

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419721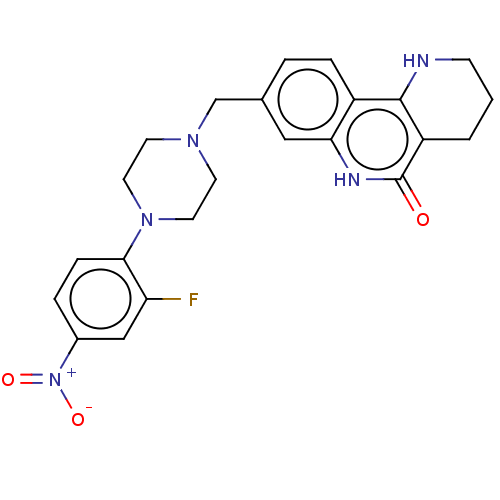 (US10464919, Example 59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419799 (US10464919, Example 137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50204580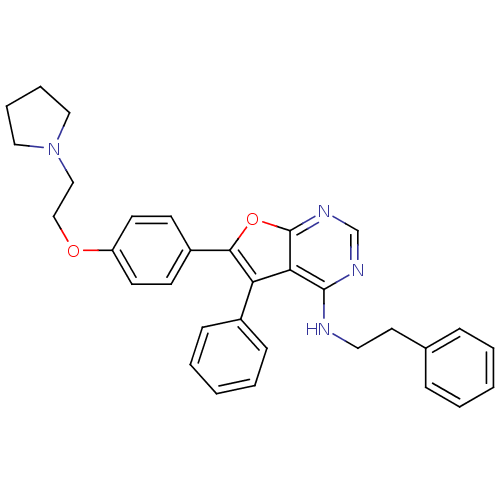 (CHEMBL247667 | N-phenethyl-5-phenyl-6-(4-(2-(pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Ack1 | Bioorg Med Chem Lett 17: 2305-9 (2007) Article DOI: 10.1016/j.bmcl.2007.01.057 BindingDB Entry DOI: 10.7270/Q2697365 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419844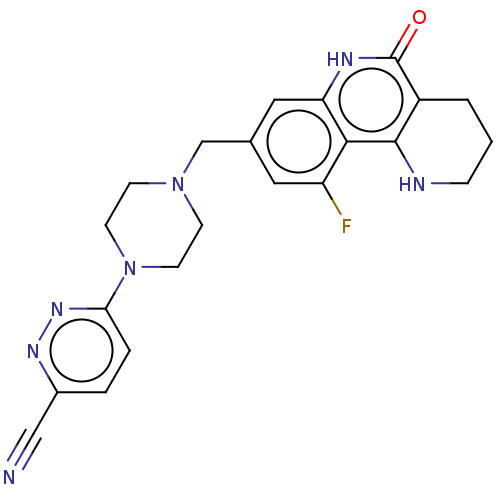 (US10464919, Example 182) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419794 (US10464919, Example 132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419731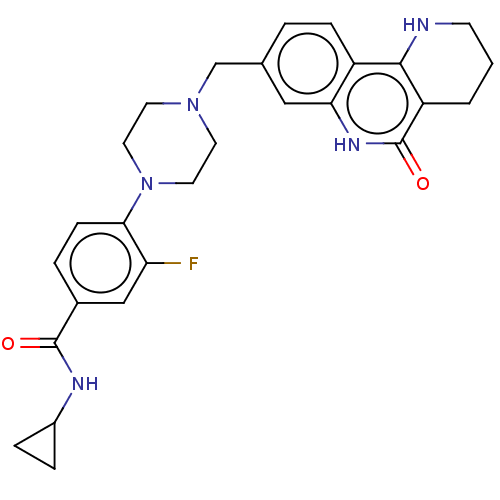 (US10464919, Example 69) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419781 (US10464919, Example 119) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419751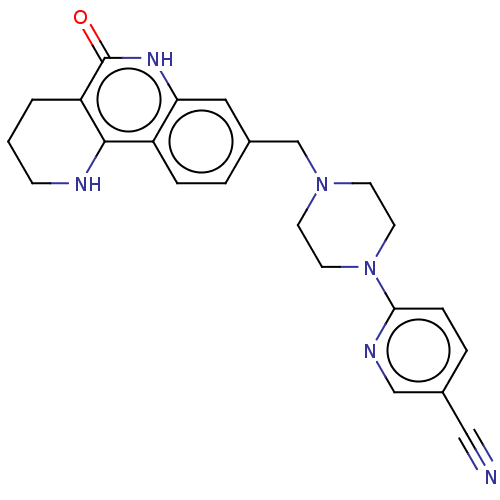 (US10464919, Example 89) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419791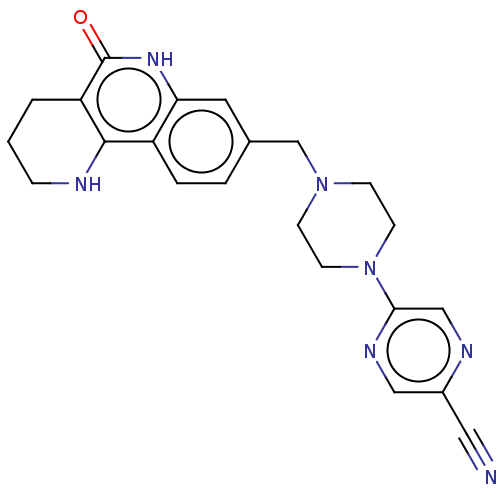 (US10464919, Example 129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.65 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419758 (US10464919, Example 96) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419797 (US10464919, Example 135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419740 (US10464919, Example 78) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.87 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419832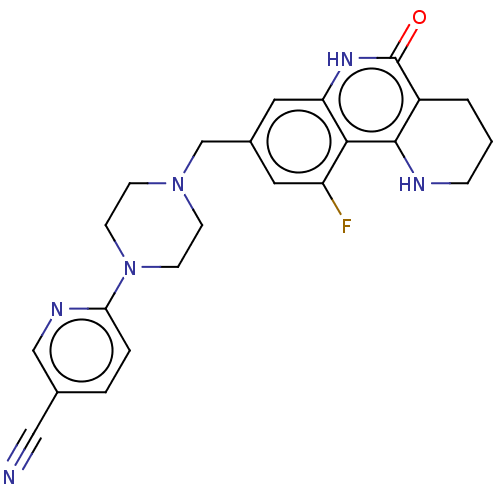 (US10464919, Example 170) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.96 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419755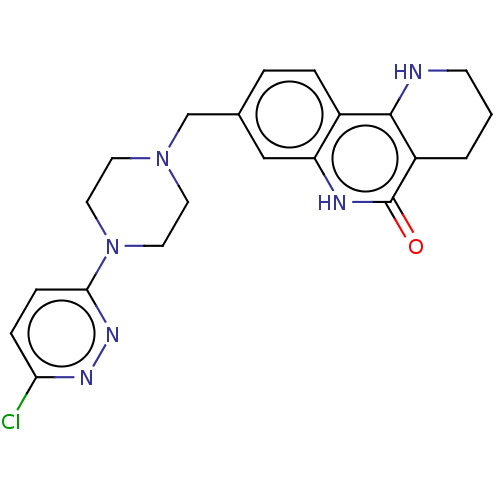 (US10464919, Example 93) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419761 (US10464919, Example 99) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50246164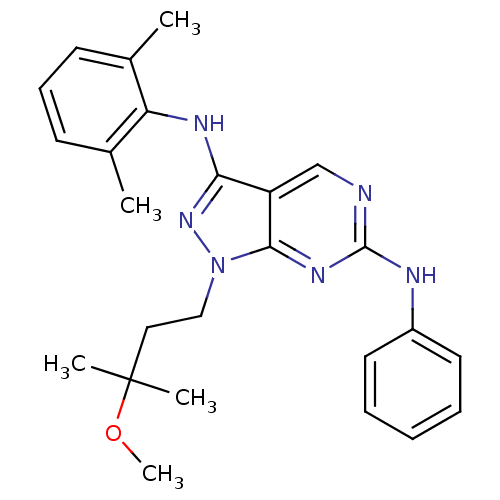 (CHEMBL487242 | N3-(2,6-dimethylphenyl)-1-(3-methox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Inhibition of ACK1 kinase (unknown origin) | J Med Chem 58: 2746-63 (2015) Article DOI: 10.1021/jm501929n BindingDB Entry DOI: 10.7270/Q2H996XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419765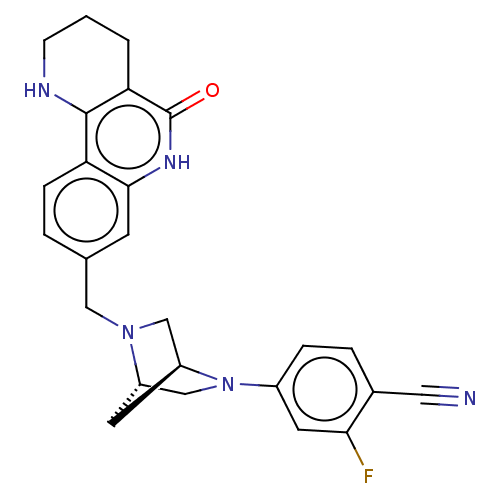 (US10464919, Example 103) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419842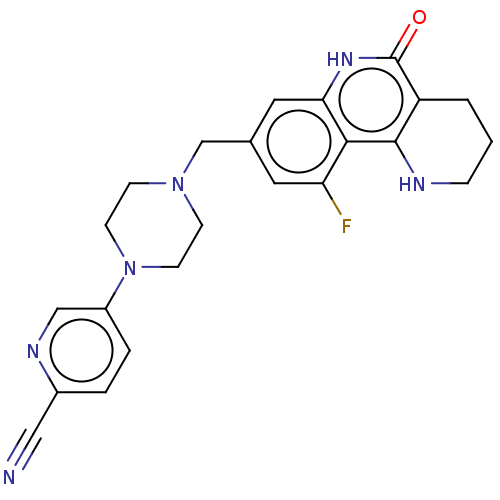 (US10464919, Example 180) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419784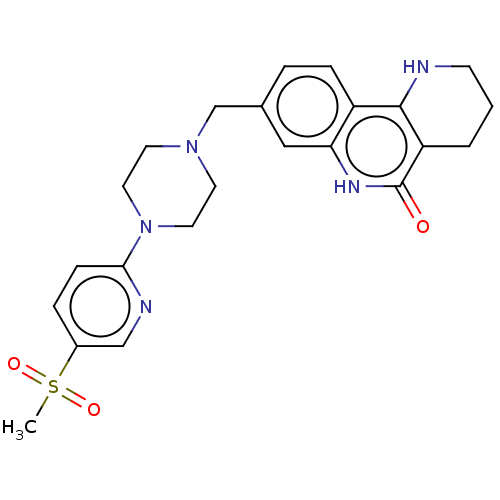 (US10464919, Example 122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.37 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419785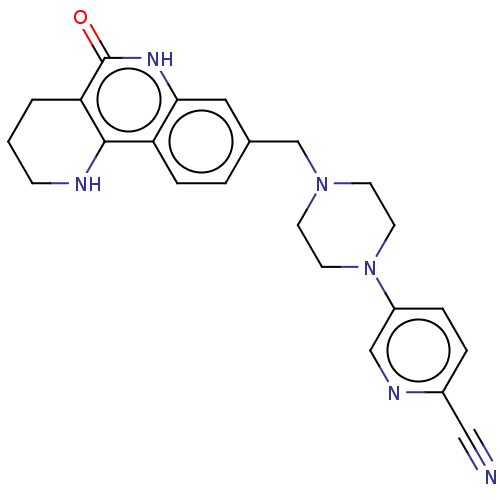 (US10464919, Example 123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419804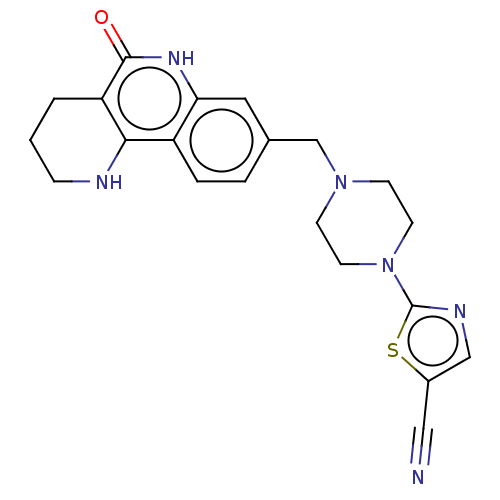 (US10464919, Example 142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419803 (US10464919, Example 141) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419796 (US10464919, Example 134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM4552 (4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Inhibition of ACK1 kinase (unknown origin) | J Med Chem 58: 2746-63 (2015) Article DOI: 10.1021/jm501929n BindingDB Entry DOI: 10.7270/Q2H996XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM4552 (4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of TNK2 | Leukemia 23: 477-85 (2009) Article DOI: 10.1038/leu.2008.334 BindingDB Entry DOI: 10.7270/Q22Z15R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419793 (US10464919, Example 131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.81 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419738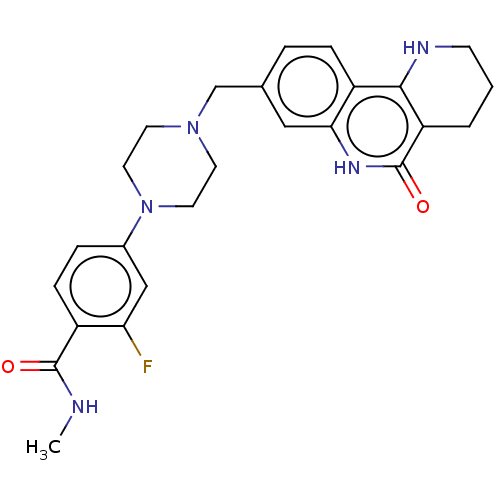 (US10464919, Example 76) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419787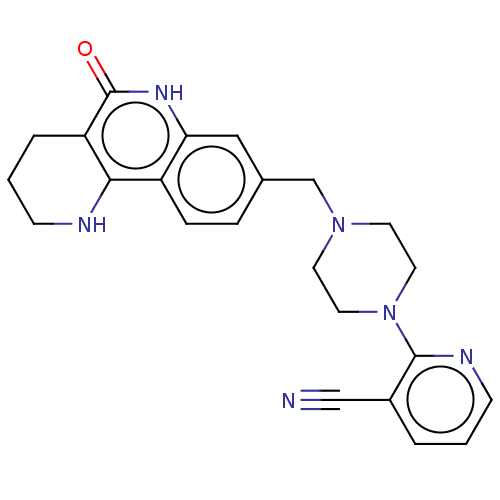 (US10464919, Example 125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419807 (US10464919, Example 145) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.24 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419726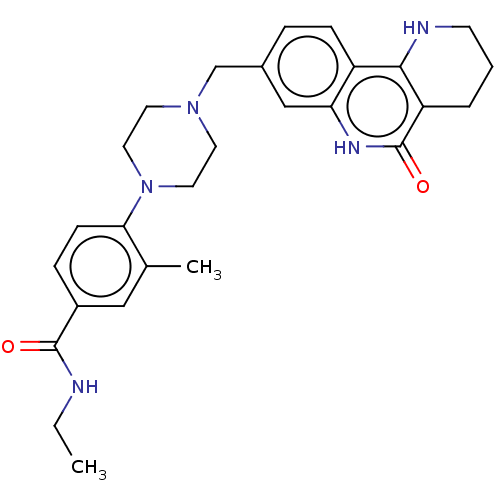 (US10464919, Example 64) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419775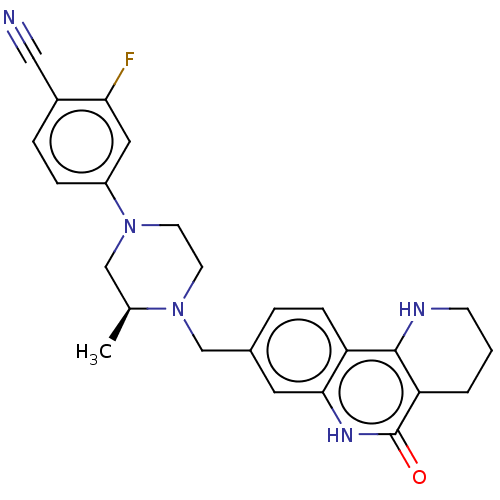 (US10464919, Example 113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.49 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419759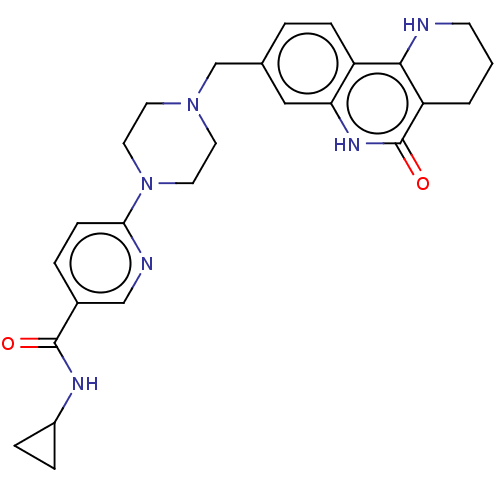 (US10464919, Example 97) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419718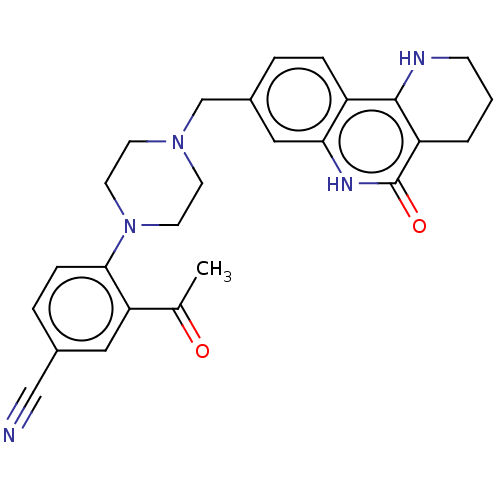 (US10464919, Example 56) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.78 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419723 (US10464919, Example 61) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50539626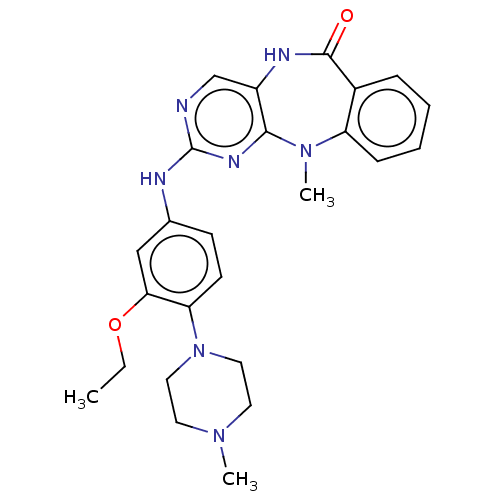 (CHEMBL4642708) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of tracer 236 binding to recombinant human GST-tagged TNK2 catalytic domain (110 to 476 residues) expressed in baculovirus expression syst... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126948 BindingDB Entry DOI: 10.7270/Q28G8Q7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50539636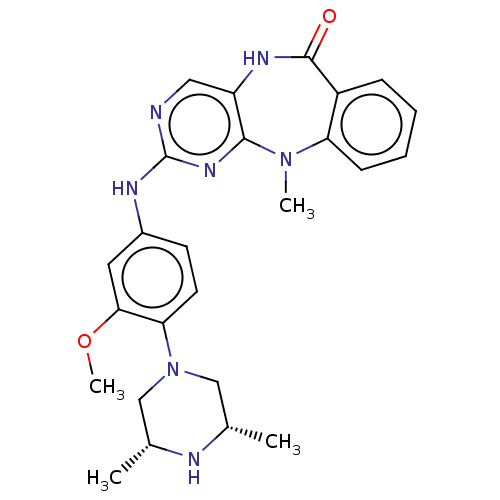 (CHEMBL4641234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of tracer 236 binding to recombinant human GST-tagged TNK2 catalytic domain (110 to 476 residues) expressed in baculovirus expression syst... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126948 BindingDB Entry DOI: 10.7270/Q28G8Q7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50539625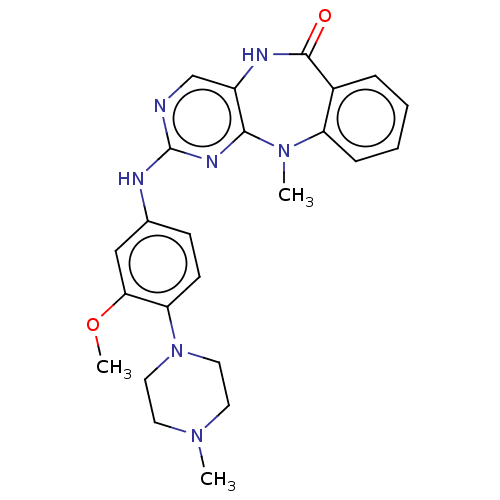 (CHEMBL4640664) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of tracer 236 binding to recombinant human GST-tagged TNK2 catalytic domain (110 to 476 residues) expressed in baculovirus expression syst... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126948 BindingDB Entry DOI: 10.7270/Q28G8Q7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419727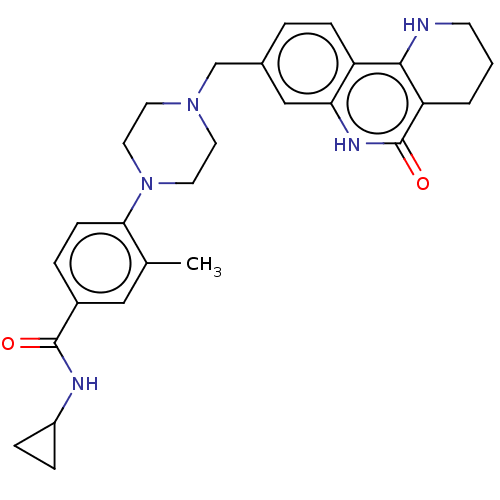 (US10464919, Example 65) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419729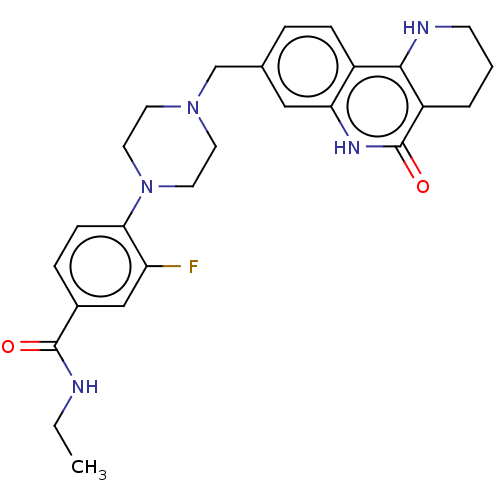 (US10464919, Example 67) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419822 (US10464919, Example 160) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419786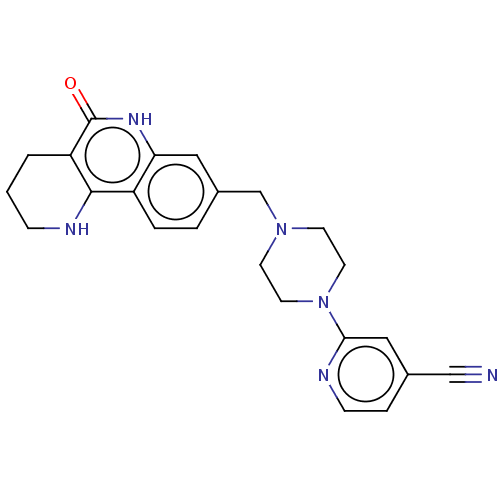 (US10464919, Example 124) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.71 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419782 (US10464919, Example 120) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.82 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419725 (US10464919, Example 63) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.96 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50459203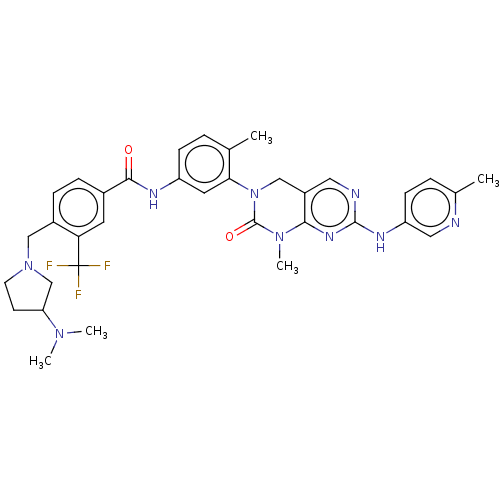 (CHEMBL4207036) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University Curated by ChEMBL | Assay Description Inhibition of ACK1 (unknown origin) by radiometric biochemical kinase assay | J Med Chem 61: 8353-8373 (2018) Article DOI: 10.1021/acs.jmedchem.8b00882 BindingDB Entry DOI: 10.7270/Q2T72M31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM50539627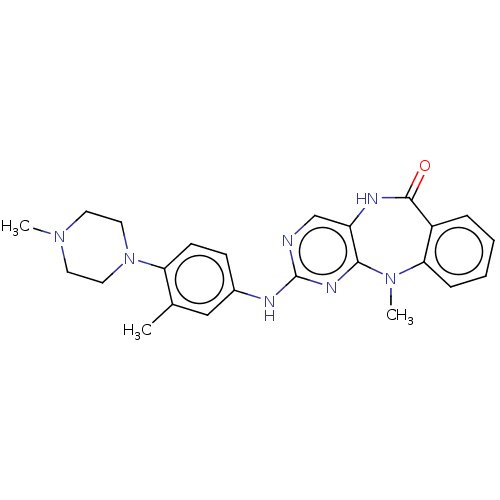 (CHEMBL4646285) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of tracer 236 binding to recombinant human GST-tagged TNK2 catalytic domain (110 to 476 residues) expressed in baculovirus expression syst... | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.126948 BindingDB Entry DOI: 10.7270/Q28G8Q7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419812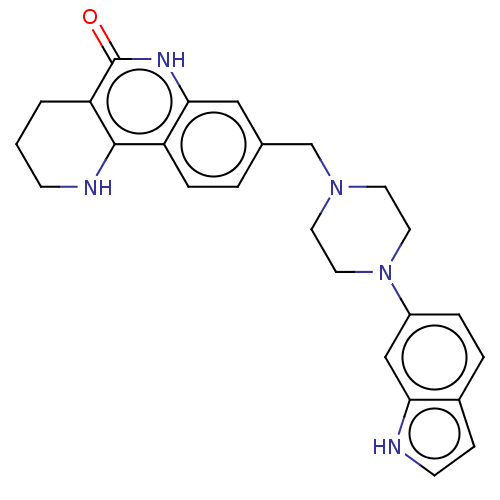 (US10464919, Example 150) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419769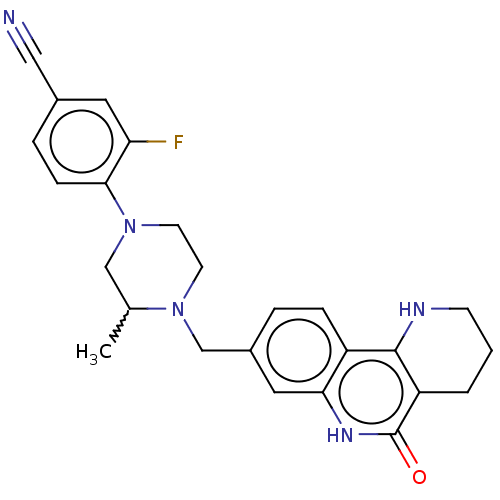 (US10464919, Example 107) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419722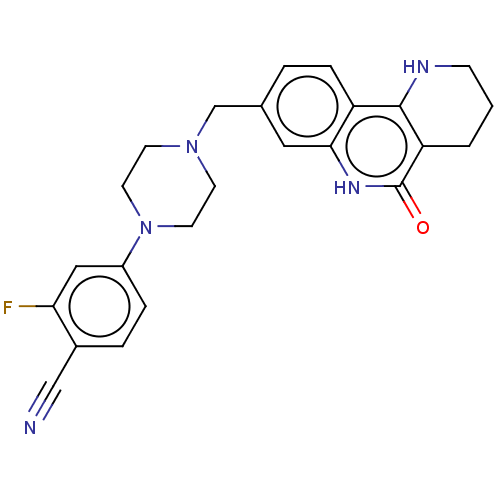 (US10464919, Example 60) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.47 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419820 (US10464919, Example 158) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.59 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activated CDC42 kinase 1 (Homo sapiens (Human)) | BDBM419811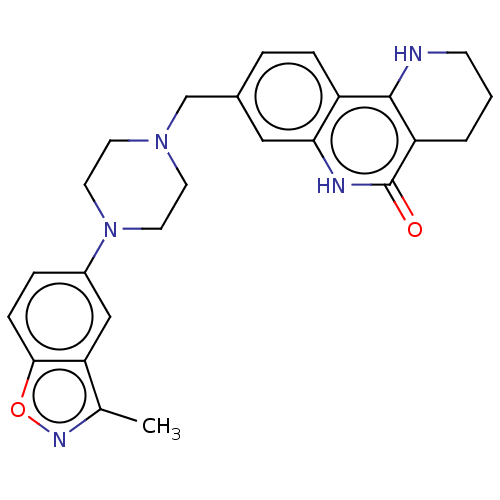 (US10464919, Example 149) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.99 | n/a | n/a | n/a | n/a | n/a | n/a |
Je Il Pharmaceutical Co., Ltd. US Patent | Assay Description The tankyrase-1 or tankyrase-2 enzyme inhibitory activities of the compounds of the present invention were assayed in the following manner by use of ... | US Patent US10464919 (2019) BindingDB Entry DOI: 10.7270/Q2736T8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 688 total ) | Next | Last >> |