

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SETDB1 (Homo sapiens (Human)) | BDBM50009672 (AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SETDB1 (unknown origin) by HMT assay | Bioorg Med Chem Lett 25: 1532-7 (2015) Article DOI: 10.1016/j.bmcl.2015.02.017 BindingDB Entry DOI: 10.7270/Q2TM7CSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETDB1 (Homo sapiens (Human)) | BDBM50530712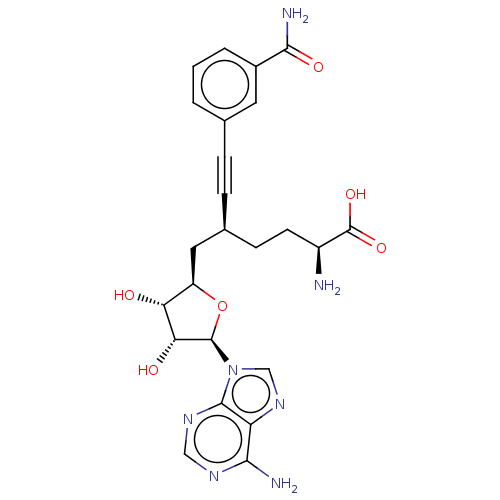 (CHEMBL4591248) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of human SETDB1 expressed in sf9 insect cells assessed as reduction in methylated histone H3 full length level using histone H3 full lengt... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETDB1 (Homo sapiens (Human)) | BDBM50530712 (CHEMBL4591248) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioKin Ltd. Curated by ChEMBL | Assay Description Inhibition of human SETDB1 expressed in sf9 insect cells assessed as reduction in methylated histone H3 full length level using histone H3 full lengt... | J Med Chem 62: 9837-9873 (2019) Article DOI: 10.1021/acs.jmedchem.9b01238 BindingDB Entry DOI: 10.7270/Q2D50RDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETDB1 (Homo sapiens (Human)) | BDBM50051117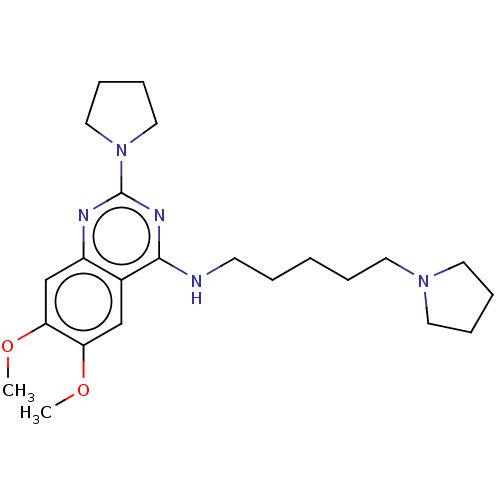 (CHEMBL3318285) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of SETDB1 (unknown origin) assessed as incorporation of tritium-labeled methyl group to lysine or arginine residues of peptide substrate b... | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETDB1 (Homo sapiens (Human)) | BDBM225230 (EED226 | US11013745, Compound EED226) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research | Assay Description All HMT reactions were performed as described previously [Nasveschuk et al., ACS Med. Chem. Lett., 5:378-383]. | Nat Chem Biol 13: 381-388 (2017) Article DOI: 10.1038/nchembio.2304 BindingDB Entry DOI: 10.7270/Q2S75F64 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETDB1 (Homo sapiens (Human)) | BDBM50063670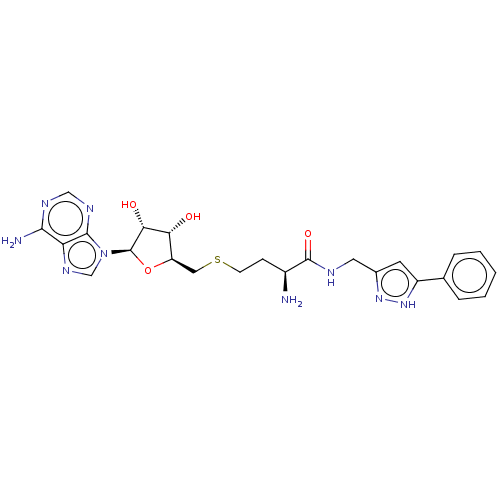 (CHEMBL3397332) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SETDB1 (unknown origin) by HMT assay | Bioorg Med Chem Lett 25: 1532-7 (2015) Article DOI: 10.1016/j.bmcl.2015.02.017 BindingDB Entry DOI: 10.7270/Q2TM7CSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||