 Found 346 hits of ic50 data for polymerid = 6547
Found 346 hits of ic50 data for polymerid = 6547 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50250586
(CHEMBL4093140)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)CN[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C78H113N21O18/c1-5-6-19-54(93-75(115)62(42-101)97-73(113)59(34-47-24-27-51(103)28-25-47)95-74(114)61(41-100)89-45(4)102)69(109)90-49(26-29-65(105)106)39-86-57(36-50-38-83-43-88-50)71(111)94-58(33-46-16-8-7-9-17-46)72(112)92-55(22-14-31-84-78(81)82)70(110)96-60(35-48-37-85-53-20-11-10-18-52(48)53)68(108)87-40-64(104)91-56(21-12-13-30-79)77(117)99-32-15-23-63(99)76(116)98-66(44(2)3)67(80)107/h7-11,16-18,20,24-25,27-28,37-38,43-44,49,54-63,66,85-86,100-101,103H,5-6,12-15,19,21-23,26,29-36,39-42,79H2,1-4H3,(H2,80,107)(H,83,88)(H,87,108)(H,89,102)(H,90,109)(H,91,104)(H,92,112)(H,93,115)(H,94,111)(H,95,114)(H,96,110)(H,97,113)(H,98,116)(H,105,106)(H4,81,82,84)/t49-,54-,55-,56-,57-,58+,59-,60-,61-,62-,63-,66-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting method |
J Med Chem 60: 9320-9329 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01295
BindingDB Entry DOI: 10.7270/Q2XG9TJB |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50134946
(CHEMBL2370964 | H-Tyr-Val-Nle-Gly-His-D-Nal(2')-Ar...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |wU:68.72,8.22,90.96,wD:82.88,32.33,42.44,101.107,12.13,57.61,4.3,(2.47,-9.76,;1.17,-9.01,;1.19,-7.5,;-.16,-6.75,;-.14,-5.24,;-1.49,-4.47,;-2.84,-5.23,;-2.85,-6.73,;-4.17,-4.45,;-5.53,-5.2,;-6.86,-4.44,;-6.86,-2.92,;-8.22,-5.18,;-9.55,-4.42,;-8.24,-6.7,;-6.9,-7.44,;-5.56,-6.71,;-4.2,-7.47,;-4.23,-8.99,;-2.87,-9.75,;-5.57,-9.73,;-6.91,-8.97,;-4.17,-2.95,;-5.5,-2.19,;-2.82,-2.2,;1.2,-4.49,;1.22,-2.98,;2.54,-5.26,;3.9,-4.5,;5.23,-5.27,;5.21,-6.79,;6.58,-4.52,;7.92,-5.29,;7.92,-6.79,;9.25,-7.55,;10.69,-6.96,;11.71,-8.09,;10.92,-9.39,;9.41,-9.05,;9.28,-4.55,;9.3,-3.03,;10.61,-5.31,;11.96,-4.56,;11.96,-3.02,;11.17,-1.68,;9.62,-1.68,;8.85,-.34,;9.62,1.02,;8.85,2.36,;9.61,3.72,;11.17,3.72,;11.95,2.36,;11.17,1.02,;11.95,-.32,;13.31,-5.32,;13.29,-6.82,;14.65,-4.56,;15.99,-5.32,;15.98,-6.85,;17.32,-7.61,;17.3,-9.11,;18.65,-9.89,;18.63,-11.4,;19.98,-12.15,;17.29,-12.13,;17.35,-4.59,;17.36,-3.08,;18.69,-5.35,;20.05,-4.61,;20.05,-3.09,;21.4,-2.35,;22.81,-2.98,;23.86,-1.85,;23.1,-.55,;23.58,.89,;22.56,2.03,;21.03,1.71,;20.53,.28,;21.57,-.85,;21.37,-5.37,;21.37,-6.88,;22.73,-4.62,;24.06,-5.4,;24.06,-6.9,;25.41,-7.67,;26.74,-6.9,;25.39,-9.17,;25.41,-4.64,;25.43,-3.14,;26.76,-5.4,;28.12,-4.66,;28.13,-3.15,;29.48,-2.4,;29.48,-.89,;30.83,-.15,;30.84,1.36,;32.2,2.11,;29.51,2.12,;29.46,-5.43,;29.45,-6.93,;30.8,-4.67,;32.14,-5.44,;32.13,-6.95,;33.47,-7.71,;34.83,-6.96,;36.17,-7.72,;36.15,-9.24,;34.8,-9.99,;33.47,-9.22,;33.5,-4.69,;33.5,-3.19,;34.83,-5.46,;36.18,-4.72,;37.53,-5.48,;38.89,-4.73,;37.51,-6.99,)| Show InChI InChI=1S/C79H104N22O15/c1-4-5-20-56(96-77(116)67(44(2)3)101-68(107)54(80)33-46-25-28-52(102)29-26-46)69(108)91-42-65(104)93-62(37-51-40-86-43-92-51)75(114)98-60(35-47-24-27-48-17-9-10-18-49(48)32-47)73(112)94-58(23-14-31-88-79(84)85)72(111)99-61(36-50-39-89-55-21-12-11-19-53(50)55)74(113)100-63(38-66(105)106)76(115)95-57(22-13-30-87-78(82)83)71(110)97-59(70(109)90-41-64(81)103)34-45-15-7-6-8-16-45/h6-12,15-19,21,24-29,32,39-40,43-44,54,56-63,67,89,102H,4-5,13-14,20,22-23,30-31,33-38,41-42,80H2,1-3H3,(H2,81,103)(H,86,92)(H,90,109)(H,91,108)(H,93,104)(H,94,112)(H,95,115)(H,96,116)(H,97,110)(H,98,114)(H,99,111)(H,100,113)(H,101,107)(H,105,106)(H4,82,83,87)(H4,84,85,88)/t54-,56-,57-,58-,59-,60?,61-,62-,63-,67-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01295
BindingDB Entry DOI: 10.7270/Q2GX4GPJ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50134946

(CHEMBL2370964 | H-Tyr-Val-Nle-Gly-His-D-Nal(2')-Ar...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |wU:68.72,8.22,90.96,wD:82.88,32.33,42.44,101.107,12.13,57.61,4.3,(2.47,-9.76,;1.17,-9.01,;1.19,-7.5,;-.16,-6.75,;-.14,-5.24,;-1.49,-4.47,;-2.84,-5.23,;-2.85,-6.73,;-4.17,-4.45,;-5.53,-5.2,;-6.86,-4.44,;-6.86,-2.92,;-8.22,-5.18,;-9.55,-4.42,;-8.24,-6.7,;-6.9,-7.44,;-5.56,-6.71,;-4.2,-7.47,;-4.23,-8.99,;-2.87,-9.75,;-5.57,-9.73,;-6.91,-8.97,;-4.17,-2.95,;-5.5,-2.19,;-2.82,-2.2,;1.2,-4.49,;1.22,-2.98,;2.54,-5.26,;3.9,-4.5,;5.23,-5.27,;5.21,-6.79,;6.58,-4.52,;7.92,-5.29,;7.92,-6.79,;9.25,-7.55,;10.69,-6.96,;11.71,-8.09,;10.92,-9.39,;9.41,-9.05,;9.28,-4.55,;9.3,-3.03,;10.61,-5.31,;11.96,-4.56,;11.96,-3.02,;11.17,-1.68,;9.62,-1.68,;8.85,-.34,;9.62,1.02,;8.85,2.36,;9.61,3.72,;11.17,3.72,;11.95,2.36,;11.17,1.02,;11.95,-.32,;13.31,-5.32,;13.29,-6.82,;14.65,-4.56,;15.99,-5.32,;15.98,-6.85,;17.32,-7.61,;17.3,-9.11,;18.65,-9.89,;18.63,-11.4,;19.98,-12.15,;17.29,-12.13,;17.35,-4.59,;17.36,-3.08,;18.69,-5.35,;20.05,-4.61,;20.05,-3.09,;21.4,-2.35,;22.81,-2.98,;23.86,-1.85,;23.1,-.55,;23.58,.89,;22.56,2.03,;21.03,1.71,;20.53,.28,;21.57,-.85,;21.37,-5.37,;21.37,-6.88,;22.73,-4.62,;24.06,-5.4,;24.06,-6.9,;25.41,-7.67,;26.74,-6.9,;25.39,-9.17,;25.41,-4.64,;25.43,-3.14,;26.76,-5.4,;28.12,-4.66,;28.13,-3.15,;29.48,-2.4,;29.48,-.89,;30.83,-.15,;30.84,1.36,;32.2,2.11,;29.51,2.12,;29.46,-5.43,;29.45,-6.93,;30.8,-4.67,;32.14,-5.44,;32.13,-6.95,;33.47,-7.71,;34.83,-6.96,;36.17,-7.72,;36.15,-9.24,;34.8,-9.99,;33.47,-9.22,;33.5,-4.69,;33.5,-3.19,;34.83,-5.46,;36.18,-4.72,;37.53,-5.48,;38.89,-4.73,;37.51,-6.99,)| Show InChI InChI=1S/C79H104N22O15/c1-4-5-20-56(96-77(116)67(44(2)3)101-68(107)54(80)33-46-25-28-52(102)29-26-46)69(108)91-42-65(104)93-62(37-51-40-86-43-92-51)75(114)98-60(35-47-24-27-48-17-9-10-18-49(48)32-47)73(112)94-58(23-14-31-88-79(84)85)72(111)99-61(36-50-39-89-55-21-12-11-19-53(50)55)74(113)100-63(38-66(105)106)76(115)95-57(22-13-30-87-78(82)83)71(110)97-59(70(109)90-41-64(81)103)34-45-15-7-6-8-16-45/h6-12,15-19,21,24-29,32,39-40,43-44,54,56-63,67,89,102H,4-5,13-14,20,22-23,30-31,33-38,41-42,80H2,1-3H3,(H2,81,103)(H,86,92)(H,90,109)(H,91,108)(H,93,104)(H,94,112)(H,95,115)(H,96,116)(H,97,110)(H,98,114)(H,99,111)(H,100,113)(H,101,107)(H,105,106)(H4,82,83,87)(H4,84,85,88)/t54-,56-,57-,58-,59-,60?,61-,62-,63-,67-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0741 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01295
BindingDB Entry DOI: 10.7270/Q2GX4GPJ |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 5462-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.015
BindingDB Entry DOI: 10.7270/Q2XD11BD |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50211392
(Ac-Nle4-cyclo(Asp5-Pro6-D-4,4'-Bip7-Pro8-Trp9-Lys1...)Show SMILES [H][C@@]12CCCN1C(=O)[C@H](CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@]1([H])CCCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC2=O)C(N)=O)NC(=O)[C@H](CCCC)NC(C)=O Show InChI InChI=1S/C54H68N10O9/c1-3-4-17-41(58-33(2)65)49(68)61-44-31-47(66)56-26-11-10-19-40(48(55)67)59-50(69)42(30-37-32-57-39-18-9-8-16-38(37)39)60-51(70)45-20-12-27-63(45)53(72)43(62-52(71)46-21-13-28-64(46)54(44)73)29-34-22-24-36(25-23-34)35-14-6-5-7-15-35/h5-9,14-16,18,22-25,32,40-46,57H,3-4,10-13,17,19-21,26-31H2,1-2H3,(H2,55,67)(H,56,66)(H,58,65)(H,59,69)(H,60,70)(H,61,68)(H,62,71)/t40?,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDPalphaMSH from human cloned melanocortin receptor 1b expressed in CHO cells |
J Med Chem 50: 2520-6 (2007)
Article DOI: 10.1021/jm0614275
BindingDB Entry DOI: 10.7270/Q2JQ10QM |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50163144
(CHEMBL414778 | H-Tyr-Val-Nle-Gly-His-D-Nal(2'')-Ar...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)ON Show InChI InChI=1S/C77H103N21O16/c1-4-5-22-54(93-75(113)65(44(2)3)98-66(104)53(78)34-47-26-29-52(99)30-27-47)67(105)87-41-62(100)90-60(38-51-40-84-43-89-51)73(111)96-59(37-48-25-28-49-20-12-13-21-50(49)33-48)71(109)91-56(24-15-32-86-77(81)82)70(108)95-58(36-46-18-10-7-11-19-46)72(110)97-61(39-63(101)102)74(112)92-55(23-14-31-85-76(79)80)69(107)94-57(35-45-16-8-6-9-17-45)68(106)88-42-64(103)114-83/h6-13,16-21,25-30,33,40,43-44,53-61,65,99H,4-5,14-15,22-24,31-32,34-39,41-42,78,83H2,1-3H3,(H,84,89)(H,87,105)(H,88,106)(H,90,100)(H,91,109)(H,92,112)(H,93,113)(H,94,107)(H,95,108)(H,96,111)(H,97,110)(H,98,104)(H,101,102)(H4,79,80,85)(H4,81,82,86)/t53-,54-,55-,56-,57-,58-,59+,60-,61-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-MSH binding to Melanocortin 1 receptor expressed in HEK293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alphaMSH from human MC1 receptor expressed in HEK293 cells |
J Med Chem 49: 6888-96 (2006)
Article DOI: 10.1021/jm060768f
BindingDB Entry DOI: 10.7270/Q2RR1XW7 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC1R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibitory activity against hMC1R transfected in HEK293 cells |
J Med Chem 49: 1946-52 (2006)
Article DOI: 10.1021/jm0510326
BindingDB Entry DOI: 10.7270/Q2C24W1F |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50205745
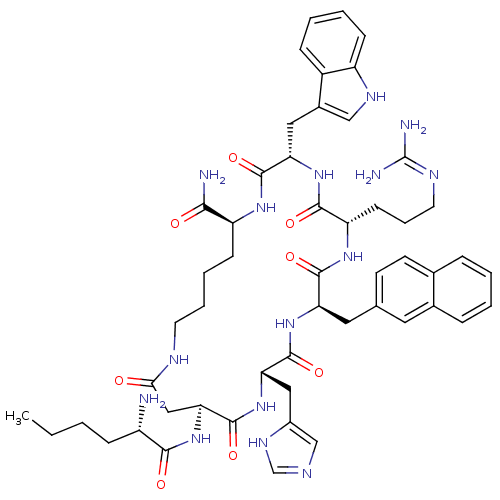
((3S,6S,9R,12S,15S,23S)-15-[(2S)-2-aminohexanamido]...)Show SMILES CCCC[C@H](N)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |wU:36.38,9.8,62.66,wD:47.49,22.22,18.77,4.4,(-.77,-36.46,;.71,-36.07,;1.11,-34.58,;2.6,-34.18,;3,-32.7,;1.91,-31.61,;4.49,-32.3,;5.58,-33.39,;4.89,-30.81,;6.38,-30.41,;5.98,-31.9,;7.27,-32.74,;7.34,-34.28,;8.46,-31.78,;7.92,-30.34,;8.73,-29.1,;10.3,-29.11,;11.1,-27.78,;12.65,-27.81,;13.44,-26.45,;12.68,-25.09,;13.46,-23.77,;11.14,-25.08,;11.89,-23.74,;12.98,-22.65,;14.51,-22.87,;15.21,-21.49,;14.11,-20.41,;14.19,-18.88,;12.9,-18.04,;11.52,-18.75,;11.45,-20.28,;12.74,-21.11,;10.39,-23.81,;8.85,-23.8,;8.08,-25.12,;8.09,-22.46,;8.86,-21.13,;8.09,-19.79,;8.86,-18.46,;8.09,-17.13,;8.86,-15.8,;8.09,-14.46,;10.41,-15.8,;6.54,-22.46,;5.75,-23.79,;6.5,-25.12,;4.21,-23.77,;3.46,-22.43,;1.92,-22.41,;1.15,-23.73,;-.39,-23.71,;-1.15,-22.36,;-2.67,-22.35,;-3.43,-21.03,;-2.66,-19.7,;-1.12,-19.71,;-.37,-21.04,;1.18,-21.06,;3.25,-24.96,;3.99,-26.33,;5.7,-26.45,;3.37,-27.76,;1.83,-27.84,;.73,-28.93,;.96,-30.46,;-.42,-31.16,;-1.51,-30.06,;-.8,-28.68,;4.12,-29.09,;5.6,-29.05,;6.36,-27.71,;13.4,-29.16,;12.61,-30.47,;14.93,-29.18,)| Show InChI InChI=1S/C52H69N15O8/c1-2-3-14-36(53)46(70)64-43-26-44(68)58-20-9-8-16-38(45(54)69)62-49(73)41(24-33-27-60-37-15-7-6-13-35(33)37)66-47(71)39(17-10-21-59-52(55)56)63-48(72)40(23-30-18-19-31-11-4-5-12-32(31)22-30)65-50(74)42(67-51(43)75)25-34-28-57-29-61-34/h4-7,11-13,15,18-19,22,27-29,36,38-43,60H,2-3,8-10,14,16-17,20-21,23-26,53H2,1H3,(H2,54,69)(H,57,61)(H,58,68)(H,62,73)(H,63,72)(H,64,70)(H,65,74)(H,66,71)(H,67,75)(H4,55,56,59)/t36-,38-,39-,40+,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDPalphaMSH from human MC1R expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 2492-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.02.020
BindingDB Entry DOI: 10.7270/Q2FN15W9 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC1 receptor expressed in HEK293 cells |
J Med Chem 51: 187-95 (2008)
Article DOI: 10.1021/jm070461w
BindingDB Entry DOI: 10.7270/Q2V125N2 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50134946

(CHEMBL2370964 | H-Tyr-Val-Nle-Gly-His-D-Nal(2')-Ar...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |wU:68.72,8.22,90.96,wD:82.88,32.33,42.44,101.107,12.13,57.61,4.3,(2.47,-9.76,;1.17,-9.01,;1.19,-7.5,;-.16,-6.75,;-.14,-5.24,;-1.49,-4.47,;-2.84,-5.23,;-2.85,-6.73,;-4.17,-4.45,;-5.53,-5.2,;-6.86,-4.44,;-6.86,-2.92,;-8.22,-5.18,;-9.55,-4.42,;-8.24,-6.7,;-6.9,-7.44,;-5.56,-6.71,;-4.2,-7.47,;-4.23,-8.99,;-2.87,-9.75,;-5.57,-9.73,;-6.91,-8.97,;-4.17,-2.95,;-5.5,-2.19,;-2.82,-2.2,;1.2,-4.49,;1.22,-2.98,;2.54,-5.26,;3.9,-4.5,;5.23,-5.27,;5.21,-6.79,;6.58,-4.52,;7.92,-5.29,;7.92,-6.79,;9.25,-7.55,;10.69,-6.96,;11.71,-8.09,;10.92,-9.39,;9.41,-9.05,;9.28,-4.55,;9.3,-3.03,;10.61,-5.31,;11.96,-4.56,;11.96,-3.02,;11.17,-1.68,;9.62,-1.68,;8.85,-.34,;9.62,1.02,;8.85,2.36,;9.61,3.72,;11.17,3.72,;11.95,2.36,;11.17,1.02,;11.95,-.32,;13.31,-5.32,;13.29,-6.82,;14.65,-4.56,;15.99,-5.32,;15.98,-6.85,;17.32,-7.61,;17.3,-9.11,;18.65,-9.89,;18.63,-11.4,;19.98,-12.15,;17.29,-12.13,;17.35,-4.59,;17.36,-3.08,;18.69,-5.35,;20.05,-4.61,;20.05,-3.09,;21.4,-2.35,;22.81,-2.98,;23.86,-1.85,;23.1,-.55,;23.58,.89,;22.56,2.03,;21.03,1.71,;20.53,.28,;21.57,-.85,;21.37,-5.37,;21.37,-6.88,;22.73,-4.62,;24.06,-5.4,;24.06,-6.9,;25.41,-7.67,;26.74,-6.9,;25.39,-9.17,;25.41,-4.64,;25.43,-3.14,;26.76,-5.4,;28.12,-4.66,;28.13,-3.15,;29.48,-2.4,;29.48,-.89,;30.83,-.15,;30.84,1.36,;32.2,2.11,;29.51,2.12,;29.46,-5.43,;29.45,-6.93,;30.8,-4.67,;32.14,-5.44,;32.13,-6.95,;33.47,-7.71,;34.83,-6.96,;36.17,-7.72,;36.15,-9.24,;34.8,-9.99,;33.47,-9.22,;33.5,-4.69,;33.5,-3.19,;34.83,-5.46,;36.18,-4.72,;37.53,-5.48,;38.89,-4.73,;37.51,-6.99,)| Show InChI InChI=1S/C79H104N22O15/c1-4-5-20-56(96-77(116)67(44(2)3)101-68(107)54(80)33-46-25-28-52(102)29-26-46)69(108)91-42-65(104)93-62(37-51-40-86-43-92-51)75(114)98-60(35-47-24-27-48-17-9-10-18-49(48)32-47)73(112)94-58(23-14-31-88-79(84)85)72(111)99-61(36-50-39-89-55-21-12-11-19-53(50)55)74(113)100-63(38-66(105)106)76(115)95-57(22-13-30-87-78(82)83)71(110)97-59(70(109)90-41-64(81)103)34-45-15-7-6-8-16-45/h6-12,15-19,21,24-29,32,39-40,43-44,54,56-63,67,89,102H,4-5,13-14,20,22-23,30-31,33-38,41-42,80H2,1-3H3,(H2,81,103)(H,86,92)(H,90,109)(H,91,108)(H,93,104)(H,94,112)(H,95,115)(H,96,116)(H,97,110)(H,98,114)(H,99,111)(H,100,113)(H,101,107)(H,105,106)(H4,82,83,87)(H4,84,85,88)/t54-,56-,57-,58-,59-,60?,61-,62-,63-,67-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting method |
J Med Chem 60: 9320-9329 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01295
BindingDB Entry DOI: 10.7270/Q2XG9TJB |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50163145
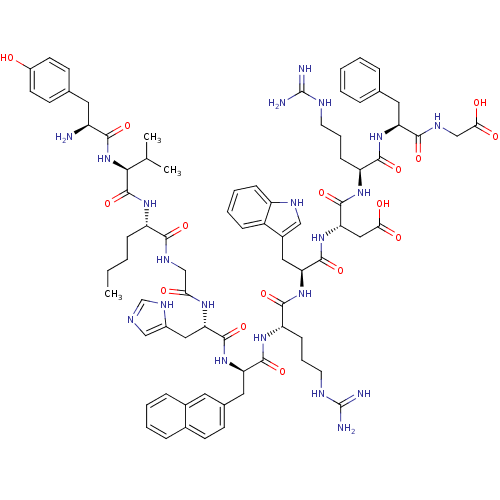
(CHEMBL413439 | H-Tyr-Val-Nle-Gly-His-D-Nal(2'')-Ar...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(O)=O Show InChI InChI=1S/C79H103N21O16/c1-4-5-20-56(95-77(116)67(44(2)3)100-68(107)54(80)33-46-25-28-52(101)29-26-46)69(108)89-41-64(102)92-62(37-51-40-85-43-91-51)75(114)97-60(35-47-24-27-48-17-9-10-18-49(48)32-47)73(112)93-58(23-14-31-87-79(83)84)72(111)98-61(36-50-39-88-55-21-12-11-19-53(50)55)74(113)99-63(38-65(103)104)76(115)94-57(22-13-30-86-78(81)82)71(110)96-59(70(109)90-42-66(105)106)34-45-15-7-6-8-16-45/h6-12,15-19,21,24-29,32,39-40,43-44,54,56-63,67,88,101H,4-5,13-14,20,22-23,30-31,33-38,41-42,80H2,1-3H3,(H,85,91)(H,89,108)(H,90,109)(H,92,102)(H,93,112)(H,94,115)(H,95,116)(H,96,110)(H,97,114)(H,98,111)(H,99,113)(H,100,107)(H,103,104)(H,105,106)(H4,81,82,86)(H4,83,84,87)/t54-,56-,57-,58-,59-,60+,61-,62-,63-,67-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-MSH binding to Melanocortin 1 receptor expressed in HEK293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50454555
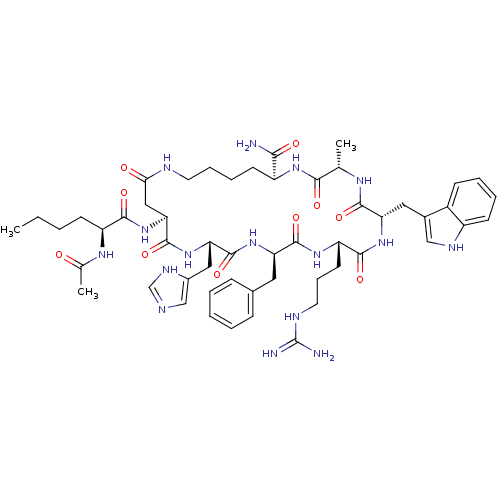
(CHEMBL2115441)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O |wU:30.30,66.69,4.4,44.46,25.25,wD:12.11,21.80,55.57,(-3.6,-3.27,;-2.8,-4.62,;-3.51,-6.01,;-2.66,-7.31,;-3.24,-8.75,;-4.77,-8.95,;-5.17,-10.44,;-6.68,-10.84,;-4.09,-11.55,;-2.3,-9.96,;-2.87,-11.38,;-.49,-9.48,;.9,-10.13,;.41,-11.59,;.29,-13.13,;-1.26,-13.19,;.53,-14.65,;1.14,-16.07,;2.06,-17.29,;3.26,-18.26,;4.65,-18.92,;6.15,-19.23,;7.69,-19.18,;9.17,-18.75,;9.77,-20.16,;10.5,-17.98,;11.43,-19.2,;11.62,-16.91,;12.44,-15.6,;13.83,-16.26,;12.93,-14.14,;14.44,-14.44,;14.92,-15.92,;16.39,-16.38,;16.4,-17.92,;14.94,-18.4,;14.33,-19.81,;12.8,-19.99,;11.88,-18.75,;12.49,-17.34,;14.02,-17.17,;13.05,-12.61,;12.8,-11.09,;14.28,-10.67,;12.2,-9.68,;13.53,-8.91,;14.87,-9.68,;16.19,-8.91,;17.53,-9.68,;18.87,-8.91,;20.19,-9.68,;18.87,-7.37,;11.27,-8.44,;10.09,-7.47,;10.9,-6.17,;8.69,-6.81,;9.17,-5.35,;10.69,-5.05,;11.17,-3.59,;12.68,-3.28,;13.71,-4.44,;13.22,-5.89,;11.7,-6.19,;7.18,-6.51,;5.64,-6.55,;5.92,-5.52,;4.17,-6.99,;3.56,-5.58,;4.48,-4.34,;6.03,-4.38,;6.52,-2.9,;5.3,-1.99,;4.03,-2.88,;2.83,-7.77,;1.72,-8.83,;.53,-7.85,;6.03,-20.77,;7.29,-21.65,;4.64,-21.43,)| Show InChI InChI=1S/C53H74N16O10/c1-4-5-17-38(63-31(3)70)47(74)69-43-26-44(71)58-21-12-11-19-37(45(54)72)64-46(73)30(2)62-49(76)41(24-33-27-60-36-18-10-9-16-35(33)36)67-48(75)39(20-13-22-59-53(55)56)65-50(77)40(23-32-14-7-6-8-15-32)66-51(78)42(68-52(43)79)25-34-28-57-29-61-34/h6-10,14-16,18,27-30,37-43,60H,4-5,11-13,17,19-26H2,1-3H3,(H2,54,72)(H,57,61)(H,58,71)(H,62,76)(H,63,70)(H,64,73)(H,65,77)(H,66,78)(H,67,75)(H,68,79)(H,69,74)(H4,55,56,59)/t30-,37-,38-,39-,40+,41-,42-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50250591
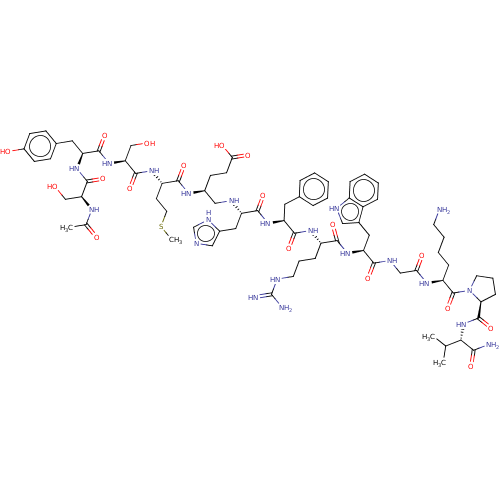
(CHEMBL4072387)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)CN[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r| Show InChI InChI=1S/C77H111N21O18S/c1-43(2)65(66(79)106)97-75(115)62-20-13-30-98(62)76(116)55(18-10-11-28-78)90-63(103)39-86-67(107)59(34-47-36-84-52-17-9-8-16-51(47)52)95-69(109)53(19-12-29-83-77(80)81)91-71(111)57(32-45-14-6-5-7-15-45)93-70(110)56(35-49-37-82-42-87-49)85-38-48(23-26-64(104)105)89-68(108)54(27-31-117-4)92-74(114)61(41-100)96-72(112)58(33-46-21-24-50(102)25-22-46)94-73(113)60(40-99)88-44(3)101/h5-9,14-17,21-22,24-25,36-37,42-43,48,53-62,65,84-85,99-100,102H,10-13,18-20,23,26-35,38-41,78H2,1-4H3,(H2,79,106)(H,82,87)(H,86,107)(H,88,101)(H,89,108)(H,90,103)(H,91,111)(H,92,114)(H,93,110)(H,94,113)(H,95,109)(H,96,112)(H,97,115)(H,104,105)(H4,80,81,83)/t48-,53-,54-,55-,56-,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting method |
J Med Chem 60: 9320-9329 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01295
BindingDB Entry DOI: 10.7270/Q2XG9TJB |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50029747
((4S)-4-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S)-1-[({[(2S...)Show SMILES CSCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C77H109N21O19S/c1-42(2)64(65(79)106)97-75(116)61-20-13-30-98(61)76(117)54(18-10-11-28-78)88-62(103)38-85-66(107)57(34-46-36-84-50-17-9-8-16-49(46)50)94-67(108)51(19-12-29-83-77(80)81)89-70(111)55(32-44-14-6-5-7-15-44)92-72(113)58(35-47-37-82-41-86-47)95-68(109)52(25-26-63(104)105)90-69(110)53(27-31-118-4)91-74(115)60(40-100)96-71(112)56(33-45-21-23-48(102)24-22-45)93-73(114)59(39-99)87-43(3)101/h5-9,14-17,21-24,36-37,41-42,51-61,64,84,99-100,102H,10-13,18-20,25-35,38-40,78H2,1-4H3,(H2,79,106)(H,82,86)(H,85,107)(H,87,101)(H,88,103)(H,89,111)(H,90,110)(H,91,115)(H,92,113)(H,93,114)(H,94,108)(H,95,109)(H,96,112)(H,97,116)(H,104,105)(H4,80,81,83)/t51-,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-MSH binding to Melanocortin 1 receptor expressed in HEK293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50268806
(Ac-Tyr-Val-Nle-Gly-His-DPhe-Arg-Trp-Asp-Arg-Phe-Gl...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |r,wU:12.21,56.58,35.35,81.85,45.46,4.4,100.104,wD:67.69,8.8,89.93,(6.82,-32.01,;5.49,-32.78,;5.49,-34.31,;4.16,-35.09,;4.16,-36.62,;2.82,-37.38,;1.49,-36.61,;1.49,-35.08,;.16,-37.38,;-1.17,-36.62,;-2.5,-37.4,;-2.5,-38.94,;-3.83,-36.63,;-3.83,-35.09,;-2.49,-34.32,;-1.16,-35.09,;.18,-34.32,;.18,-32.78,;1.51,-32,;-1.15,-32.01,;-2.49,-32.78,;-5.18,-37.37,;-6.51,-36.6,;-7.85,-37.37,;-6.51,-35.06,;.16,-38.93,;1.49,-39.69,;-1.18,-39.7,;5.49,-37.39,;5.49,-38.94,;6.83,-36.63,;8.16,-37.4,;9.49,-36.63,;9.49,-35.09,;10.83,-37.4,;12.17,-36.64,;12.17,-35.1,;13.5,-34.33,;14.91,-34.96,;15.95,-33.81,;15.17,-32.48,;13.66,-32.79,;13.5,-37.41,;13.5,-38.95,;14.83,-36.63,;16.17,-37.4,;16.17,-38.95,;17.5,-39.71,;18.84,-38.95,;20.17,-39.72,;20.17,-41.26,;18.83,-42.03,;17.5,-41.26,;17.5,-36.63,;17.5,-35.1,;18.83,-37.4,;20.18,-36.65,;20.18,-35.11,;21.51,-34.34,;21.51,-32.81,;22.84,-32.03,;22.85,-30.49,;21.51,-29.71,;24.18,-29.72,;21.51,-37.42,;21.51,-38.97,;22.84,-36.64,;24.17,-37.41,;24.17,-38.95,;25.5,-39.72,;26.91,-39.1,;27.94,-40.24,;27.17,-41.58,;27.64,-43.04,;26.61,-44.18,;25.1,-43.86,;24.63,-42.39,;25.66,-41.25,;25.5,-36.64,;25.5,-35.1,;26.84,-37.41,;28.17,-36.64,;28.17,-35.11,;29.5,-34.34,;30.83,-35.11,;29.5,-32.8,;29.5,-37.42,;29.5,-38.96,;30.84,-36.64,;32.17,-37.41,;32.17,-38.96,;33.49,-39.72,;33.49,-41.27,;34.82,-42.04,;34.83,-43.58,;33.49,-44.35,;36.16,-44.35,;33.49,-36.64,;33.49,-35.11,;34.83,-37.41,;36.16,-36.64,;36.16,-35.1,;37.5,-34.34,;38.83,-35.11,;40.16,-34.34,;40.17,-32.8,;38.84,-32.03,;37.5,-32.79,;37.5,-37.42,;37.5,-38.95,;38.83,-36.64,;40.16,-37.41,;41.48,-36.64,;42.83,-37.42,;41.48,-35.11,)| Show InChI InChI=1S/C77H104N22O16/c1-5-6-22-53(94-75(115)65(43(2)3)99-74(114)57(90-44(4)100)34-47-26-28-50(101)29-27-47)66(106)88-41-63(103)91-60(36-49-39-83-42-89-49)72(112)96-58(33-46-19-11-8-12-20-46)70(110)92-55(25-16-31-85-77(81)82)69(109)97-59(35-48-38-86-52-23-14-13-21-51(48)52)71(111)98-61(37-64(104)105)73(113)93-54(24-15-30-84-76(79)80)68(108)95-56(67(107)87-40-62(78)102)32-45-17-9-7-10-18-45/h7-14,17-21,23,26-29,38-39,42-43,53-61,65,86,101H,5-6,15-16,22,24-25,30-37,40-41H2,1-4H3,(H2,78,102)(H,83,89)(H,87,107)(H,88,106)(H,90,100)(H,91,103)(H,92,110)(H,93,113)(H,94,115)(H,95,108)(H,96,112)(H,97,109)(H,98,111)(H,99,114)(H,104,105)(H4,79,80,84)(H4,81,82,85)/t53-,54-,55-,56-,57-,58+,59-,60-,61-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC1R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50163146
(Ac-Tyr-Val-Nle-Gly-His-D-Phe-Arg-Trp-Asp-Arg-Phe-G...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)ON Show InChI InChI=1S/C77H104N22O17/c1-5-6-22-53(94-75(115)65(43(2)3)99-74(114)57(90-44(4)100)34-47-26-28-50(101)29-27-47)66(106)87-40-62(102)91-60(36-49-39-83-42-89-49)72(112)96-58(33-46-19-11-8-12-20-46)70(110)92-55(25-16-31-85-77(80)81)69(109)97-59(35-48-38-86-52-23-14-13-21-51(48)52)71(111)98-61(37-63(103)104)73(113)93-54(24-15-30-84-76(78)79)68(108)95-56(32-45-17-9-7-10-18-45)67(107)88-41-64(105)116-82/h7-14,17-21,23,26-29,38-39,42-43,53-61,65,86,101H,5-6,15-16,22,24-25,30-37,40-41,82H2,1-4H3,(H,83,89)(H,87,106)(H,88,107)(H,90,100)(H,91,102)(H,92,110)(H,93,113)(H,94,115)(H,95,108)(H,96,112)(H,97,109)(H,98,111)(H,99,114)(H,103,104)(H4,78,79,84)(H4,80,81,85)/t53-,54-,55-,56-,57-,58-,59-,60-,61-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-MSH binding to Melanocortin 1 receptor expressed in HEK293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50029748
((2R,3R)-15-(2-Acetylamino-hexanoylamino)-9-benzyl-...)Show SMILES [H][C@]1(NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@H](CC(=O)NCCCC[C@H](NC1=O)C(N)=O)NC(=O)[C@H](CCCC)NC(C)=O)[C@@H](C)c1c[nH]c2ccccc12 |wU:27.28,56.63,65.68,5.5,wD:37.55,46.52,16.16,1.0,(19.89,-7.11,;18.68,-8.53,;18.45,-7.02,;17.78,-5.61,;19.09,-4.78,;16.79,-4.45,;17.81,-3.3,;19.32,-3.62,;20.34,-2.47,;21.85,-2.76,;22.87,-1.63,;24.37,-1.93,;22.39,-.17,;15.51,-3.6,;14.03,-3.12,;13.68,-4.91,;12.5,-3.04,;12.37,-1.5,;13.62,-.62,;13.49,.92,;14.74,1.81,;16.15,1.17,;16.27,-.36,;15.03,-1.26,;11.02,-3.39,;9.65,-4.13,;10.31,-5.51,;8.56,-5.2,;7.34,-4.26,;7.57,-2.73,;8.91,-2,;8.62,-.49,;7.12,-.27,;6.45,-1.66,;7.79,-6.54,;7.41,-8.01,;5.87,-7.85,;7.44,-9.54,;7.89,-11.05,;8.72,-12.34,;7.57,-13.33,;9.87,-13.36,;11.25,-14.03,;12.78,-14.32,;14.29,-14.18,;15.73,-13.64,;16.98,-12.71,;17.94,-11.49,;18.52,-10.06,;19.99,-10.45,;18.07,-13.81,;17.65,-15.3,;19.54,-13.43,;5.93,-9.81,;5.36,-11.25,;6.35,-12.45,;3.85,-11.49,;2.89,-10.29,;3.44,-8.87,;2.44,-7.66,;2.99,-6.22,;3.31,-12.93,;1.8,-13.27,;1.32,-14.71,;.78,-12.11,;20.21,-8.52,;20.6,-7.02,;21.56,-9.26,;23,-8.75,;23.93,-9.99,;23.03,-11.25,;23.41,-12.75,;22.26,-13.81,;20.79,-13.36,;20.47,-11.86,;21.59,-10.8,)| Show InChI InChI=1S/C51H71N15O9/c1-4-5-17-37(60-30(3)67)45(70)65-41-25-42(68)56-21-12-11-19-36(44(52)69)61-50(75)43(29(2)34-27-58-35-18-10-9-16-33(34)35)66-46(71)38(20-13-22-57-51(53)54)62-47(72)39(23-31-14-7-6-8-15-31)63-48(73)40(64-49(41)74)24-32-26-55-28-59-32/h6-10,14-16,18,26-29,36-41,43,58H,4-5,11-13,17,19-25H2,1-3H3,(H2,52,69)(H,55,59)(H,56,68)(H,60,67)(H,61,75)(H,62,72)(H,63,73)(H,64,74)(H,65,70)(H,66,71)(H4,53,54,57)/t29-,36-,37-,38-,39+,40-,41-,43-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
The concentration that inhibits 50% specific binding was determined against the Melanocortin 1 receptor |
J Med Chem 38: 4720-9 (1995)
BindingDB Entry DOI: 10.7270/Q2B56HRS |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
The concentration that inhibits 50% specific binding was determined against the Melanocortin 1 receptor |
J Med Chem 38: 4720-9 (1995)
BindingDB Entry DOI: 10.7270/Q2B56HRS |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50268806

(Ac-Tyr-Val-Nle-Gly-His-DPhe-Arg-Trp-Asp-Arg-Phe-Gl...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |r,wU:12.21,56.58,35.35,81.85,45.46,4.4,100.104,wD:67.69,8.8,89.93,(6.82,-32.01,;5.49,-32.78,;5.49,-34.31,;4.16,-35.09,;4.16,-36.62,;2.82,-37.38,;1.49,-36.61,;1.49,-35.08,;.16,-37.38,;-1.17,-36.62,;-2.5,-37.4,;-2.5,-38.94,;-3.83,-36.63,;-3.83,-35.09,;-2.49,-34.32,;-1.16,-35.09,;.18,-34.32,;.18,-32.78,;1.51,-32,;-1.15,-32.01,;-2.49,-32.78,;-5.18,-37.37,;-6.51,-36.6,;-7.85,-37.37,;-6.51,-35.06,;.16,-38.93,;1.49,-39.69,;-1.18,-39.7,;5.49,-37.39,;5.49,-38.94,;6.83,-36.63,;8.16,-37.4,;9.49,-36.63,;9.49,-35.09,;10.83,-37.4,;12.17,-36.64,;12.17,-35.1,;13.5,-34.33,;14.91,-34.96,;15.95,-33.81,;15.17,-32.48,;13.66,-32.79,;13.5,-37.41,;13.5,-38.95,;14.83,-36.63,;16.17,-37.4,;16.17,-38.95,;17.5,-39.71,;18.84,-38.95,;20.17,-39.72,;20.17,-41.26,;18.83,-42.03,;17.5,-41.26,;17.5,-36.63,;17.5,-35.1,;18.83,-37.4,;20.18,-36.65,;20.18,-35.11,;21.51,-34.34,;21.51,-32.81,;22.84,-32.03,;22.85,-30.49,;21.51,-29.71,;24.18,-29.72,;21.51,-37.42,;21.51,-38.97,;22.84,-36.64,;24.17,-37.41,;24.17,-38.95,;25.5,-39.72,;26.91,-39.1,;27.94,-40.24,;27.17,-41.58,;27.64,-43.04,;26.61,-44.18,;25.1,-43.86,;24.63,-42.39,;25.66,-41.25,;25.5,-36.64,;25.5,-35.1,;26.84,-37.41,;28.17,-36.64,;28.17,-35.11,;29.5,-34.34,;30.83,-35.11,;29.5,-32.8,;29.5,-37.42,;29.5,-38.96,;30.84,-36.64,;32.17,-37.41,;32.17,-38.96,;33.49,-39.72,;33.49,-41.27,;34.82,-42.04,;34.83,-43.58,;33.49,-44.35,;36.16,-44.35,;33.49,-36.64,;33.49,-35.11,;34.83,-37.41,;36.16,-36.64,;36.16,-35.1,;37.5,-34.34,;38.83,-35.11,;40.16,-34.34,;40.17,-32.8,;38.84,-32.03,;37.5,-32.79,;37.5,-37.42,;37.5,-38.95,;38.83,-36.64,;40.16,-37.41,;41.48,-36.64,;42.83,-37.42,;41.48,-35.11,)| Show InChI InChI=1S/C77H104N22O16/c1-5-6-22-53(94-75(115)65(43(2)3)99-74(114)57(90-44(4)100)34-47-26-28-50(101)29-27-47)66(106)88-41-63(103)91-60(36-49-39-83-42-89-49)72(112)96-58(33-46-19-11-8-12-20-46)70(110)92-55(25-16-31-85-77(81)82)69(109)97-59(35-48-38-86-52-23-14-13-21-51(48)52)71(111)98-61(37-64(104)105)73(113)93-54(24-15-30-84-76(79)80)68(108)95-56(67(107)87-40-62(78)102)32-45-17-9-7-10-18-45/h7-14,17-21,23,26-29,38-39,42-43,53-61,65,86,101H,5-6,15-16,22,24-25,30-37,40-41H2,1-4H3,(H2,78,102)(H,83,89)(H,87,107)(H,88,106)(H,90,100)(H,91,103)(H,92,110)(H,93,113)(H,94,115)(H,95,108)(H,96,112)(H,97,109)(H,98,111)(H,99,114)(H,104,105)(H4,79,80,84)(H4,81,82,85)/t53-,54-,55-,56-,57-,58+,59-,60-,61-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting method |
J Med Chem 60: 9320-9329 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01295
BindingDB Entry DOI: 10.7270/Q2XG9TJB |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM82411
(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM82411

(CAS_75921-69-6 | NDP-MSH)Show SMILES CCCC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(N)=O |r,wU:66.68,55.56,45.45,111.116,4.4,14.23,wD:77.79,95.99,107.113,36.36,8.10,26.29,(-7.66,4.36,;-8.98,3.65,;-9.15,2.12,;-10.5,1.37,;-10.5,-.23,;-11.93,-.94,;-13.21,-.15,;-13.17,1.41,;-14.6,-.83,;-14.64,-2.37,;-13.43,-3.18,;-15.78,-.09,;-17.19,-.68,;-17.34,-2.22,;-18.55,.02,;-18.4,1.67,;-17.08,2.44,;-17.08,3.97,;-15.61,4.72,;-14.35,3.82,;-13,4.55,;-14.45,2.26,;-15.78,1.52,;-19.9,-.62,;-21.14,.17,;-21.14,1.73,;-22.53,-.62,;-22.53,-2.12,;-21.33,-2.88,;-23.81,.17,;-25.13,-.41,;-26.27,.45,;-25.2,-1.9,;-9.26,-1,;-9.3,-2.5,;-7.94,-.34,;-6.59,-1.15,;-6.59,-2.71,;-7.94,-3.46,;-7.94,-5,;-9.3,-5.6,;-6.63,-5.81,;-5.24,-.34,;-5.2,1.15,;-3.86,-1.11,;-2.57,-.23,;-2.57,1.26,;-1.19,2.05,;.1,1.47,;1.29,2.58,;.52,3.87,;-.97,3.65,;-1.19,-1,;-1.19,-2.5,;.1,-.3,;1.55,-1.15,;1.55,-2.65,;2.79,-3.4,;2.79,-4.89,;4.22,-5.7,;5.56,-4.89,;5.56,-3.4,;4.22,-2.65,;2.79,-.34,;2.79,1.2,;4.22,-1,;5.33,-.09,;5.29,1.47,;6.63,2.26,;6.53,3.82,;7.81,4.72,;7.81,6.13,;9.13,7.03,;6.46,6.92,;6.85,-.73,;7,-2.37,;8,.13,;9.35,-.41,;9.69,-2.07,;10.95,-2.71,;12.29,-1.9,;13.51,-2.99,;12.83,-4.36,;13.43,-5.81,;12.55,-7.03,;10.95,-6.77,;10.33,-5.43,;11.29,-4.21,;10.69,.41,;10.48,2.16,;12.04,-.41,;13.38,.3,;14.58,-.51,;14.47,-2.03,;15.99,.17,;17.25,-.68,;17.25,-2.26,;18.49,-3.08,;18.45,-4.57,;19.73,-5.55,;19.69,-7.03,;18.66,.02,;18.77,1.52,;20.01,-.68,;20.09,-2.22,;21.72,-2.54,;22.29,-1.11,;21.5,-.09,;21.78,1.47,;20.65,2.84,;23.32,1.64,;23.83,2.97,;23,4.19,;23.6,5.58,;21.5,4.04,;25.41,3.12,;26.03,4.44,;26.27,1.94,)| Show InChI InChI=1S/C78H111N21O19/c1-5-6-19-52(91-75(116)61(41-101)97-72(113)57(34-46-24-26-49(103)27-25-46)94-74(115)60(40-100)88-44(4)102)68(109)92-54(28-29-64(105)106)70(111)96-59(36-48-38-83-42-87-48)73(114)93-56(33-45-16-8-7-9-17-45)71(112)90-53(22-14-31-84-78(81)82)69(110)95-58(35-47-37-85-51-20-11-10-18-50(47)51)67(108)86-39-63(104)89-55(21-12-13-30-79)77(118)99-32-15-23-62(99)76(117)98-65(43(2)3)66(80)107/h7-11,16-18,20,24-27,37-38,42-43,52-62,65,85,100-101,103H,5-6,12-15,19,21-23,28-36,39-41,79H2,1-4H3,(H2,80,107)(H,83,87)(H,86,108)(H,88,102)(H,89,104)(H,90,112)(H,91,116)(H,92,109)(H,93,114)(H,94,115)(H,95,110)(H,96,111)(H,97,113)(H,98,117)(H,105,106)(H4,81,82,84)/t52-,53-,54-,55-,56+,57-,58-,59-,60-,61-,62-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-MSH from Melanocortin 1 receptor at 10 uM |
J Med Chem 40: 2133-9 (1997)
Article DOI: 10.1021/jm960840h
BindingDB Entry DOI: 10.7270/Q28K79R6 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50163141
(CHEMBL438920 | H-Tyr-Val-Nle-Gly-His-D-Phe-Arg-D-N...)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)ON Show InChI InChI=1S/C77H103N21O16/c1-4-5-22-54(93-75(113)65(44(2)3)98-66(104)53(78)34-47-26-29-52(99)30-27-47)67(105)87-41-62(100)90-60(38-51-40-84-43-89-51)73(111)96-58(36-46-18-10-7-11-19-46)71(109)91-56(24-15-32-86-77(81)82)70(108)95-59(37-48-25-28-49-20-12-13-21-50(49)33-48)72(110)97-61(39-63(101)102)74(112)92-55(23-14-31-85-76(79)80)69(107)94-57(35-45-16-8-6-9-17-45)68(106)88-42-64(103)114-83/h6-13,16-21,25-30,33,40,43-44,53-61,65,99H,4-5,14-15,22-24,31-32,34-39,41-42,78,83H2,1-3H3,(H,84,89)(H,87,105)(H,88,106)(H,90,100)(H,91,109)(H,92,112)(H,93,113)(H,94,107)(H,95,108)(H,96,111)(H,97,110)(H,98,104)(H,101,102)(H4,79,80,85)(H4,81,82,86)/t53-,54-,55-,56-,57-,58-,59+,60-,61-,65-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-MSH binding to Melanocortin 1 receptor expressed in HEK293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50468287
(CHEMBL4292317)Show SMILES CCCC[C@H](NC(=O)[C@@H](Cc1ccc(F)cc1)NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| Show InChI InChI=1S/C33H41FN6O5/c1-3-4-9-26(31(43)38-27(30(35)42)18-22-19-36-25-10-6-5-8-24(22)25)37-32(44)28(17-21-12-14-23(34)15-13-21)39-33(45)29-11-7-16-40(29)20(2)41/h5-6,8,10,12-15,19,26-29,36H,3-4,7,9,11,16-18H2,1-2H3,(H2,35,42)(H,37,44)(H,38,43)(H,39,45)/t26-,27-,28+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle4,d-Phe7]-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting analysis |
Eur J Med Chem 151: 815-823 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.021
BindingDB Entry DOI: 10.7270/Q20K2C9T |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027179
(CHEMBL2096759)Show SMILES CCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(=O)ON Show InChI InChI=1S/C75H102N22O16/c1-4-5-21-52(92-73(112)63(42(2)3)97-64(103)50(76)31-45-25-27-48(98)28-26-45)65(104)86-39-60(99)89-58(35-47-38-82-41-88-47)71(110)94-56(33-44-18-10-7-11-19-44)69(108)90-54(24-15-30-84-75(79)80)68(107)95-57(34-46-37-85-51-22-13-12-20-49(46)51)70(109)96-59(36-61(100)101)72(111)91-53(23-14-29-83-74(77)78)67(106)93-55(32-43-16-8-6-9-17-43)66(105)87-40-62(102)113-81/h6-13,16-20,22,25-28,37-38,41-42,50,52-59,63,85,98H,4-5,14-15,21,23-24,29-36,39-40,76,81H2,1-3H3,(H,82,88)(H,86,104)(H,87,105)(H,89,99)(H,90,108)(H,91,111)(H,92,112)(H,93,106)(H,94,110)(H,95,107)(H,96,109)(H,97,103)(H,100,101)(H4,77,78,83)(H4,79,80,84)/t50-,52-,53-,54-,55-,56+,57+,58-,59-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-MSH binding to Melanocortin 1 receptor expressed in HEK293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle4,d-Phe7]-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting analysis |
Eur J Med Chem 151: 815-823 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.021
BindingDB Entry DOI: 10.7270/Q20K2C9T |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1 receptor expressed in HEK293 cells by gamma counting analysis |
ACS Med Chem Lett 6: 192-7 (2015)
Article DOI: 10.1021/ml500436s
BindingDB Entry DOI: 10.7270/Q24B330P |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Binding affinity towards human melanocortin receptor (hMC1R) using NDP-MSH as radioligand |
Bioorg Med Chem Lett 13: 1307-11 (2003)
BindingDB Entry DOI: 10.7270/Q21J994C |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50121268
(21-(2-Acetylamino-hexanoylamino)-7-[3-(diaminometh...)Show SMILES CCCC[C@@H](NC(C)=O)C(=O)N[C@@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2c[nH]cn2)NC1=O)C(N)=O Show InChI InChI=1S/C54H71N15O9/c1-3-4-15-40(63-31(2)70)48(73)69-45-27-46(71)59-21-10-9-17-39(47(55)72)64-51(76)43(25-35-28-61-38-16-8-7-14-37(35)38)67-49(74)41(18-11-22-60-54(56)57)65-50(75)42(24-32-19-20-33-12-5-6-13-34(33)23-32)66-52(77)44(68-53(45)78)26-36-29-58-30-62-36/h5-8,12-14,16,19-20,23,28-30,39-45,61H,3-4,9-11,15,17-18,21-22,24-27H2,1-2H3,(H2,55,72)(H,58,62)(H,59,71)(H,63,70)(H,64,76)(H,65,75)(H,66,77)(H,67,74)(H,68,78)(H,69,73)(H4,56,57,60)/t39-,40+,41-,42+,43-,44-,45+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I][Nle4,Dphe7]-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50250590
(CHEMBL4081900)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C72H100N16O15/c1-41(2)32-51(86-71(103)61(43(5)6)87-62(94)49(73)34-47-26-28-48(89)29-27-47)63(95)79-40-59(91)88-31-17-25-57(88)70(102)85-55(37-46-22-14-9-15-23-46)68(100)81-52(33-42(3)4)66(98)83-54(36-45-20-12-8-13-21-45)67(99)84-56(38-60(92)93)69(101)80-50(24-16-30-77-72(75)76)65(97)82-53(64(96)78-39-58(74)90)35-44-18-10-7-11-19-44/h7-15,18-23,26-29,41-43,49-57,61,89H,16-17,24-25,30-40,73H2,1-6H3,(H2,74,90)(H,78,96)(H,79,95)(H,80,101)(H,81,100)(H,82,97)(H,83,98)(H,84,99)(H,85,102)(H,86,103)(H,87,94)(H,92,93)(H4,75,76,77)/t49-,50-,51-,52-,53-,54-,55-,56-,57-,61-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting method |
J Med Chem 60: 9320-9329 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01295
BindingDB Entry DOI: 10.7270/Q2XG9TJB |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50268796
(Ac-Nle-c[Asp-cis-4-guanidinyl-Pro-DPhe-Arg-Trp-Lys...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H]2C[C@@H](CN2C1=O)N=C(N)N)C(N)=O |r,wU:4.4,50.52,12.11,21.76,wD:25.25,39.41,61.68,63.72,(20.34,-25.22,;18.98,-24.49,;18.93,-22.95,;17.57,-22.23,;17.52,-20.69,;18.83,-19.87,;18.78,-18.33,;20.08,-17.51,;17.42,-17.6,;16.16,-19.96,;16.11,-18.42,;14.85,-20.77,;13.49,-20.05,;12.18,-20.86,;12.24,-22.4,;13.59,-23.13,;10.92,-23.22,;9.56,-22.49,;8.26,-23.31,;6.89,-22.58,;5.58,-23.39,;4.23,-22.66,;4.17,-21.12,;2.82,-20.4,;1.5,-21.21,;2.76,-18.86,;1.39,-18.12,;-.07,-18.63,;-1.33,-17.73,;-2.56,-18.65,;-2.05,-20.13,;-2.79,-21.47,;-1.99,-22.78,;-.45,-22.73,;.28,-21.39,;-.52,-20.09,;4.07,-18.04,;5.39,-18.27,;6.72,-19.07,;4.02,-16.5,;2.66,-15.78,;2.61,-14.23,;1.25,-13.51,;1.2,-11.97,;-.17,-11.24,;-.21,-9.7,;-1.47,-12.06,;5.33,-15.69,;6.69,-16.41,;6.74,-17.95,;8,-15.6,;7.95,-14.06,;6.59,-13.33,;6.54,-11.79,;5.18,-11.07,;3.87,-11.88,;3.92,-13.43,;5.27,-14.15,;9.36,-16.32,;10.67,-15.52,;10.62,-13.97,;12.04,-16.25,;13.12,-15.14,;14.62,-15.54,;14.61,-17.08,;12.07,-17.77,;13.44,-18.51,;14.75,-17.69,;15.84,-14.61,;15.64,-13.08,;14.21,-12.48,;16.87,-12.14,;2.92,-23.48,;1.55,-22.75,;2.96,-25.02,)| Show InChI InChI=1S/C50H72N16O9/c1-3-4-16-35(59-28(2)67)43(70)65-39-25-41(68)56-20-11-10-18-34(42(51)69)61-46(73)38(23-30-26-58-33-17-9-8-15-32(30)33)63-44(71)36(19-12-21-57-49(52)53)62-45(72)37(22-29-13-6-5-7-14-29)64-47(74)40-24-31(60-50(54)55)27-66(40)48(39)75/h5-9,13-15,17,26,31,34-40,58H,3-4,10-12,16,18-25,27H2,1-2H3,(H2,51,69)(H,56,68)(H,59,67)(H,61,73)(H,62,72)(H,63,71)(H,64,74)(H,65,70)(H4,52,53,57)(H4,54,55,60)/t31-,34-,35-,36-,37+,38-,39-,40-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I][Nle4,Dphe7]-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Napoli Federico II
Curated by ChEMBL
| Assay Description
Displacement of 125I-labeled [Nle4,DPhe7]-alpha-MSH from human melanocortin 1 receptor expressed in HEK293 cells incubated for 40 mins by gamma count... |
J Med Chem 58: 9773-8 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01285
BindingDB Entry DOI: 10.7270/Q24Q7Z0B |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50134948
(CHEMBL437050 | H-Tyr-Val-Met-Gly-His-Phe-Arg-Trp-A...)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(O)=O Show InChI InChI=1S/C74H99N21O16S/c1-41(2)62(95-63(102)49(75)30-44-22-24-47(96)25-23-44)72(111)90-53(26-29-112-3)64(103)84-38-59(97)87-57(34-46-37-80-40-86-46)70(109)92-55(32-43-16-8-5-9-17-43)68(107)88-52(21-13-28-82-74(78)79)67(106)93-56(33-45-36-83-50-19-11-10-18-48(45)50)69(108)94-58(35-60(98)99)71(110)89-51(20-12-27-81-73(76)77)66(105)91-54(65(104)85-39-61(100)101)31-42-14-6-4-7-15-42/h4-11,14-19,22-25,36-37,40-41,49,51-58,62,83,96H,12-13,20-21,26-35,38-39,75H2,1-3H3,(H,80,86)(H,84,103)(H,85,104)(H,87,97)(H,88,107)(H,89,110)(H,90,111)(H,91,105)(H,92,109)(H,93,106)(H,94,108)(H,95,102)(H,98,99)(H,100,101)(H4,76,77,81)(H4,78,79,82)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,62-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-MSH binding to Melanocortin 1 receptor expressed in HEK293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50268802
(Ac-Nle-c[Asp-cis-4-guanidinyl-Pro-DNal(2')-Arg-Trp...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H]2C[C@@H](CN2C1=O)N=C(N)N)C(N)=O |r,wU:4.4,12.11,21.81,50.52,wD:25.25,39.41,65.73,67.77,(39.57,-47.74,;38.21,-47.02,;38.16,-45.48,;36.8,-44.75,;36.75,-43.21,;38.06,-42.4,;38,-40.86,;39.31,-40.04,;36.65,-40.13,;35.39,-42.49,;35.34,-40.95,;34.08,-43.3,;32.72,-42.58,;31.42,-43.39,;31.47,-44.93,;32.83,-45.65,;30.16,-45.74,;28.8,-45.02,;27.49,-45.83,;26.13,-45.1,;24.82,-45.91,;23.47,-45.19,;23.42,-43.65,;22.06,-42.92,;20.75,-43.74,;22.01,-41.38,;20.63,-40.65,;19.18,-41.16,;17.92,-40.26,;16.69,-41.18,;17.19,-42.65,;16.46,-44,;17.25,-45.3,;18.8,-45.26,;19.52,-43.91,;18.73,-42.62,;23.31,-40.57,;24.63,-40.8,;25.96,-41.59,;23.26,-39.03,;21.9,-38.31,;21.85,-36.77,;20.5,-36.04,;20.44,-34.5,;19.08,-33.78,;19.04,-32.24,;17.77,-34.59,;24.57,-38.22,;25.93,-38.94,;25.98,-40.48,;27.24,-38.13,;27.19,-36.59,;25.83,-35.86,;24.52,-36.68,;23.17,-35.96,;23.11,-34.42,;21.76,-33.69,;21.71,-32.15,;23.02,-31.33,;24.38,-32.07,;24.43,-33.61,;25.78,-34.33,;28.59,-38.85,;29.91,-38.05,;29.86,-36.5,;31.27,-38.78,;32.35,-37.67,;33.85,-38.07,;33.84,-39.61,;31.31,-40.3,;32.67,-41.04,;33.98,-40.22,;35.07,-37.14,;34.87,-35.61,;33.45,-35.01,;36.1,-34.67,;22.16,-46,;20.8,-45.28,;22.21,-47.54,)| Show InChI InChI=1S/C54H74N16O9/c1-3-4-15-39(63-30(2)71)47(74)69-43-27-45(72)60-21-10-9-17-38(46(55)73)65-50(77)42(25-34-28-62-37-16-8-7-14-36(34)37)67-48(75)40(18-11-22-61-53(56)57)66-49(76)41(24-31-19-20-32-12-5-6-13-33(32)23-31)68-51(78)44-26-35(64-54(58)59)29-70(44)52(43)79/h5-8,12-14,16,19-20,23,28,35,38-44,62H,3-4,9-11,15,17-18,21-22,24-27,29H2,1-2H3,(H2,55,73)(H,60,72)(H,63,71)(H,65,77)(H,66,76)(H,67,75)(H,68,78)(H,69,74)(H4,56,57,61)(H4,58,59,64)/t35-,38-,39-,40-,41+,42-,43-,44-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I][Nle4,Dphe7]-alpha-MSH from human MC1R expressed in HEK293 cells |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples "Federico II"
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting method |
J Med Chem 61: 4263-4269 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00488
BindingDB Entry DOI: 10.7270/Q22J6FF7 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50250585
(CHEMBL4104092)Show SMILES CSCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(=O)NCC(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccccc1)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C74H100N22O15S/c1-41(2)62(96-63(102)49(75)30-44-22-24-47(97)25-23-44)72(111)91-53(26-29-112-3)64(103)86-39-60(99)88-57(34-46-37-81-40-87-46)70(109)93-55(32-43-16-8-5-9-17-43)68(107)89-52(21-13-28-83-74(79)80)67(106)94-56(33-45-36-84-50-19-11-10-18-48(45)50)69(108)95-58(35-61(100)101)71(110)90-51(20-12-27-82-73(77)78)66(105)92-54(65(104)85-38-59(76)98)31-42-14-6-4-7-15-42/h4-11,14-19,22-25,36-37,40-41,49,51-58,62,84,97H,12-13,20-21,26-35,38-39,75H2,1-3H3,(H2,76,98)(H,81,87)(H,85,104)(H,86,103)(H,88,99)(H,89,107)(H,90,110)(H,91,111)(H,92,105)(H,93,109)(H,94,106)(H,95,108)(H,96,102)(H,100,101)(H4,77,78,82)(H4,79,80,83)/t49-,51-,52-,53-,54-,55-,56-,57-,58-,62-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting method |
J Med Chem 60: 9320-9329 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01295
BindingDB Entry DOI: 10.7270/Q2XG9TJB |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50193836
(1-((3R,8aS)-3-benzyl-hexahydropyrrolo[1,2-a]pyrazi...)Show SMILES O=C(Cc1ccccc1)N1C[C@@H]2CCCN2C[C@H]1Cc1ccccc1 Show InChI InChI=1S/C22H26N2O/c25-22(15-19-10-5-2-6-11-19)24-17-20-12-7-13-23(20)16-21(24)14-18-8-3-1-4-9-18/h1-6,8-11,20-21H,7,12-17H2/t20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 5462-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.015
BindingDB Entry DOI: 10.7270/Q2XD11BD |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50112890
(CHEMBL3600833)Show SMILES CCCC[C@@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)N(C)C(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| Show InChI InChI=1S/C55H73N15O9/c1-4-5-16-41(64-32(2)71)49(74)67-44-28-47(72)60-22-11-10-18-40(48(56)73)65-50(75)42(26-36-29-62-39-17-9-8-15-38(36)39)68-53(78)46(19-12-23-61-55(57)58)70(3)54(79)45(25-33-20-21-34-13-6-7-14-35(34)24-33)69-51(76)43(66-52(44)77)27-37-30-59-31-63-37/h6-9,13-15,17,20-21,24,29-31,40-46,62H,4-5,10-12,16,18-19,22-23,25-28H2,1-3H3,(H2,56,73)(H,59,63)(H,60,72)(H,64,71)(H,65,75)(H,66,77)(H,67,74)(H,68,78)(H,69,76)(H4,57,58,61)/t40-,41+,42-,43-,44-,45+,46-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle4,D-Phe7]-alpha-MSH from human MC1 receptor expressed in HEK293 cells after 40 mins by luminescence counting |
J Med Chem 58: 6359-67 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00102
BindingDB Entry DOI: 10.7270/Q251410F |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50468288
(CHEMBL4286957)Show SMILES CCCC[C@H](NC(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(N)=O |r| Show InChI InChI=1S/C33H41ClN6O5/c1-3-4-9-26(31(43)38-27(30(35)42)18-22-19-36-25-10-6-5-8-24(22)25)37-32(44)28(17-21-12-14-23(34)15-13-21)39-33(45)29-11-7-16-40(29)20(2)41/h5-6,8,10,12-15,19,26-29,36H,3-4,7,9,11,16-18H2,1-2H3,(H2,35,42)(H,37,44)(H,38,43)(H,39,45)/t26-,27-,28+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-[Nle4,d-Phe7]-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by gamma counting analysis |
Eur J Med Chem 151: 815-823 (2018)
Article DOI: 10.1016/j.ejmech.2018.04.021
BindingDB Entry DOI: 10.7270/Q20K2C9T |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027084

(Melatonan)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01848
BindingDB Entry DOI: 10.7270/Q2J67N1M |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50058160
((3R,6S,9R,12S,15R,18R,26R)-18-Acetylamino-12-benzy...)Show SMILES C[C@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H](CC(=O)NCCCC[C@@H](NC1=O)C(N)=O)NC(C)=O Show InChI InChI=1S/C47H63N15O9/c1-26-41(66)58-33(40(48)65)15-8-9-17-52-39(64)22-38(57-27(2)63)46(71)62-37(21-30-24-51-25-55-30)45(70)60-35(19-28-11-4-3-5-12-28)44(69)59-34(16-10-18-53-47(49)50)42(67)61-36(43(68)56-26)20-29-23-54-32-14-7-6-13-31(29)32/h3-7,11-14,23-26,33-38,54H,8-10,15-22H2,1-2H3,(H2,48,65)(H,51,55)(H,52,64)(H,56,68)(H,57,63)(H,58,66)(H,59,69)(H,60,70)(H,61,67)(H,62,71)(H4,49,50,53)/t26-,33-,34-,35+,36+,37-,38-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Michigan Medical Center
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human Melanocortin 1 receptor (hMC1R) |
J Med Chem 40: 1738-48 (1997)
Article DOI: 10.1021/jm960845e
BindingDB Entry DOI: 10.7270/Q29K4BWK |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50027178
(CHEMBL2371712)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccc(-[#8])cc1)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#8]-[#7] Show InChI InChI=1S/C80H105N19O16/c1-4-5-22-57(93-78(114)68(46(2)3)98-69(105)56(81)39-48-28-32-55(100)33-29-48)70(106)89-44-65(101)99-36-15-25-64(99)77(113)97-62(42-50-27-31-52-19-10-12-21-54(52)38-50)74(110)91-59(24-14-35-88-80(84)85)73(109)95-61(41-49-26-30-51-18-9-11-20-53(51)37-49)75(111)96-63(43-66(102)103)76(112)92-58(23-13-34-87-79(82)83)72(108)94-60(40-47-16-7-6-8-17-47)71(107)90-45-67(104)115-86/h6-12,16-21,26-33,37-38,46,56-64,68,100H,4-5,13-15,22-25,34-36,39-45,81,86H2,1-3H3,(H,89,106)(H,90,107)(H,91,110)(H,92,112)(H,93,114)(H,94,108)(H,95,109)(H,96,111)(H,97,113)(H,98,105)(H,102,103)(H4,82,83,87)(H4,84,85,88)/t56-,57-,58-,59-,60-,61+,62+,63-,64-,68-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of [125I]NDP-MSH binding to Melanocortin 1 receptor expressed in HEK293 cells; (N = 4) |
J Med Chem 48: 1839-48 (2005)
Article DOI: 10.1021/jm049579s
BindingDB Entry DOI: 10.7270/Q2XD12F5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50454019
(CHEMBL2370906)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@H]([C@H](C)c1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCN)C(N)=O |wU:12.11,30.30,67.70,4.3,wD:52.53,20.19,41.42,53.55,(-5.23,-3.6,;-3.9,-2.83,;-2.56,-3.6,;-1.23,-2.83,;.1,-3.6,;.1,-5.14,;-1.23,-5.91,;-2.56,-5.14,;-1.23,-7.45,;1.44,-2.83,;1.44,-1.29,;2.77,-3.6,;4.1,-2.83,;4.1,-1.29,;5.44,-.52,;6.77,-1.29,;5.44,1.02,;5.44,-3.6,;5.44,-5.14,;6.77,-2.83,;8.1,-3.6,;8.1,-5.14,;9.44,-5.91,;9.6,-7.44,;11.11,-7.76,;11.88,-6.42,;10.85,-5.28,;9.44,-2.83,;9.44,-1.29,;10.77,-3.6,;12.11,-2.83,;12.11,-1.29,;13.44,-.52,;14.77,-1.29,;16.11,-.52,;16.11,1.02,;14.77,1.79,;13.44,1.02,;13.44,-3.6,;13.44,-5.14,;14.77,-2.83,;16.11,-3.6,;16.11,-5.14,;14.77,-5.91,;14.77,-7.45,;13.44,-8.22,;13.44,-9.76,;12.11,-10.53,;14.77,-10.53,;17.44,-2.83,;17.44,-1.29,;18.77,-3.6,;20.11,-2.83,;20.11,-1.29,;18.77,-.52,;21.44,-.52,;22.85,-1.14,;23.88,0,;23.11,1.34,;23.58,2.8,;22.55,3.94,;21.05,3.62,;20.57,2.16,;21.6,1.02,;21.44,-3.6,;21.44,-5.14,;22.78,-2.83,;24.11,-3.6,;25.44,-2.83,;26.78,-3.6,;28.11,-2.83,;29.44,-3.6,;30.78,-2.83,;24.11,-5.14,;22.78,-5.91,;25.44,-5.91,)| Show InChI InChI=1S/C51H73N15O10/c1-4-5-17-37(60-30(3)67)45(71)65-41(25-42(68)69)49(75)64-40(24-32-26-56-28-59-32)48(74)63-39(23-31-14-7-6-8-15-31)47(73)62-38(20-13-22-57-51(54)55)46(72)66-43(50(76)61-36(44(53)70)19-11-12-21-52)29(2)34-27-58-35-18-10-9-16-33(34)35/h6-10,14-16,18,26-29,36-41,43,58H,4-5,11-13,17,19-25,52H2,1-3H3,(H2,53,70)(H,56,59)(H,60,67)(H,61,76)(H,62,73)(H,63,74)(H,64,75)(H,65,71)(H,66,72)(H,68,69)(H4,54,55,57)/t29-,36+,37+,38+,39+,40+,41+,43-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
The concentration that inhibits 50% specific binding was determined against the Melanocortin 1 receptor |
J Med Chem 38: 4720-9 (1995)
BindingDB Entry DOI: 10.7270/Q2B56HRS |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50193835
(1-((3S,8aS)-3-benzyl-hexahydropyrrolo[1,2-a]pyrazi...)Show SMILES O=C(Cc1ccccc1)N1C[C@@H]2CCCN2C[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C22H26N2O/c25-22(15-19-10-5-2-6-11-19)24-17-20-12-7-13-23(20)16-21(24)14-18-8-3-1-4-9-18/h1-6,8-11,20-21H,7,12-17H2/t20-,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human MC1R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 5462-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.015
BindingDB Entry DOI: 10.7270/Q2XD11BD |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50268801
(Ac-Nle-c[Asp-trans-4-guanidinyl-Pro-DNal(2')-Arg-T...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H]2C[C@H](CN2C1=O)N=C(N)N)C(N)=O |r,wU:4.4,12.11,67.77,21.81,50.52,wD:25.25,39.41,65.73,(14,-47.54,;12.64,-46.82,;12.59,-45.28,;11.23,-44.55,;11.18,-43.02,;12.48,-42.2,;12.43,-40.66,;13.74,-39.85,;11.08,-39.94,;9.82,-42.29,;9.77,-40.75,;8.51,-43.1,;7.15,-42.38,;5.85,-43.19,;5.9,-44.73,;7.25,-45.46,;4.58,-45.55,;3.23,-44.82,;1.92,-45.64,;.56,-44.9,;-.75,-45.72,;-2.1,-44.99,;-2.16,-43.45,;-3.51,-42.73,;-4.83,-43.54,;-3.56,-41.19,;-4.94,-40.46,;-6.39,-40.96,;-7.65,-40.07,;-8.88,-40.99,;-8.38,-42.46,;-9.11,-43.8,;-8.32,-45.1,;-6.78,-45.06,;-6.05,-43.72,;-6.84,-42.42,;-2.26,-40.37,;-.94,-40.6,;.38,-41.4,;-2.31,-38.83,;-3.67,-38.11,;-3.72,-36.57,;-5.08,-35.85,;-5.13,-34.31,;-6.49,-33.58,;-6.54,-32.04,;-7.8,-34.4,;-1,-38.02,;.36,-38.75,;.41,-40.29,;1.67,-37.93,;1.61,-36.39,;.26,-35.67,;-1.06,-36.48,;-2.41,-35.77,;-2.46,-34.22,;-3.82,-33.49,;-3.87,-31.96,;-2.55,-31.14,;-1.19,-31.87,;-1.15,-33.41,;.21,-34.13,;3.02,-38.66,;4.34,-37.85,;4.28,-36.3,;5.7,-38.58,;6.78,-37.48,;8.27,-37.87,;8.27,-39.41,;5.74,-40.1,;7.1,-40.84,;8.41,-40.03,;9.5,-36.94,;9.3,-35.41,;7.88,-34.82,;10.52,-34.48,;-3.41,-45.81,;-4.77,-45.08,;-3.37,-47.35,)| Show InChI InChI=1S/C54H74N16O9/c1-3-4-15-39(63-30(2)71)47(74)69-43-27-45(72)60-21-10-9-17-38(46(55)73)65-50(77)42(25-34-28-62-37-16-8-7-14-36(34)37)67-48(75)40(18-11-22-61-53(56)57)66-49(76)41(24-31-19-20-32-12-5-6-13-33(32)23-31)68-51(78)44-26-35(64-54(58)59)29-70(44)52(43)79/h5-8,12-14,16,19-20,23,28,35,38-44,62H,3-4,9-11,15,17-18,21-22,24-27,29H2,1-2H3,(H2,55,73)(H,60,72)(H,63,71)(H,65,77)(H,66,76)(H,67,75)(H,68,78)(H,69,74)(H4,56,57,61)(H4,58,59,64)/t35-,38+,39+,40+,41-,42+,43+,44+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC1R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50184359

((3S,6S,9R,12S,15S,23S)-15-((S)-2-acetylamino-hexan...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |r,wU:21.75,50.52,12.11,4.4,39.41,wD:25.25,61.64,(19.68,-11.7,;18.32,-10.97,;18.27,-9.43,;16.91,-8.71,;16.86,-7.17,;18.17,-6.36,;18.11,-4.82,;19.42,-4,;16.76,-4.09,;15.5,-6.44,;15.45,-4.9,;14.19,-7.26,;12.83,-6.53,;11.53,-7.35,;11.58,-8.88,;12.94,-9.61,;10.27,-9.7,;8.91,-8.97,;7.6,-9.79,;6.25,-9.06,;4.94,-9.87,;3.58,-9.15,;3.53,-7.61,;2.17,-6.88,;.86,-7.7,;2.12,-5.35,;.76,-4.62,;.71,-3.08,;1.93,-2.14,;1.41,-.69,;-.14,-.74,;-1.21,.35,;-2.69,-.02,;-3.1,-1.51,;-2.03,-2.6,;-.56,-2.22,;3.43,-4.53,;4.79,-5.26,;4.84,-6.8,;6.98,-5.22,;8.76,-4.36,;10.12,-5.08,;10.17,-6.62,;8.86,-7.43,;7.5,-6.71,;6.19,-7.52,;8.83,-5.93,;6.09,-4.44,;6.04,-2.9,;4.68,-2.18,;7.35,-2.09,;7.3,-.55,;5.94,.17,;5.89,1.71,;4.53,2.44,;3.23,1.62,;3.28,.07,;4.64,-.64,;8.71,-2.82,;10.02,-2,;9.96,-.46,;11.37,-2.73,;12.68,-1.91,;12.64,-.37,;13.86,.56,;13.35,2.01,;11.81,1.97,;11.37,.5,;11.42,-4.27,;12.78,-4.99,;14.09,-4.18,;2.27,-9.96,;.91,-9.24,;2.32,-11.5,)| Show InChI InChI=1S/C50H69N15O9/c1-3-4-16-36(59-29(2)66)44(69)65-41-25-42(67)55-20-11-10-18-35(43(51)68)60-47(72)39(23-31-26-57-34-17-9-8-15-33(31)34)63-45(70)37(19-12-21-56-50(52)53)61-46(71)38(22-30-13-6-5-7-14-30)62-48(73)40(64-49(41)74)24-32-27-54-28-58-32/h5-9,13-15,17,26-28,35-41,57H,3-4,10-12,16,18-25H2,1-2H3,(H2,51,68)(H,54,58)(H,55,67)(H,59,66)(H,60,72)(H,61,71)(H,62,73)(H,63,70)(H,64,74)(H,65,69)(H4,52,53,56)/t35-,36-,37-,38+,39-,40-,41-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I]-NDP-alpha-MSH from human MC1R expressed in HEK293 cells after 40 mins by Wallac 1470 gamma counting |
Bioorg Med Chem Lett 21: 3099-102 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.019
BindingDB Entry DOI: 10.7270/Q2BC3ZVF |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50268795
(Ac-Nle-c[Asp-trans-4-guanidinyl-Pro-DPhe-Arg-Trp-L...)Show SMILES CCCC[C@H](NC(C)=O)C(=O)N[C@H]1CC(=O)NCCCC[C@H](NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H]2C[C@H](CN2C1=O)N=C(N)N)C(N)=O |r,wU:4.4,50.52,63.72,12.11,21.76,wD:25.25,39.41,61.68,(21.05,-10.24,;19.69,-9.52,;19.64,-7.97,;18.28,-7.25,;18.23,-5.71,;19.54,-4.89,;19.48,-3.35,;20.79,-2.54,;18.13,-2.63,;16.87,-4.98,;16.82,-3.44,;15.56,-5.8,;14.2,-5.07,;12.89,-5.89,;12.94,-7.43,;14.3,-8.15,;11.63,-8.24,;10.27,-7.52,;8.96,-8.33,;7.6,-7.6,;6.29,-8.41,;4.93,-7.69,;4.88,-6.15,;3.52,-5.42,;2.21,-6.24,;3.47,-3.88,;2.1,-3.15,;.64,-3.65,;-.62,-2.76,;-1.85,-3.68,;-1.35,-5.15,;-2.08,-6.49,;-1.28,-7.8,;.26,-7.76,;.99,-6.41,;.19,-5.12,;4.78,-3.07,;6.1,-3.3,;7.43,-4.09,;4.73,-1.52,;3.37,-.8,;3.32,.74,;1.96,1.47,;1.91,3.01,;.54,3.73,;.5,5.28,;-.76,2.92,;6.03,-.71,;7.4,-1.43,;7.45,-2.98,;8.71,-.62,;8.66,.92,;7.3,1.65,;7.25,3.18,;5.89,3.91,;4.58,3.1,;4.63,1.55,;5.98,.83,;10.06,-1.35,;11.38,-.54,;11.33,1.01,;12.75,-1.27,;13.83,-.17,;15.32,-.56,;15.32,-2.1,;12.78,-2.79,;14.15,-3.53,;15.45,-2.72,;16.54,.37,;16.34,1.9,;14.92,2.5,;17.57,2.84,;3.63,-8.5,;2.26,-7.78,;3.67,-10.04,)| Show InChI InChI=1S/C50H72N16O9/c1-3-4-16-35(59-28(2)67)43(70)65-39-25-41(68)56-20-11-10-18-34(42(51)69)61-46(73)38(23-30-26-58-33-17-9-8-15-32(30)33)63-44(71)36(19-12-21-57-49(52)53)62-45(72)37(22-29-13-6-5-7-14-29)64-47(74)40-24-31(60-50(54)55)27-66(40)48(39)75/h5-9,13-15,17,26,31,34-40,58H,3-4,10-12,16,18-25,27H2,1-2H3,(H2,51,69)(H,56,68)(H,59,67)(H,61,73)(H,62,72)(H,63,71)(H,64,74)(H,65,70)(H4,52,53,57)(H4,54,55,60)/t31-,34+,35+,36+,37-,38+,39+,40+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Agonist activity at human MC1R expressed in HEK293 cells assessed as stimulation of intracellular cAMP level |
J Med Chem 52: 3627-35 (2009)
Article DOI: 10.1021/jm801300c
BindingDB Entry DOI: 10.7270/Q2D21ZJ5 |
More data for this
Ligand-Target Pair | |
Melanocyte-stimulating hormone receptor
(Homo sapiens (Human)) | BDBM50125750
(CHEMBL13910 | N-[1-[2-(4-Butyryl-4-phenyl-piperidi...)Show SMILES CCCC(=O)C1(CCN(CC1)C(=O)[C@@H](Cc1ccc(OC)cc1)NC(=O)[C@@H](Cc1cnc[nH]1)NC(=O)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C38H43N5O5/c1-3-10-34(44)38(29-13-8-5-9-14-29)19-21-43(22-20-38)37(47)33(23-27-15-17-31(48-2)18-16-27)42-36(46)32(24-30-25-39-26-40-30)41-35(45)28-11-6-4-7-12-28/h4-9,11-18,25-26,32-33H,3,10,19-24H2,1-2H3,(H,39,40)(H,41,45)(H,42,46)/t32-,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration of the compound against human Melanocortin 1 receptor (MC1R) |
J Med Chem 46: 1123-6 (2003)
Article DOI: 10.1021/jm025600i
BindingDB Entry DOI: 10.7270/Q2KK9B43 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































