

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50128131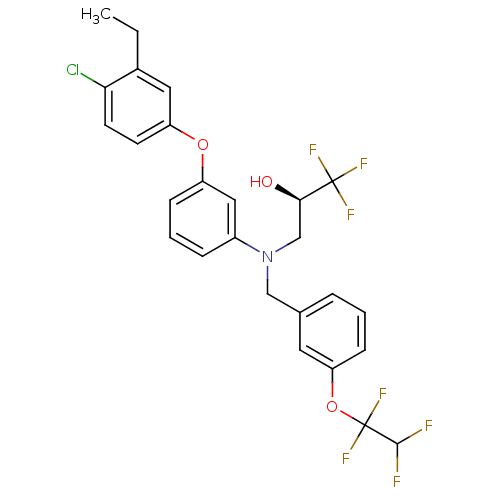 ((R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development) Curated by ChEMBL | Assay Description Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer with <1 nM [CETP] for 18 ... | J Med Chem 46: 2152-68 (2003) Article DOI: 10.1021/jm020528+ BindingDB Entry DOI: 10.7270/Q2QV3KWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516404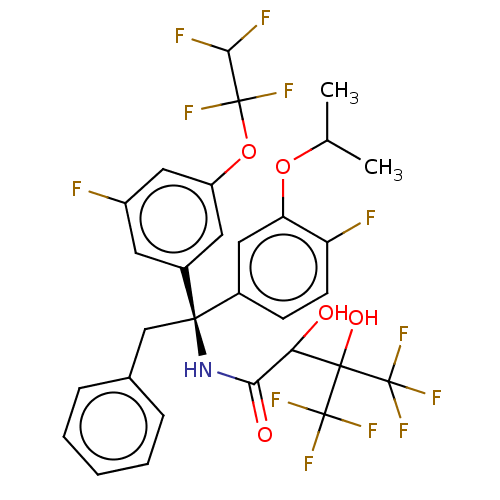 (CHEMBL4439823) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392514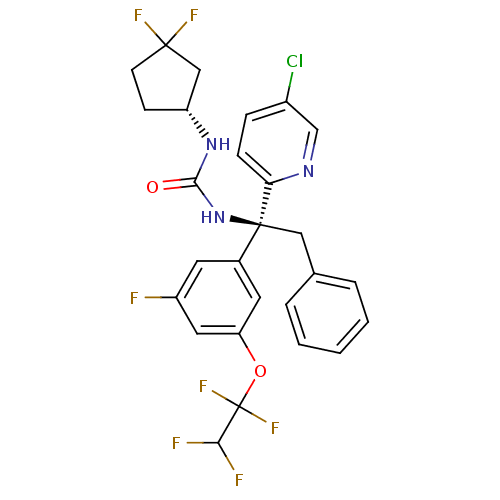 (CHEMBL2152167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178701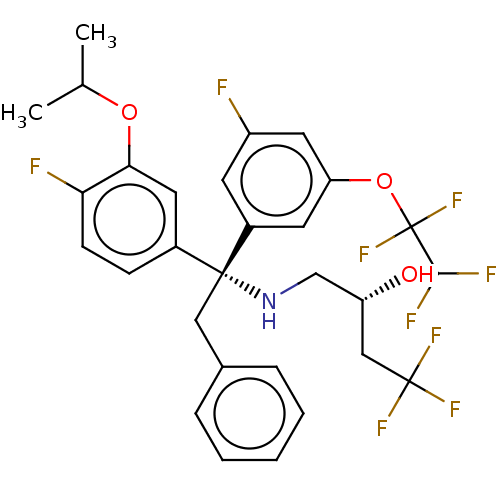 (CHEMBL3814374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254040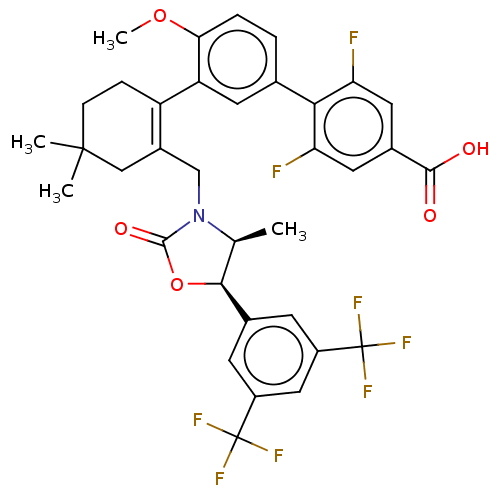 (US9493430, 593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178698 (CHEMBL3813720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254048 (US9493430, 618) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178699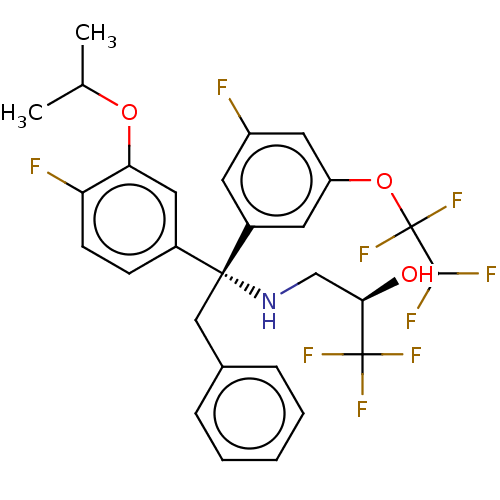 (CHEMBL3813836) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254058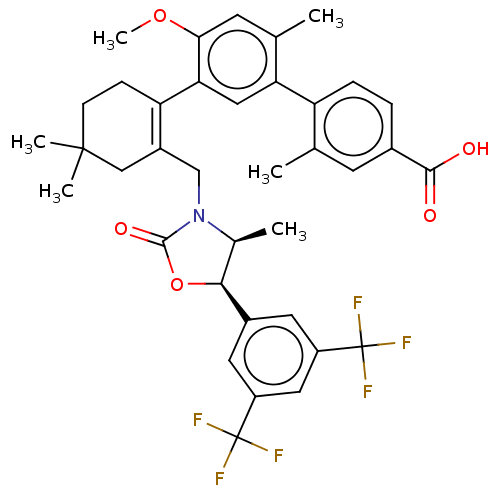 (US9493430, 684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254050 (US9493430, 631) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392527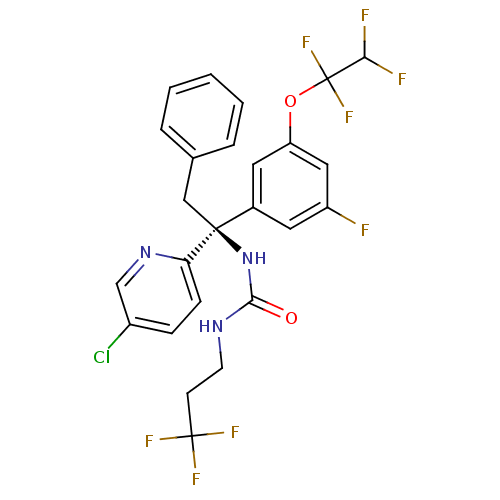 (CHEMBL2152180) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516410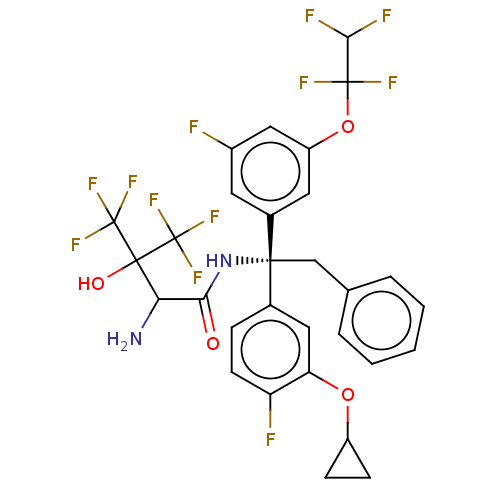 (CHEMBL4574163) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254054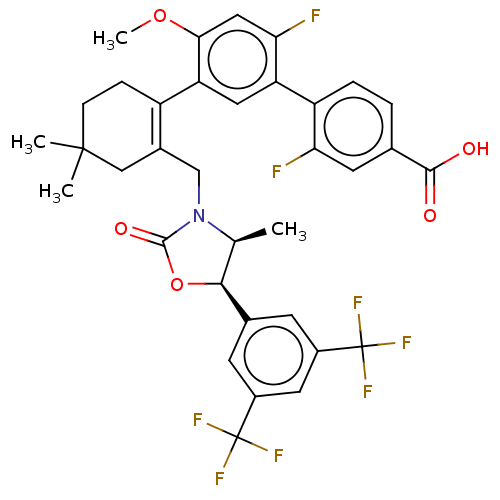 (US9493430, 661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178702 (CHEMBL3814963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50178700 (CHEMBL3814418) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]cholesteryl ester transfer from [3H]CE-HDL to biotinylated LDL by scintillation pro... | Bioorg Med Chem Lett 26: 3278-3281 (2016) Article DOI: 10.1016/j.bmcl.2016.05.058 BindingDB Entry DOI: 10.7270/Q2TQ63FN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392530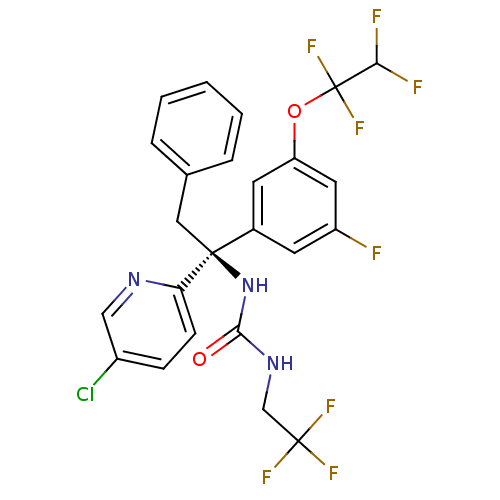 (CHEMBL2152183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516398 (CHEMBL4457286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50094519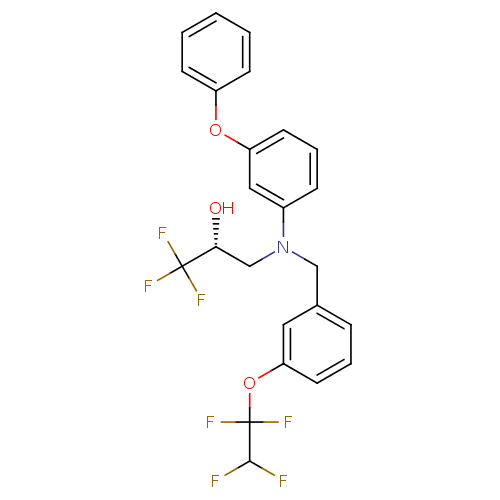 ((R)-1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human CETP by scintillation proximity assay | Bioorg Med Chem Lett 18: 2640-4 (2008) Article DOI: 10.1016/j.bmcl.2008.03.030 BindingDB Entry DOI: 10.7270/Q2V40W3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50312718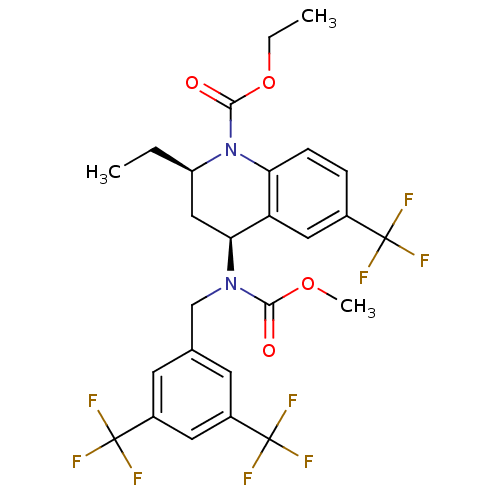 (CHEMBL479527 | torcetrapib) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG Curated by ChEMBL | Assay Description Inhibition of human plasma CETP assessed as [3H]cholesterol ester transfer after 18 hrs by scintillation proximity assay | Bioorg Med Chem Lett 20: 1740-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.071 BindingDB Entry DOI: 10.7270/Q28S4Q1P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50128164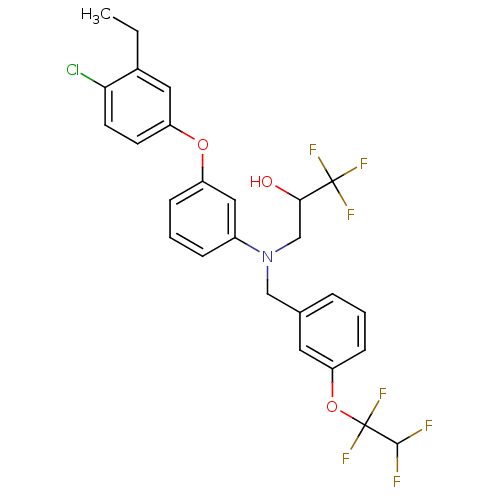 (3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CETP (unknown origin)-mediated transfer of [3H]cholesteryl ester from HDL donar particles to LDL acceptor particles in presence of buff... | J Med Chem 52: 1768-72 (2009) Article DOI: 10.1021/jm801319d BindingDB Entry DOI: 10.7270/Q2QZ29VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50128164 (3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of CETP (unknown origin)-mediated transfer of [3H]cholesteryl ester from HDL donar particles to LDL acceptor particles | J Med Chem 52: 1768-72 (2009) Article DOI: 10.1021/jm801319d BindingDB Entry DOI: 10.7270/Q2QZ29VG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234607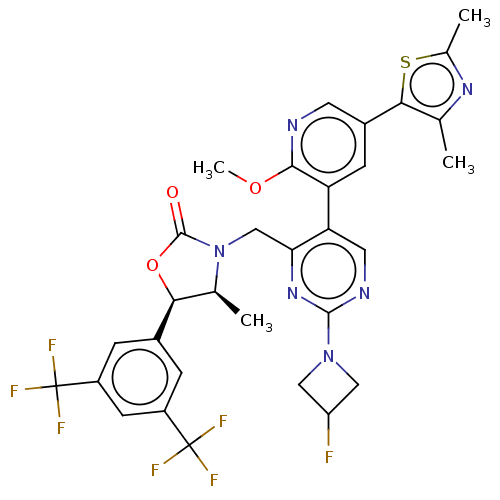 (US9353101, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234610 (US9353101, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234611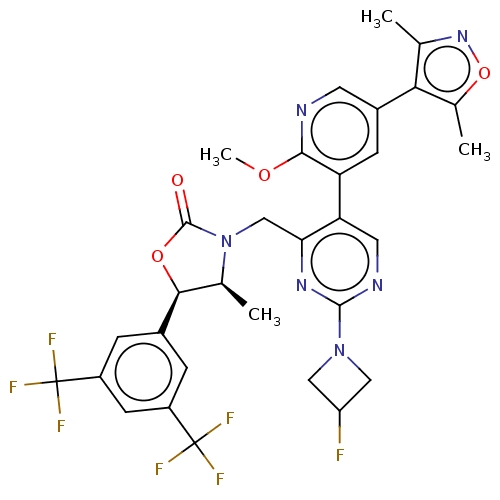 (US9353101, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254034 (US9493430, 574) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516405 (CHEMBL4552555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516406 (CHEMBL4469874) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392513 (CHEMBL2152166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234621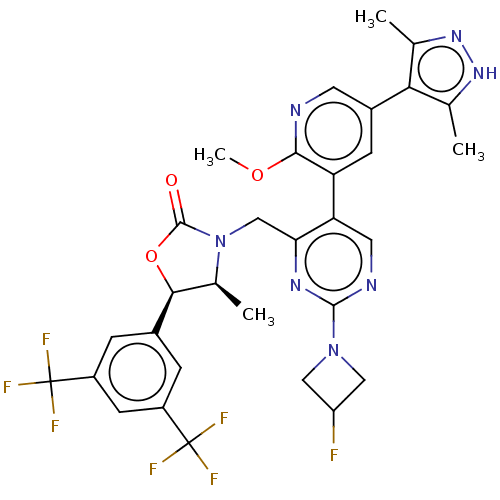 (US9353101, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234664 (US9353101, 60) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234661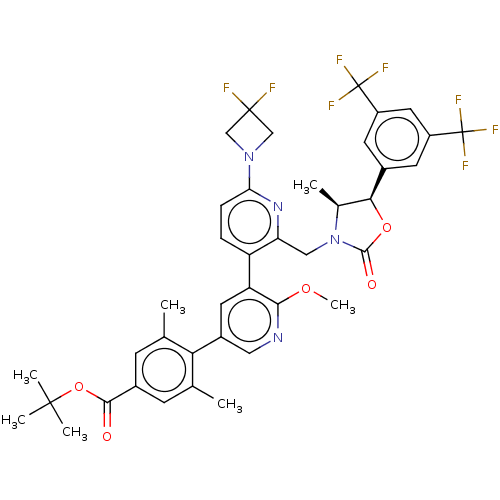 (US9353101, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234635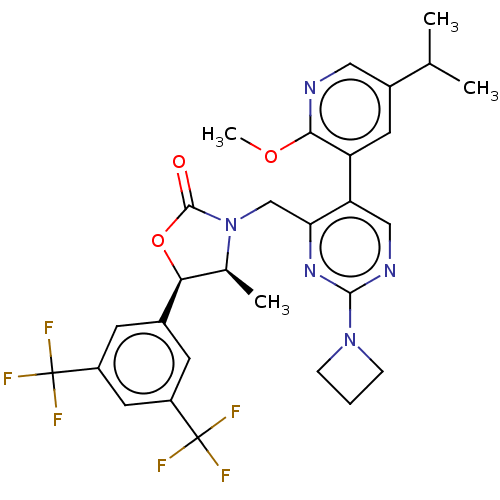 (US9353101, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254055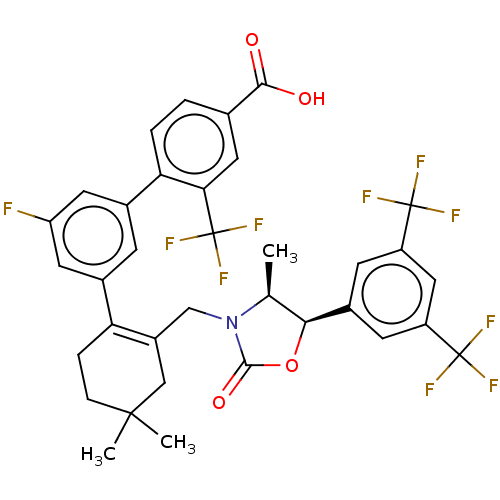 (US9493430, 668) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234632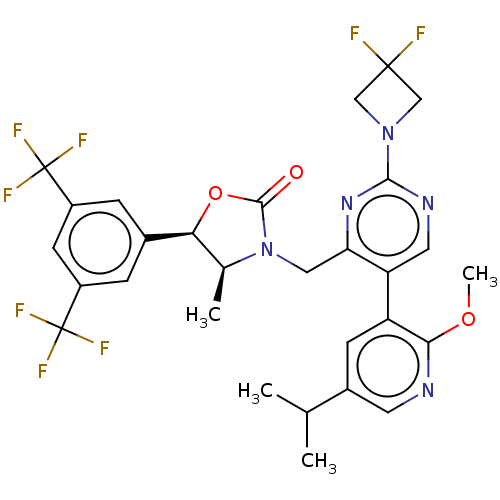 (US9353101, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254037 (US9493430, 584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254030 (US9493430, 557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254028 (US9493430, 554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254066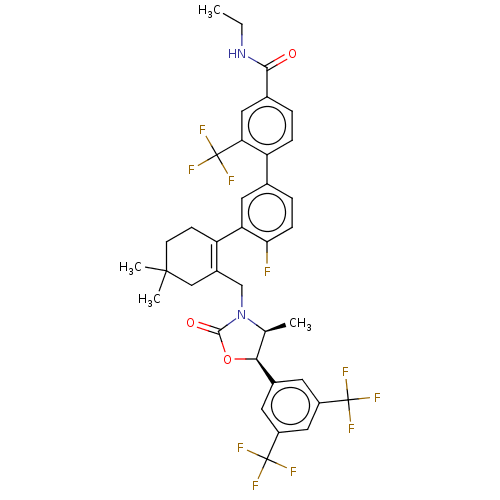 (US9493430, 756) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192305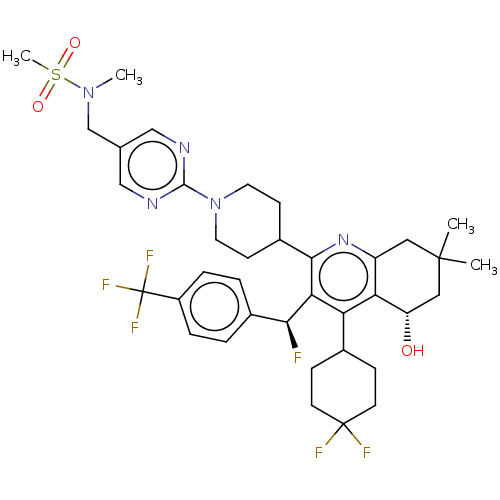 (US9187450, 51) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50516400 (CHEMBL4439856) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP assessed as reduction in [3H]CE/HDL transfer incubated for 4 hrs by scintillation proximity assay | ACS Med Chem Lett 10: 911-916 (2019) Article DOI: 10.1021/acsmedchemlett.9b00086 BindingDB Entry DOI: 10.7270/Q2TX3JQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392504 (CHEMBL2152158) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392512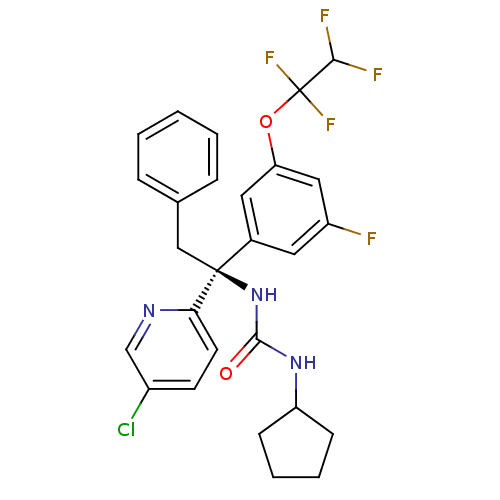 (CHEMBL2152165) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234666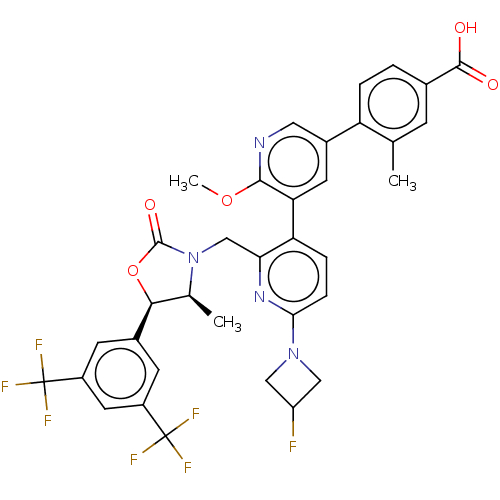 (US9353101, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM192306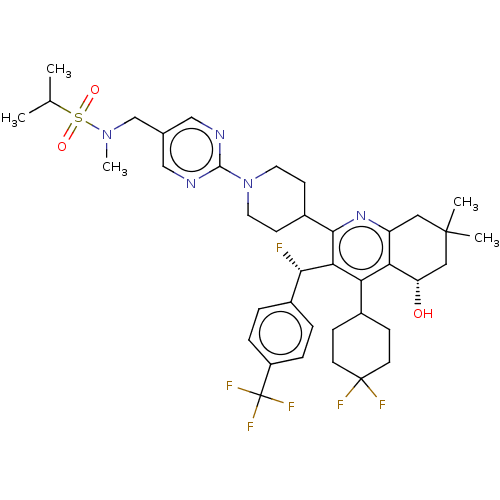 (US9187450, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | 37 |
Daiichi Sankyo Company, Limited US Patent | Assay Description A recombinant human CETP protein (manufactured by Roar Biomedical Inc.; 4.5 ng), the acceptor lipoprotein described in (2) above (32.5 ug) and 5,5'... | US Patent US9187450 (2015) BindingDB Entry DOI: 10.7270/Q29022KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392528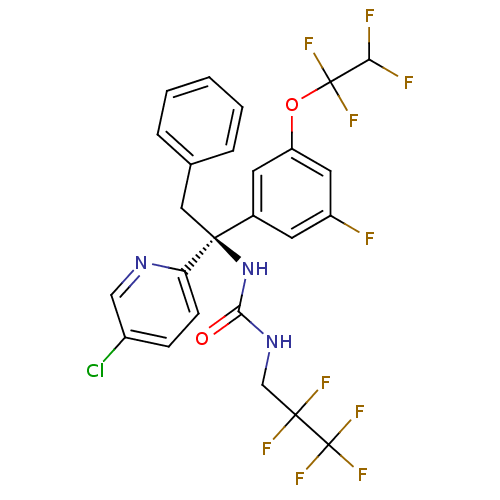 (CHEMBL2152181) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP using [3H]cholesterol ester/HDL as substrate by scintillation proximity assay | Bioorg Med Chem Lett 22: 6503-8 (2012) Article DOI: 10.1016/j.bmcl.2012.08.011 BindingDB Entry DOI: 10.7270/Q2XP761G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254060 (US9493430, 695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254057 (US9493430, 681) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50392512 (CHEMBL2152165) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human recombinant CETP-mediated [3H]cholesteryl ester transfer from HDL to biotinylated LDL by scintillation proximity assay | J Med Chem 55: 6162-75 (2012) Article DOI: 10.1021/jm300611v BindingDB Entry DOI: 10.7270/Q2KH0PF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM254043 (US9493430, 612) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chong Kun Dang Pharmaceutical Corp. US Patent | Assay Description As a protein source for cholesteryl ester transfer, plasma from healthy persons was used, and as a cholesteryl ester receptor, LDL from healthy perso... | US Patent US9493430 (2016) BindingDB Entry DOI: 10.7270/Q2W37V7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM234636 (US9353101, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC ... | US Patent US9353101 (2016) BindingDB Entry DOI: 10.7270/Q2765D7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1947 total ) | Next | Last >> |