

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073989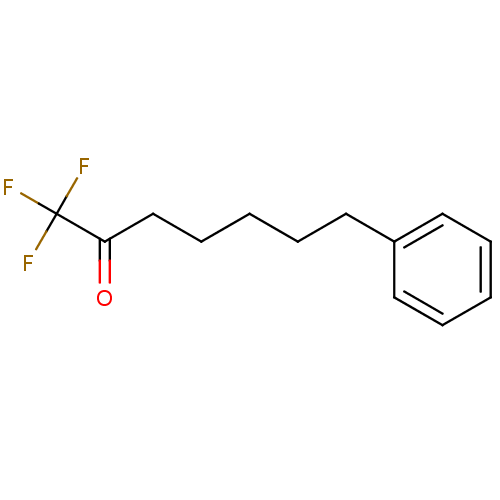 (1,1,1-Trifluoro-7-phenyl-heptan-2-one | 1,1,1-Trif...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073989 (1,1,1-Trifluoro-7-phenyl-heptan-2-one | 1,1,1-Trif...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50262188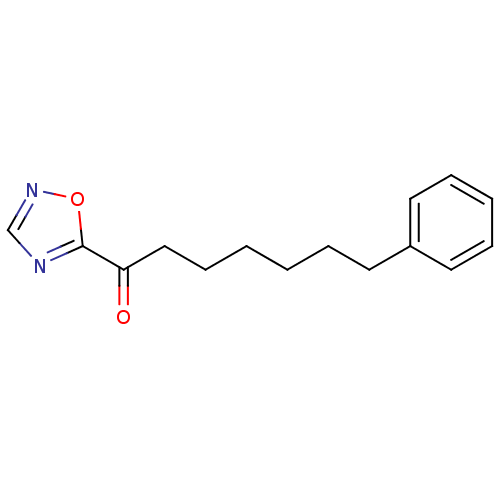 (1-(1,2,4-Oxadiazol-5-yl)-7-phenylheptan-1-one | CH...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 51: 4392-403 (2008) Article DOI: 10.1021/jm800136b BindingDB Entry DOI: 10.7270/Q26D5TW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073974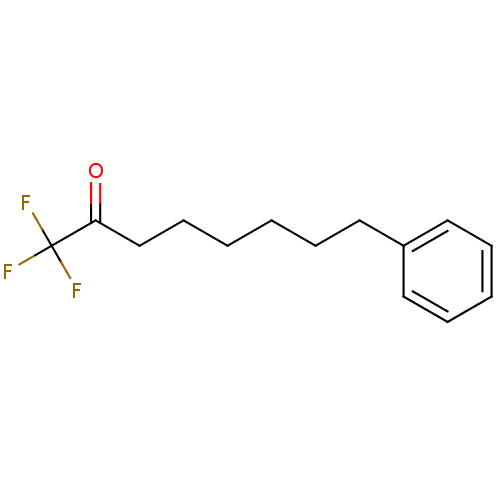 (1,1,1-Trifluoro-8-phenyl-octan-2-one | 1,1,1-Trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073974 (1,1,1-Trifluoro-8-phenyl-octan-2-one | 1,1,1-Trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073977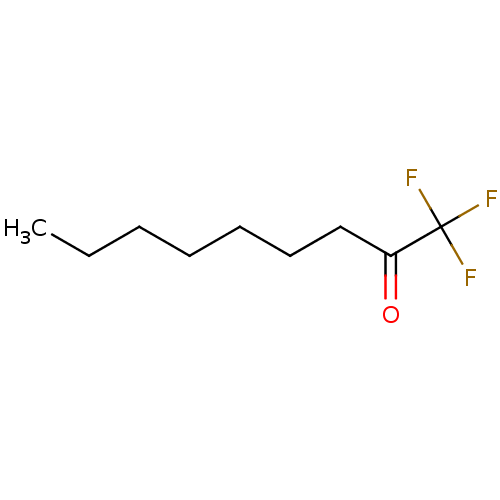 (1,1,1-Trifluoro-nonan-2-one | 1,1,1-trifluorononan...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073977 (1,1,1-Trifluoro-nonan-2-one | 1,1,1-trifluorononan...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570550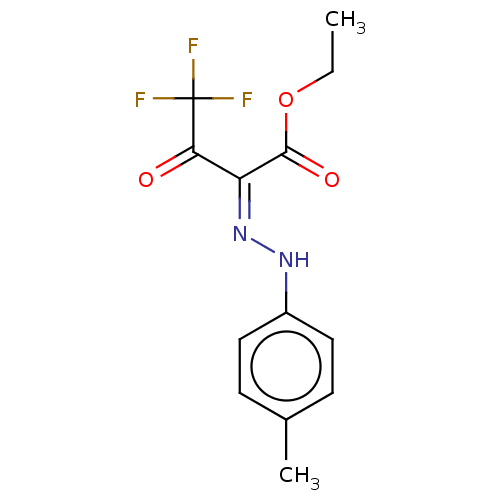 (CHEMBL4849361) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM23316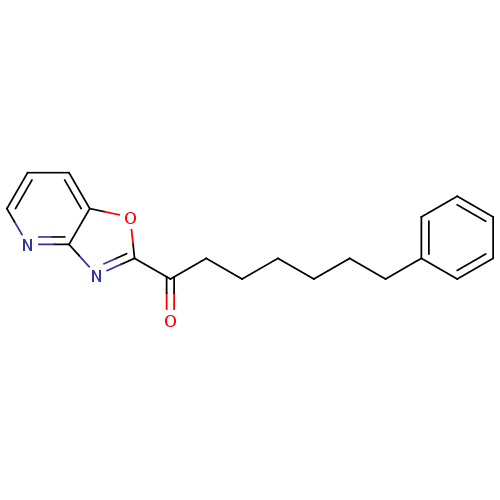 (7-phenyl-1-{pyrido[2,3-d][1,3]oxazol-2-yl}heptan-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM23316 (7-phenyl-1-{pyrido[2,3-d][1,3]oxazol-2-yl}heptan-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50163190 (2,2,2-Trifluoro-1-phenyl-ethanone | CHEMBL293277) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570552 (CHEMBL4855755) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50372373 (CHEMBL261172) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50161525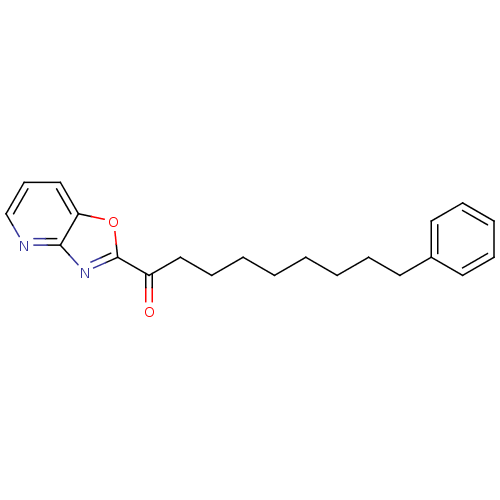 (1-(oxazolo[4,5-b]pyridin-2-yl)-9-phenylnonan-1-one...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073987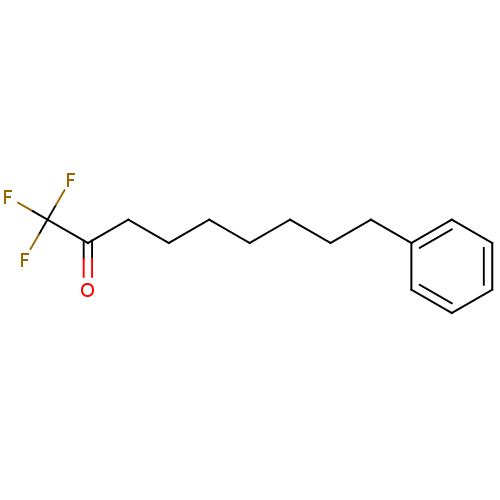 (1,1,1-Trifluoro-9-phenyl-nonan-2-one | 1,1,1-trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073987 (1,1,1-Trifluoro-9-phenyl-nonan-2-one | 1,1,1-trifl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195572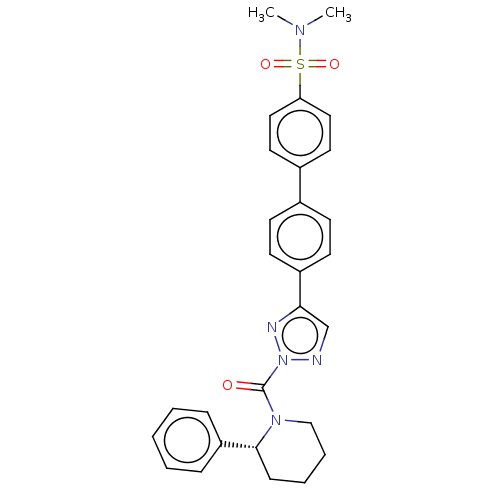 ((R)-N,N-dimethyl-4'-(2-(2-phenylpiperidine-1-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195571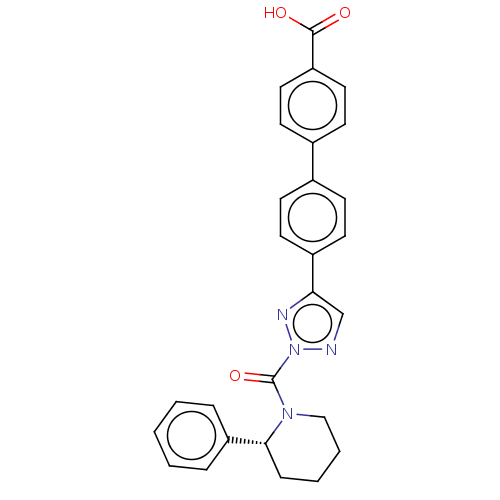 ((R)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50161520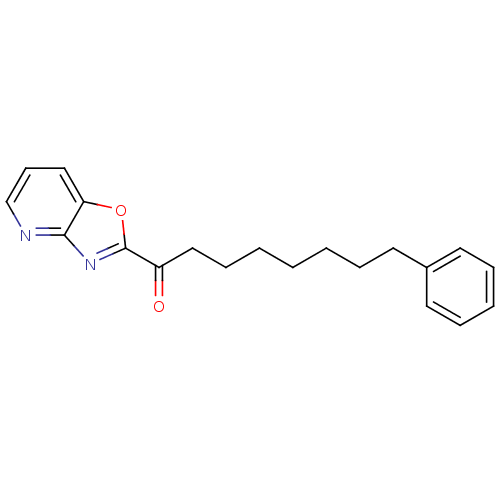 (1-(oxazolo[4,5-b]pyridin-2-yl)-8-phenyloctan-1-one...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195575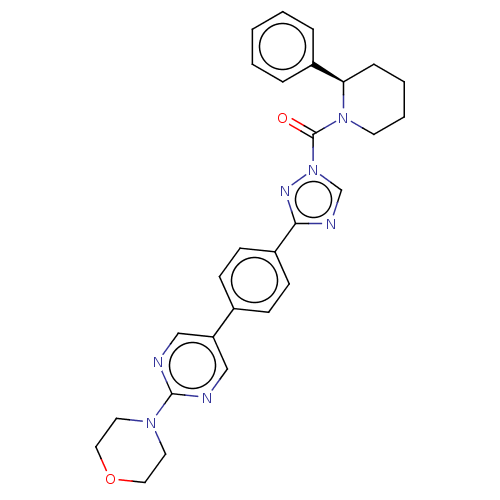 ((R)-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195574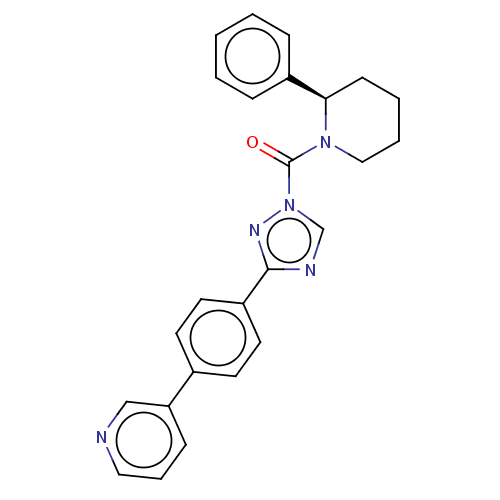 ((R)-(2-phenylpiperidin-1-yl)(3-(4-(pyridin-3-yl)ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195570 ((S)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570551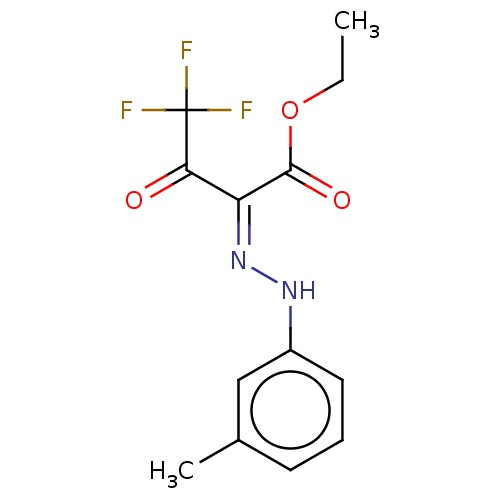 (CHEMBL4853637) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50371964 (CHEMBL273139) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant carboxylesterase 1 after 15 mins | Bioorg Med Chem 16: 2114-30 (2008) Article DOI: 10.1016/j.bmc.2007.10.081 BindingDB Entry DOI: 10.7270/Q2K938DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50371961 (CHEMBL270373) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant carboxylesterase 1 after 15 mins | Bioorg Med Chem 16: 2114-30 (2008) Article DOI: 10.1016/j.bmc.2007.10.081 BindingDB Entry DOI: 10.7270/Q2K938DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570549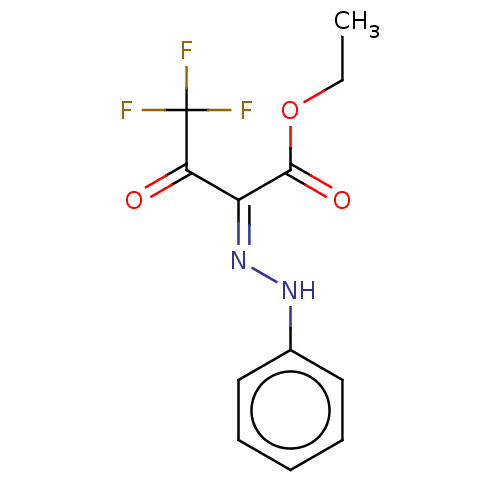 (CHEMBL4853742) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195573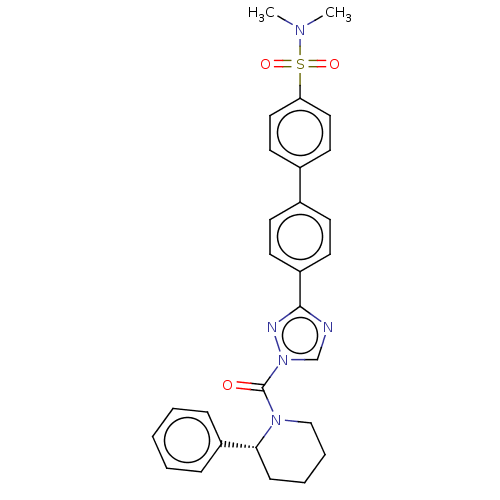 ((R)-4'-(1-(2-phenylpiperidine-1-carbonyl)-1H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073985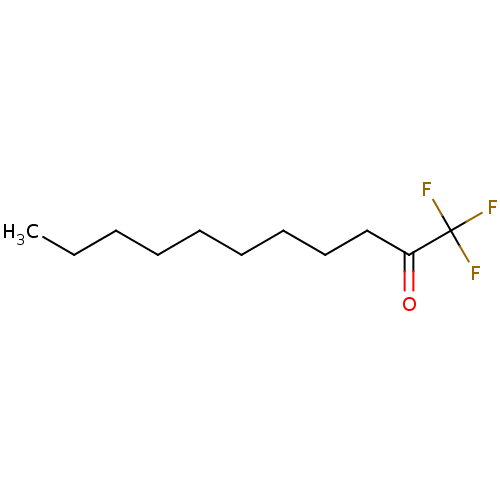 (1,1,1-Trifluoro-undecan-2-one | 1,1,1-trifluoround...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50163153 (1,1,1-Trifluoro-3-phenyl-propan-2-one | CHEMBL2924...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50073985 (1,1,1-Trifluoro-undecan-2-one | 1,1,1-trifluoround...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50161524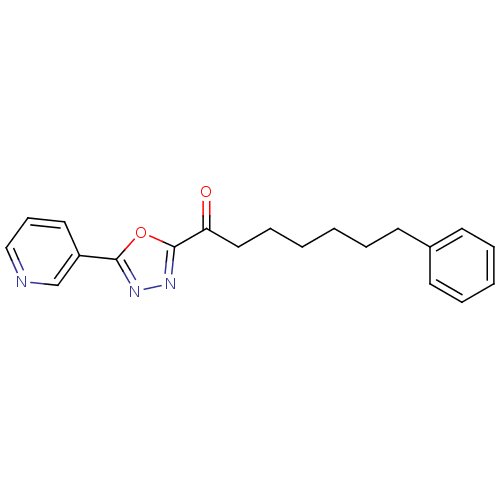 (7-Phenyl-1-(5-pyridin-3-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50262305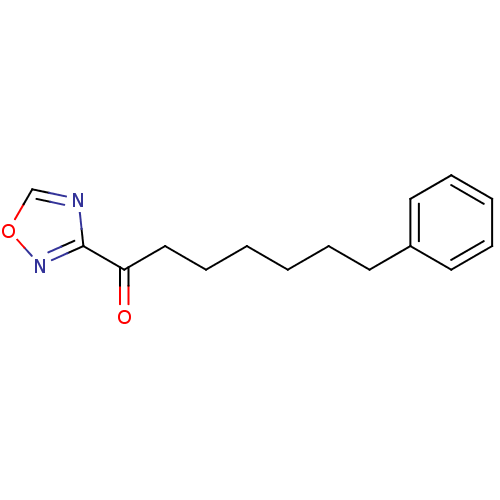 (1-(1,2,4-Oxadiazol-3-yl)-7-phenylheptan-1-one | CH...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 51: 4392-403 (2008) Article DOI: 10.1021/jm800136b BindingDB Entry DOI: 10.7270/Q26D5TW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570558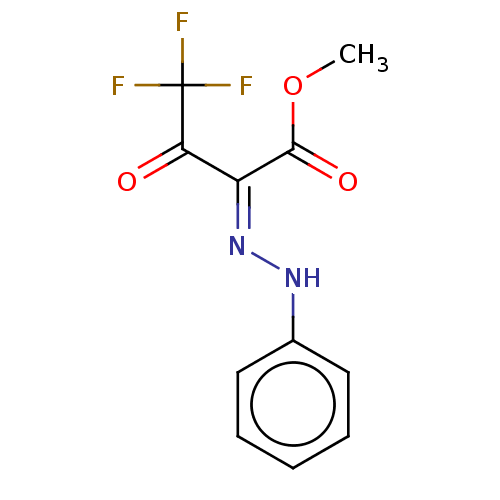 (CHEMBL4849436) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50371970 (CHEMBL89506) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant carboxylesterase 1 after 15 mins | Bioorg Med Chem 16: 2114-30 (2008) Article DOI: 10.1016/j.bmc.2007.10.081 BindingDB Entry DOI: 10.7270/Q2K938DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570555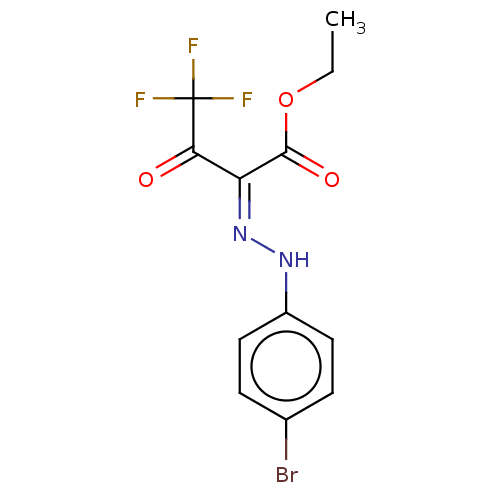 (CHEMBL4868014) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570559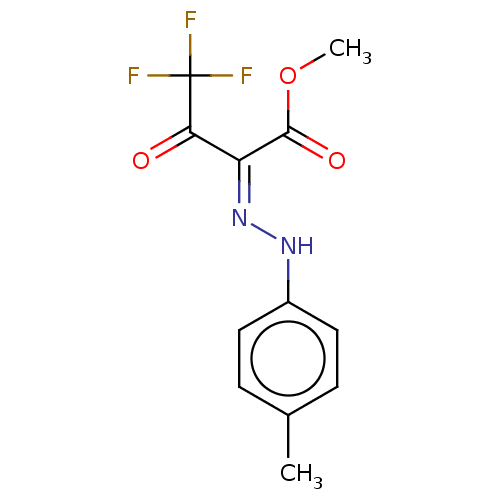 (CHEMBL4868687) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50372372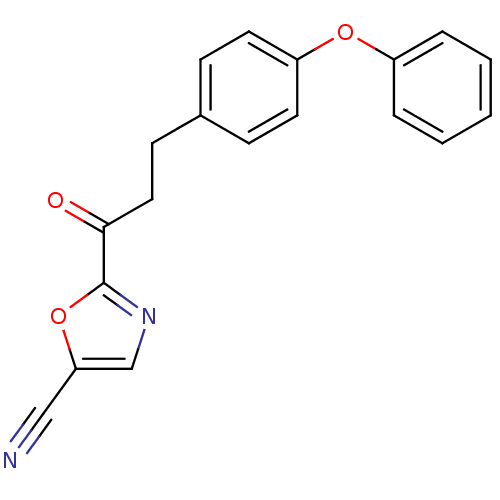 (CHEMBL272038) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50371964 (CHEMBL273139) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human recombinant carboxylesterase 1 after 5 mins | Bioorg Med Chem 16: 2114-30 (2008) Article DOI: 10.1016/j.bmc.2007.10.081 BindingDB Entry DOI: 10.7270/Q2K938DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50204512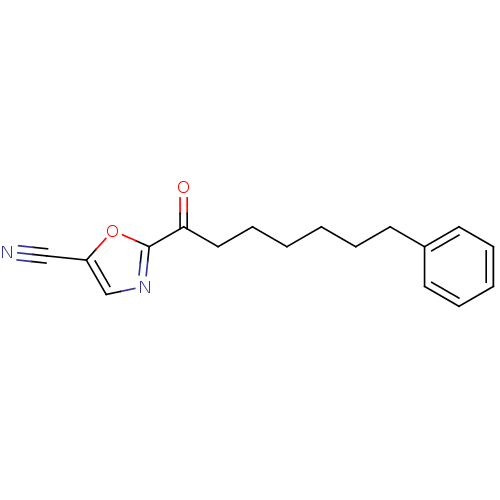 (2-(7-phenylheptanoyl)oxazole-5-carbonitrile | CHEM...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50157107 (1-Benzooxazol-2-yl-6-phenyl-hexan-1-one | CHEMBL36...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of triacylgylcerol hydrolase using FP-Rh radioligand | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50204493 (1-(oxazol-2-yl)-7-phenylheptan-1-one | CHEMBL22012...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 50: 1058-68 (2007) Article DOI: 10.1021/jm0611509 BindingDB Entry DOI: 10.7270/Q26H4J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50204512 (2-(7-phenylheptanoyl)oxazole-5-carbonitrile | CHEM...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 50: 1058-68 (2007) Article DOI: 10.1021/jm0611509 BindingDB Entry DOI: 10.7270/Q26H4J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50204493 (1-(oxazol-2-yl)-7-phenylheptan-1-one | CHEMBL22012...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 51: 937-47 (2008) Article DOI: 10.1021/jm701210y BindingDB Entry DOI: 10.7270/Q2QV3NCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50204493 (1-(oxazol-2-yl)-7-phenylheptan-1-one | CHEMBL22012...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of TGH (unknown origin) | Bioorg Med Chem Lett 18: 5842-6 (2008) Article DOI: 10.1016/j.bmcl.2008.06.084 BindingDB Entry DOI: 10.7270/Q2DN44V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50157107 (1-Benzooxazol-2-yl-6-phenyl-hexan-1-one | CHEMBL36...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against Triacylglycerol hydrolase | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50204493 (1-(oxazol-2-yl)-7-phenylheptan-1-one | CHEMBL22012...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Chemical Biology Curated by ChEMBL | Assay Description Inhibition of TGH | J Med Chem 51: 4392-403 (2008) Article DOI: 10.1021/jm800136b BindingDB Entry DOI: 10.7270/Q26D5TW3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50204512 (2-(7-phenylheptanoyl)oxazole-5-carbonitrile | CHEM...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of TGH (unknown origin) | Bioorg Med Chem Lett 18: 5842-6 (2008) Article DOI: 10.1016/j.bmcl.2008.06.084 BindingDB Entry DOI: 10.7270/Q2DN44V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM50570560 (CHEMBL4872772) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113385 BindingDB Entry DOI: 10.7270/Q2FJ2MK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 248 total ) | Next | Last >> |