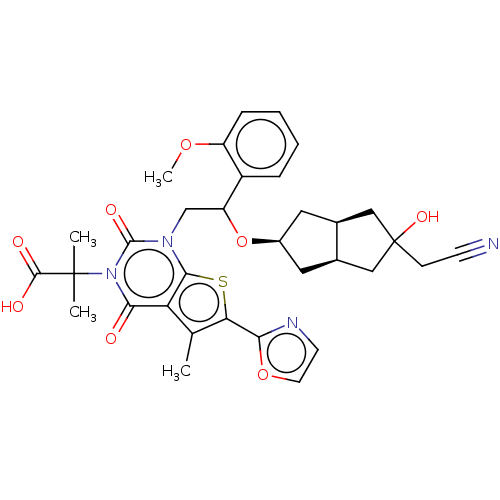 (2-[1-[2-[[(3aS,6aR)-5-(cyanomethyl)-5-hydroxy-2,3,...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527542 (US11186587, Example 25) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459204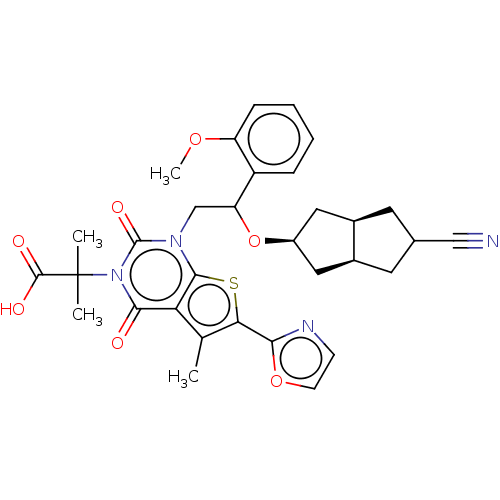 (2-[1-[2-[(5-cyano-1,2,3,3a,4,5,6,6a-octahydropenta...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527094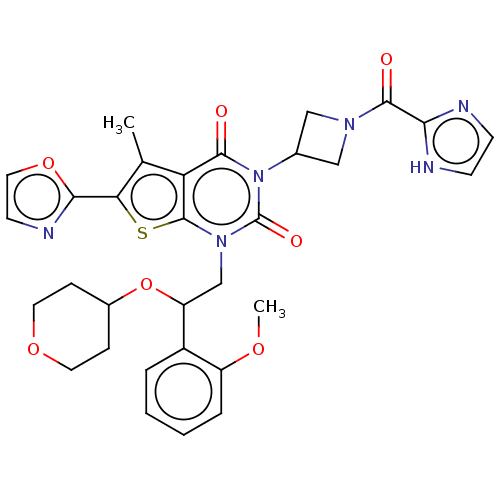 (US11186587, Example 14) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.69 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50069756 (CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ACC1 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM459202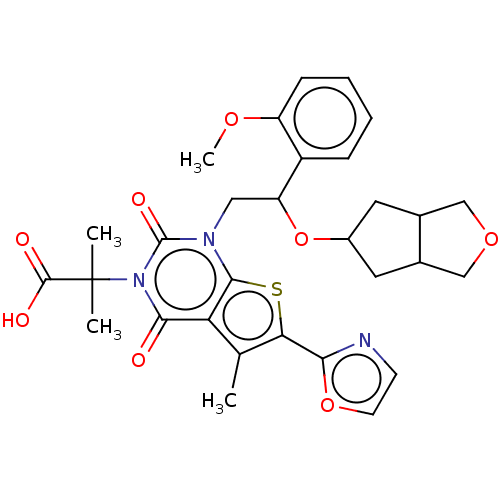 (2-[1-[2-(3,3a,4,5,6,6a-hexahydro-1H-cyclopenteno[c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC1 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527050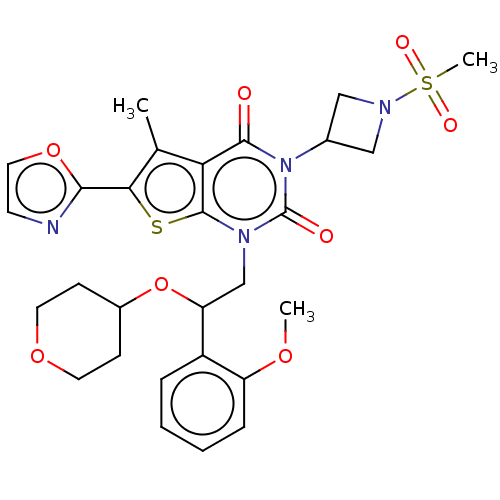 (US11186587, Example 9) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.03 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50529224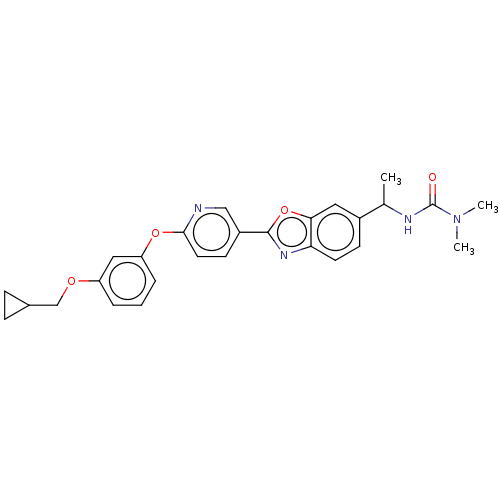 (CHEMBL4435891) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins follo... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM527546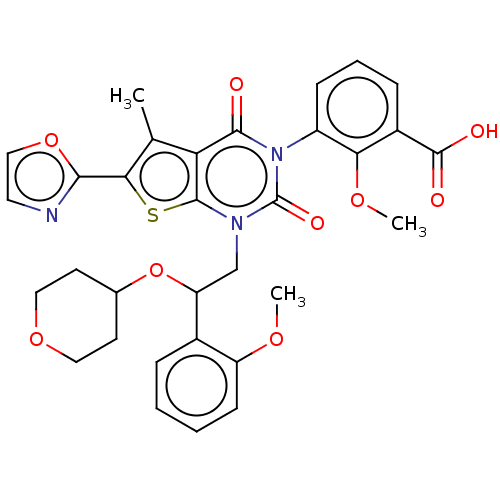 (US11186587, Example 29) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description 4.5 μL of 2.2×ACC1 enzyme (2 nM) working solution was added to a 384-well plate; and then 0.5 μL of different concentrations of compound we... | Citation and Details BindingDB Entry DOI: 10.7270/Q25H7KFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247080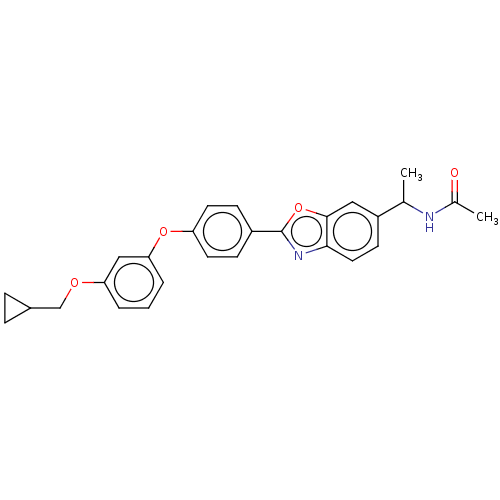 (CHEMBL4098647) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50426521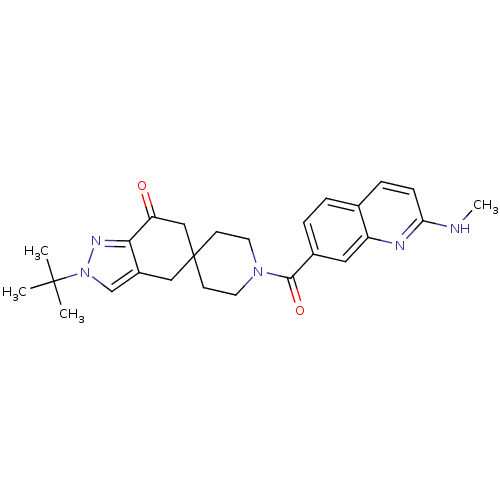 (CHEMBL2323626) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC1 | ACS Med Chem Lett 4: 16-7 (2013) Article DOI: 10.1021/ml3004044 BindingDB Entry DOI: 10.7270/Q2833TBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50562555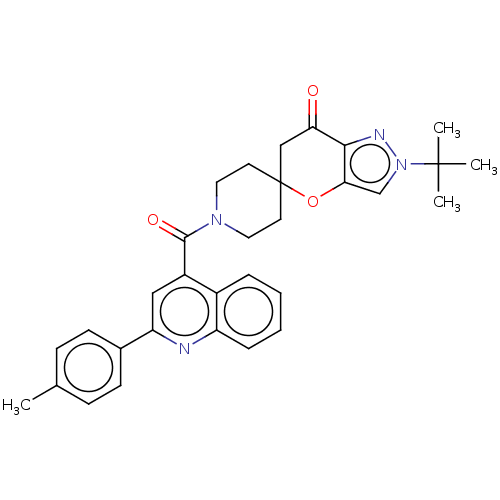 (CHEMBL4792495) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human C-terminal His-tagged ACC1 (1 to 2383 residues) expressed in baculovirus infected Sf9 insect cells preincubated for 1... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113036 BindingDB Entry DOI: 10.7270/Q2GX4G85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 955 total ) | Next | Last >> |