

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50203953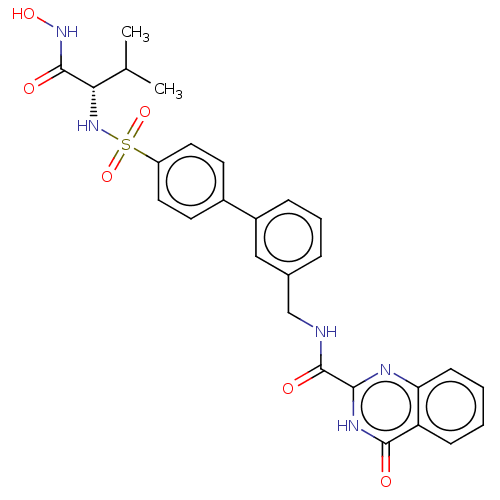 (CHEMBL3932562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50204002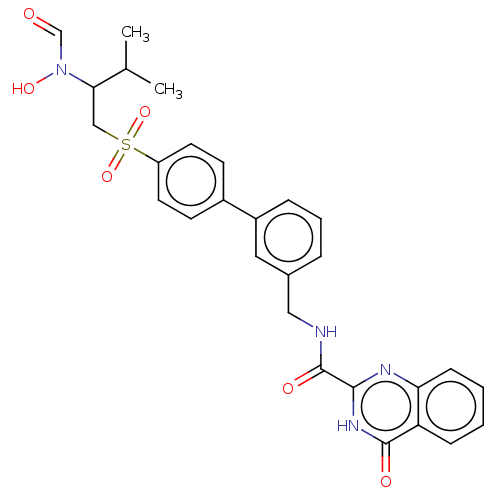 (CHEMBL3889936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM11863 (4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate after 40 mins by spectrofluorimetry | J Med Chem 57: 8886-902 (2014) Article DOI: 10.1021/jm500981k BindingDB Entry DOI: 10.7270/Q28P628P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM11863 (4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant MMP10 using fluorescence peptide Cy3-PLGLK(Cy5Q)AR-NH2 substrate by fluorescence assay | Bioorg Med Chem 22: 5487-505 (2014) Article DOI: 10.1016/j.bmc.2014.07.025 BindingDB Entry DOI: 10.7270/Q27S7QBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM11863 (4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-10 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric ... | J Med Chem 60: 608-626 (2017) Article DOI: 10.1021/acs.jmedchem.6b01007 BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM11863 (4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50203956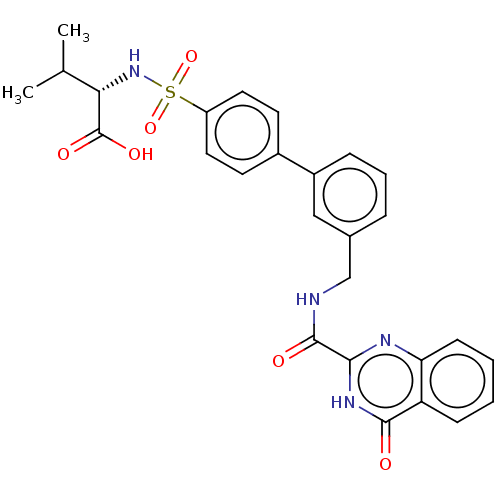 (CHEMBL3971135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50203961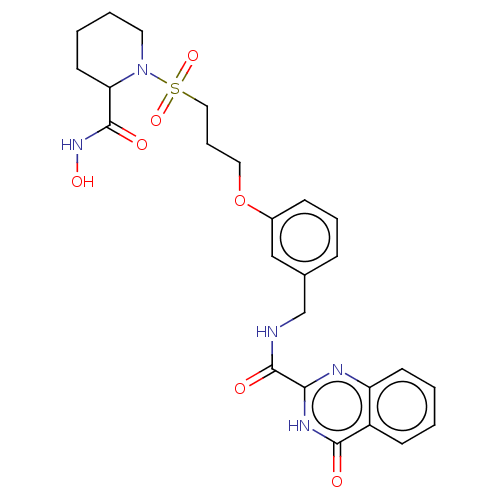 (CHEMBL3958969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078191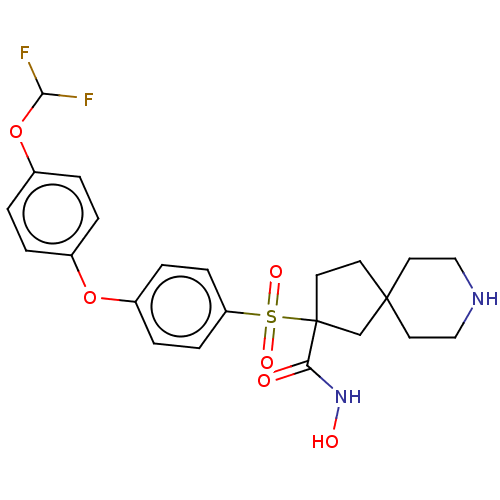 (CHEMBL3417750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078196 (CHEMBL3417745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Navarra Curated by ChEMBL | Assay Description Inhibition of MMP10 (unknown origin) using fluorescently-labeled substrate measured every min for 1 min | ACS Med Chem Lett 9: 428-433 (2018) Article DOI: 10.1021/acsmedchemlett.7b00549 BindingDB Entry DOI: 10.7270/Q2BK1FXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078196 (CHEMBL3417745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50203959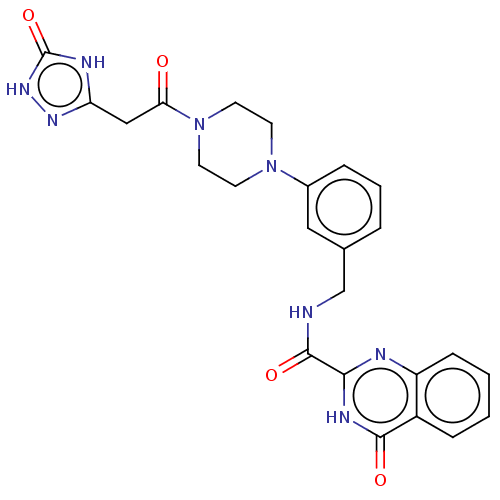 (CHEMBL3955430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078193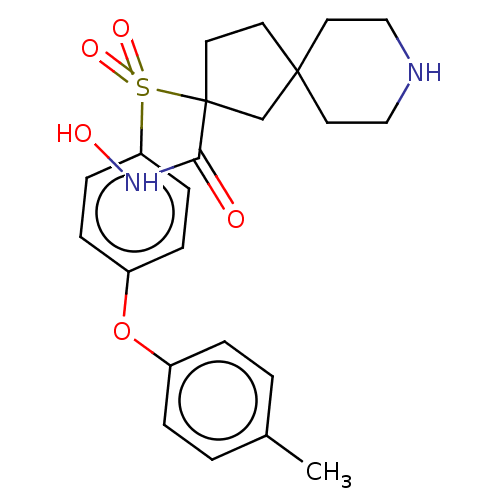 (CHEMBL3417748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50592903 (CHEMBL5199189) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01855 BindingDB Entry DOI: 10.7270/Q2GB281X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078175 (CHEMBL3417766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50203962 (CHEMBL3917494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50204009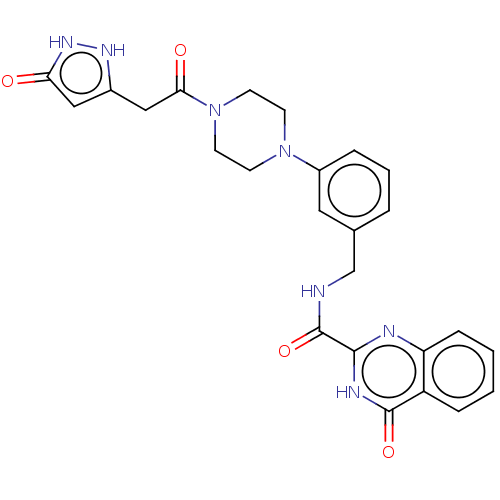 (CHEMBL3927817) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50203963 (CHEMBL3907881) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078171 (CHEMBL3417770) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078199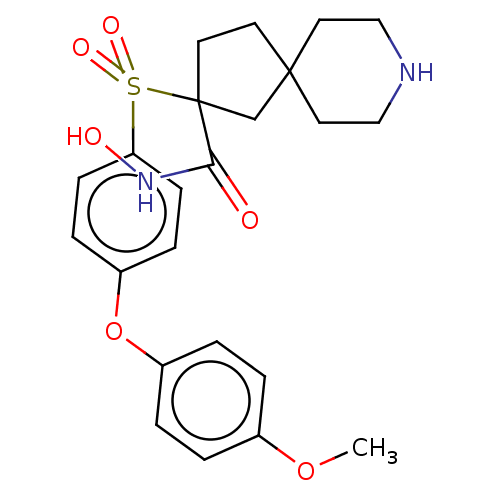 (CHEMBL3417742) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078189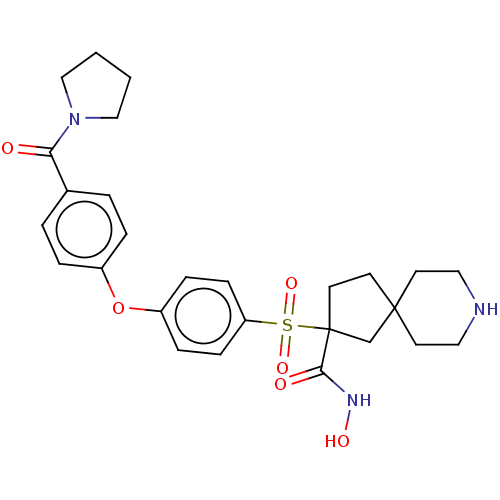 (CHEMBL3417752) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078186 (CHEMBL3417755) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078176 (CHEMBL3417765) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078177 (CHEMBL3417764) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078172 (CHEMBL3417769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078174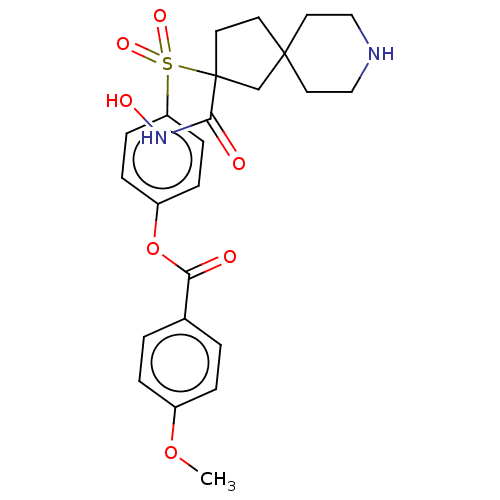 (CHEMBL3417767) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078190 (CHEMBL3417751) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50233892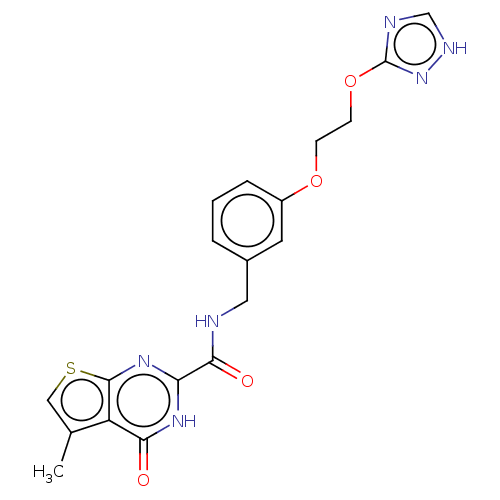 (CHEMBL4068241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-10 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric ... | J Med Chem 60: 608-626 (2017) Article DOI: 10.1021/acs.jmedchem.6b01007 BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50250161 (CHEMBL4071820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Biotechnology, Center for Organic and Medicinal Chemistry, ZHAW Zurich University of Applied Sciences , Einsiedlerstrasse 31, 8820 Wädenswil, Switzerland. Curated by ChEMBL | Assay Description Inhibition of MMP10 (unknown origin) assessed using (5-FAM/QX) FRET peptide as substrate by fluorescence assay | J Med Chem 60: 9585-9598 (2017) Article DOI: 10.1021/acs.jmedchem.7b01001 BindingDB Entry DOI: 10.7270/Q280551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50250161 (CHEMBL4071820) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Biotechnology, Center for Organic and Medicinal Chemistry, ZHAW Zurich University of Applied Sciences , Einsiedlerstrasse 31, 8820 Wädenswil, Switzerland. Curated by ChEMBL | Assay Description Inhibition of MMP10 (unknown origin) assessed using (5-FAM/QX) FRET peptide as substrate by fluorescence assay | J Med Chem 60: 9585-9598 (2017) Article DOI: 10.1021/acs.jmedchem.7b01001 BindingDB Entry DOI: 10.7270/Q280551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078195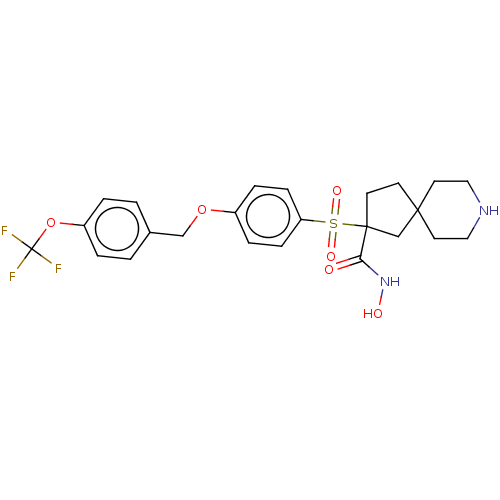 (CHEMBL3417746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50203957 (CHEMBL3900916) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078192 (CHEMBL3417749) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50592901 (CHEMBL5176328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01855 BindingDB Entry DOI: 10.7270/Q2GB281X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50592902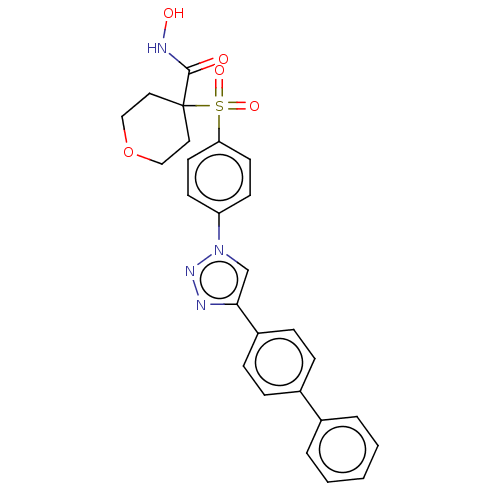 (CHEMBL5198463) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01855 BindingDB Entry DOI: 10.7270/Q2GB281X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078181 (CHEMBL3417760) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50233877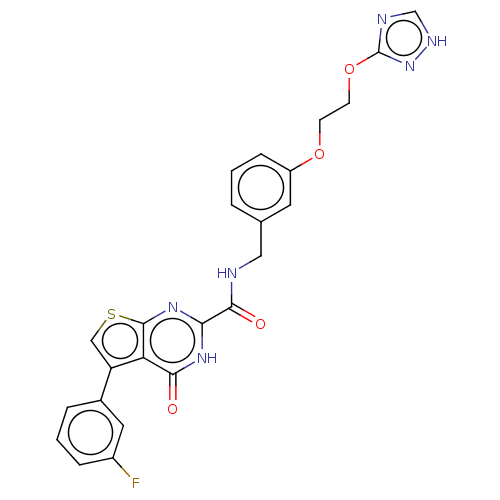 (CHEMBL4068176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-10 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric ... | J Med Chem 60: 608-626 (2017) Article DOI: 10.1021/acs.jmedchem.6b01007 BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078187 (CHEMBL3417754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50204005 (CHEMBL3907007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human AMPA-activated MMP10 using Cy3-PLGLK(Cy5Q)AR-NH2 as substrate measured after 40 mins by spectrofluorimetric method | Bioorg Med Chem 24: 6149-6165 (2016) Article DOI: 10.1016/j.bmc.2016.09.009 BindingDB Entry DOI: 10.7270/Q2H1341V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078180 (CHEMBL3417761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078200 (CHEMBL3417741) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078184 (CHEMBL3417757) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50078173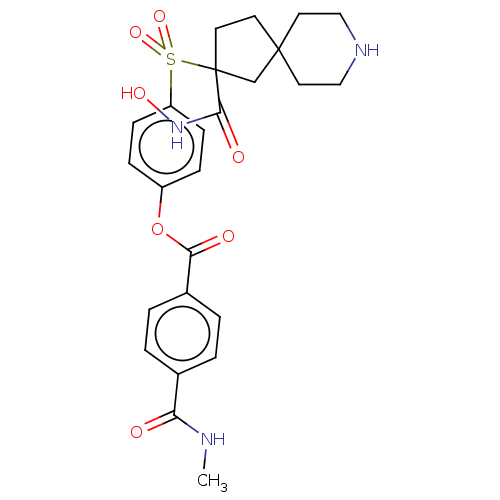 (CHEMBL3417768) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Navarra Curated by ChEMBL | Assay Description Inhibition of MMP-10 (unknown origin) measured every minute for 1 hr by fluorescence assay | J Med Chem 58: 2465-88 (2015) Article DOI: 10.1021/jm501940y BindingDB Entry DOI: 10.7270/Q28C9XXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50250175 (CHEMBL4080183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Biotechnology, Center for Organic and Medicinal Chemistry, ZHAW Zurich University of Applied Sciences , Einsiedlerstrasse 31, 8820 Wädenswil, Switzerland. Curated by ChEMBL | Assay Description Inhibition of MMP10 (unknown origin) assessed using (5-FAM/QX) FRET peptide as substrate by fluorescence assay | J Med Chem 60: 9585-9598 (2017) Article DOI: 10.1021/acs.jmedchem.7b01001 BindingDB Entry DOI: 10.7270/Q280551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50250175 (CHEMBL4080183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Biotechnology, Center for Organic and Medicinal Chemistry, ZHAW Zurich University of Applied Sciences , Einsiedlerstrasse 31, 8820 Wädenswil, Switzerland. Curated by ChEMBL | Assay Description Inhibition of MMP10 (unknown origin) assessed using (5-FAM/QX) FRET peptide as substrate by fluorescence assay | J Med Chem 60: 9585-9598 (2017) Article DOI: 10.1021/acs.jmedchem.7b01001 BindingDB Entry DOI: 10.7270/Q280551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50250162 (CHEMBL4098213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Biotechnology, Center for Organic and Medicinal Chemistry, ZHAW Zurich University of Applied Sciences , Einsiedlerstrasse 31, 8820 Wädenswil, Switzerland. Curated by ChEMBL | Assay Description Inhibition of MMP10 (unknown origin) assessed using (5-FAM/QX) FRET peptide as substrate by fluorescence assay | J Med Chem 60: 9585-9598 (2017) Article DOI: 10.1021/acs.jmedchem.7b01001 BindingDB Entry DOI: 10.7270/Q280551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50233900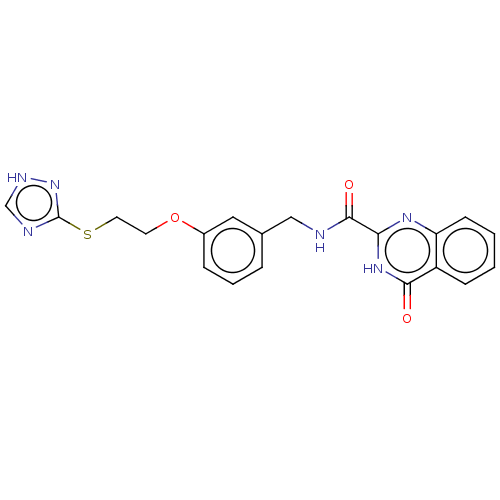 (CHEMBL4083954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-10 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric ... | J Med Chem 60: 608-626 (2017) Article DOI: 10.1021/acs.jmedchem.6b01007 BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50233888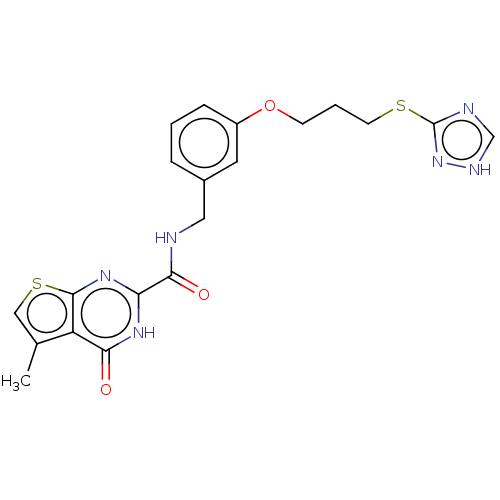 (CHEMBL4092215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of APMA-activated recombinant human MMP-10 using Cy3-PLGLK(Cy5Q)AR-NH2 peptide as substrate measured after 40 mins by spectrofluorimetric ... | J Med Chem 60: 608-626 (2017) Article DOI: 10.1021/acs.jmedchem.6b01007 BindingDB Entry DOI: 10.7270/Q2W95CF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50250162 (CHEMBL4098213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Biotechnology, Center for Organic and Medicinal Chemistry, ZHAW Zurich University of Applied Sciences , Einsiedlerstrasse 31, 8820 Wädenswil, Switzerland. Curated by ChEMBL | Assay Description Inhibition of MMP10 (unknown origin) assessed using (5-FAM/QX) FRET peptide as substrate by fluorescence assay | J Med Chem 60: 9585-9598 (2017) Article DOI: 10.1021/acs.jmedchem.7b01001 BindingDB Entry DOI: 10.7270/Q280551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM50250174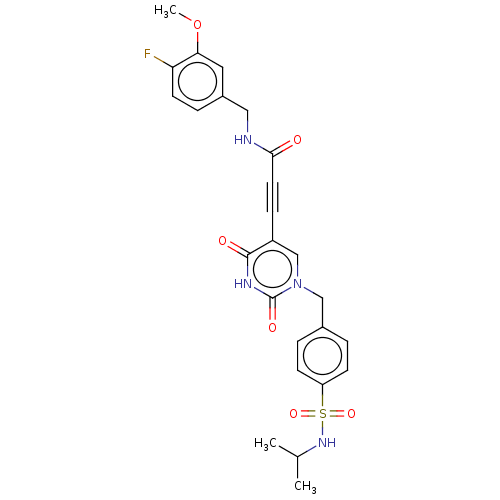 (CHEMBL4079271) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Chemistry and Biotechnology, Center for Organic and Medicinal Chemistry, ZHAW Zurich University of Applied Sciences , Einsiedlerstrasse 31, 8820 Wädenswil, Switzerland. Curated by ChEMBL | Assay Description Inhibition of MMP10 (unknown origin) assessed using (5-FAM/QX) FRET peptide as substrate by fluorescence assay | J Med Chem 60: 9585-9598 (2017) Article DOI: 10.1021/acs.jmedchem.7b01001 BindingDB Entry DOI: 10.7270/Q280551H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |